Abstract
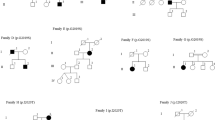
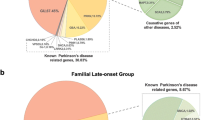
Parkinson’s disease (PD) is caused by a combination of genetic and environmental factors. Notably, genetic risk factors vary according to ethnicity and geographical regions, and few studies have analyzed the frequency of PD causative genes in Japanese patients. Therefore, we performed genetic analyses of Japanese patients with PD. We recruited 221 participants, including 26 patients with familial PD. Genetic risk factors were evaluated by target sequencing and gene dosage analysis. We detected the genetic risk factors in 58 cases (26.2%) and classified patients into three groups to clarify the differences in genetic risk factors by age at onset (AAO). The early-onset group (AAO < 50 years) included 18 cases (44.7%), who tended to have a larger number of genetic risk factors than the later-onset groups. Regarding the AAO for each causative gene, patients with PRKN variants were significantly younger at onset than those bearing LRRK2 variants. LRRK2 variants showed similar frequency in each AAO group. Of note, we identified two novel variants. Patients with early-onset PD have more genetic risk factors than patients with late-onset PD. In Japanese patients with PD, PRKN, and LRRK2 were the major PD-related genes. Particularly, LRRK2 was a common genetic factor in all age groups because of the presence of the Asian-specific variant such as LRRK2 p.G2385R. Accumulation of genetic and clinical data can contribute to the development of treatments for PD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Yamawaki M, Kusumi M, Kowa H, Nakashima K. Changes in prevalence and incidence of Parkinson’s disease in Japan during a quarter of a century. Neuroepidemiology. 2009;32:263–9.
Deng H, Wang P, Jankovic J. The genetics of Parkinson disease. Ageing Res Rev. 2018;42:72–85.
Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson’s disease and parkinsonism. Ann Neurol. 2006;60:389–98.
Rudenko IN, Kaganovich A, Langston RG, Beilina A, Ndukwe K, Kumaran R, et al. The G2385R risk factor for Parkinson’s disease enhances CHIP-dependent intracellular degradation of LRRK2. Biochem J. 2017;474:1547–58.
Shu L, Zhang Y, Sun Q, Pan H, Tang B. A comprehensive analysis of population differences in LRRK2 variant distribution in Parkinson’s disease. Front Aging Neurosci. 2019;11:13.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
Lin CH, Chen PL, Tai CH, Lin HI, Chen CS, Chen ML, et al. A clinical and genetic study of early-onset and familial parkinsonism in Taiwan: an integrated approach combining gene dosage analysis and next-generation sequencing. Mov Disord. 2019;34:506–15.
Mitsui J, Mizuta I, Toyoda A, Ashida R, Takahashi Y, Goto J, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson’s disease. Arch Neurol. 2009;6:571–6.
Tan MMX, Malek N, Lawton MA, Hubbard L, Pittman AM, Joseph T, et al. Genetic analysis of Mendelian mutations in a large UK population-based Parkinson’s disease study. Brain. 2019;142:2828–44.
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annsei G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. Engl J Med. 2009;361:1651–61.
Zabetian CP, Hutter CM, Factor SA, Nutt JG, Higgins DS, Griffith A, et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson’s disease. Ann Neurol. 2007;62:137–44.
Thacker EL, Ascherio A. Familial aggregation of Parkinson’s disease: a meta-analysis. Mov Disord. 2008;23:1174–83.
Wirdefeldt K, Gatz M, Reynolds CA, Prescott CA, Pedersen NL. Heritability of Parkinson disease in Swedish twins: a longitudinal study. Neurobiol Aging. 2011;32:e1–8.
Ishikawa A, Takahashi H, Tanaka H, Hayashi T, Tsuji S. Clinical features of familial diffuse Lewy body disease. Eur Neurol. 1997;38:34–8.
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8.
Kumazawa R, Tomiyama H, Li Y, Imamichi Y, Funayama M, Yoshino H, et al. Mutation analysis of the PINK1 gene in 391 patients with Parkinson disease. Arch Neurol. 2008;65:802–8.
Tomiyama H, Li Y, Funayama M, Hasegawa K, Yoshino H, Kubo S, et al. Clinicogenetic study of mutations in LRRK2 exon 41 in Parkinson’s disease patients from 18 countries. Mov Disord. 2006;21:1102–8.
Ning YP, Kanai K, Tomiyama H, Li Y, Funayama M, Yoshino H, et al. PARK9-linked parkinsonism in eastern Asia: mutation detection in ATP13A2 and clinical phenotype. Neurology. 2008;70:1491–3.
Yoshino H, Tomiyama H, Tachibana N, Ogaki K, Li Y, Funayama M, et al. Phenotypic spectrum of patients with PLA2G6 mutation and PARK14-linked parkinsonism. Neurology. 2010;75:1356–61.
Ando M, Funayama M, Li Y, Kashihara K, Murakami Y, Ishizu N, et al. VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov Disord. 2012;27:1413–7.
Funayama M, Ohe K, Amo T, Furuya N, Yamaguchi J, Saiki S, et al. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol. 2015;14:274–82.
Taghavi S, Chaouni R, Tafakhori A, Azcona LJ, Firouzabadi SG, Omrani MD, et al. A clinical and molecular genetic study of 50 families with autosomal recessive parkinsonism revealed known and novel gene mutations. Mol Neurobiol. 2018;55:3477–89.
Hattori N, Mizuno Y. Pathogenetic mechanisms of parkin in Parkinson’s disease. Lancet. 2004;364:722–4.
Klein C, Lohmann-Hedrich K, Rogaeva E, Schlossmacher MG, Lang AE. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 2007;6:652–62.
Huttenlocher J, Stefansson H, Steinberg S, Helgadottir HT, Sveinbjornsdottir S, Riess O, et al. Heterozygote carriers for CNVs in PARK2 are at increased risk of Parkinson’s disease. Hum Mol Genet. 2015;24:5637–43.
Abou-Sleiman PM, Muqit MM, McDonald NQ, Yang YX, Gandhi S, Healy DG, et al. A heterozygous effect for PINK1 mutations in Parkinson’s disease? Ann Neurol. 2006;60:414–9.
Funayama M, Li Y, Tomiyama H, Yoshino H, Imamichi Y, Yamamoto M, et al. Leucine-rich repeat kinase 2 G2385R variant is a risk factor for Parkinson disease in Asian population. Neuroreport. 2007;18:273–5.
Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J. 2007;405:307–17.
Li NN, Tan EK, Chang XL, Mao XY, Zhang JH, Zhao DM, et al. Genetic analysis of LRRK2 A419V variant in ethnic Chinese. Neurobiol Aging. 2012;33:e1–3.
Li K, Tang BS, Liu ZH, Kang JF, Zhang Y, Shen L, et al. LRRK2 A419V variant is a risk factor for Parkinson’s disease in Asian population. Neurobiol Aging. 2015;36:e11–5.
Gopalai AA, Lim SY, Aziz ZA, Lim SK, Tan LP, Chong YB, et al. Lack of association between the LRRK2 A419V variant and Asian Parkinson’s disease. Ann Acad Med Singap. 2013;42:237–40.
Wu YR, Tan LC, Fu X, Chen CM, Au WL, Chen L, et al. LRRK2 A419V is not associated with Parkinson’s disease in different Chinese populations. PLoS ONE. 2012;7:e36123.
Acknowledgements
We are grateful to the patients who participated in this study. We thank Ms. Eiko Nakajima for providing technical assistance.
Funding
YI receives support from Grants-in-Aid from the Research Committee of CNS Degenerative Diseases, the Ministry of Health, Labour and Welfare of Japan (20FC1049) and HK receives support from The Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
The study was designed by YK, HMorino, and HK. Statistical analysis and interpretation were performed by YK, HMorino, and RO. Sample preparation and genotyping were performed by YK and KK. Patient recruitment was performed by TK, MK, TT, YI, HMorino, and HK. Review and critique were performed by HMaruyama and HK. Drafting and revising of the manuscript were performed by all authors. All authors have read and approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kanaya, Y., Kume, K., Morino, H. et al. Analysis of genetic risk factors in Japanese patients with Parkinson’s disease. J Hum Genet 66, 957–964 (2021). https://doi.org/10.1038/s10038-021-00910-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-021-00910-4
This article is cited by
-
Gene Panel Sequencing Analysis Revealed a Strong Contribution of Rare Coding Variants to the Risk of Parkinson’s Disease in Sporadic Moroccan Patients
Journal of Molecular Neuroscience (2023)