Abstract
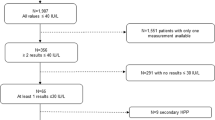
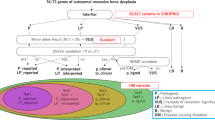
Although alkaline phosphatase (ALP) activity is relatively low in carriers of recessive type hypophosphatasia (HPP), most are asymptomatic and therefore do not undergo medical evaluations. We analyzed the association of ALP-encoding ALPL variants with serum ALP and bone traits in the general Japanese population. Study participants (n = 9671) were from the Nagahama Study, which was a longitudinal cohort study of an apparently healthy general Japanese population. ALPL variants were analyzed by whole-genome sequencing or TaqMan probe assays using DNA extracted from peripheral blood samples. The speed of sound in calcaneal bone was assessed by quantitative ultrasound (QUS) and used as surrogate measures of bone mineral density. We identified 13 ALPL variants. Minor allele frequencies of three variants were higher than expected. Variant c.529G > A has been reported as a possible pathogenic variant for adult type HPP. Variants c.979C > T and c.1559delT are reported as pathogenic variants for perinatal severe HPP or infantile HPP. The allele frequencies of c.529G > A, c.979C > T, and c.1559delT were 0.0107, 0.0040, and 0.0014, respectively. Serum ALP activity was significantly lower and differed among the three variants (P < 0.001), as well as between individuals with and without any of the three variants (P < 0.001). Serum ALP activity was inversely associated with QUS values, although no direct association was observed between the ALPL variants and QUS values. An association between serum ALP activity and QUS was confirmed; however, we failed to detect an association between ALPL variants and bone traits in the general Japanese population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mornet E. Hypophosphatasia. Metabolism. 2018;82:142–55.
Mornet E, Yvard A, Taillandier A, Fauvert D, Simon-Bouy B. A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Genet. 2011;75:439–45.
Okazaki Y, Kitajima H, Mochizuki N, Kitaoka T, Michigami T, Ozono K. Lethal hypophosphatasia successfully treated with enzyme replacement from day 1 after birth. Eur J Pediatr. 2016;175:433–7.
The Tissue Nonspecific Alkaline Phosphatase Gene Mutations Database. http://www.sesep.uvsq.fr/03_hypo_mutations.php [Accessed 30 Aug 2019].
Taketani T, Onigata K, Kobayashi H, Mushimoto Y, Fukuda S, Yamaguchi S. Clinical and genetic aspects of hypophosphatasia in Japanese patients. Arch Dis Child. 2014;99:211–5.
Watanabe A, Karasugi T, Sawai H, Naing BT, Ikegawa S, Orimo H, et al. Prevalence of c.1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasia in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet. 2011;56:166–8.
Takahashi Y, Sawai H, Murotsuki J, Satoh S, Yamada T, Hayakawa H, et al. Parental serum alkaline phosphatase activity as an auxiliary tool for prenatal diagnosis of hypophosphatasia. Prenat Diagn. 2017;37:491–6.
Hofmann C, Girschick H, Mornet E, Schneider D, Jakob F, Mentrup B. Unexpected high intrafamilial phenotypic variability observed in hypophosphatasia. Eur J Hum Genet. 2014;22:1160–4.
Michigami T, Uchihashi T, Suzuki A, Tachikawa K, Nakajima S, Ozono K. Common mutations F310L and T1559del in the tissue-nonspecific alkaline phosphatase gene are related to distinct phenotypes in Japanese patients with hypophosphatasia. Eur J Pediatr. 2005;164:277–82.
Gehring B, Mornet E, Plath H, Hansmann M, Bartmann P, Brenner RE. Perinatal hypophosphatasia: diagnosis and detection of heterozygote carriers within the family. Clin Genet. 1999;56:313–7.
Spentchian M, Merrien Y, Herasse M, Dobbie Z, Gläser D, Holder SE, et al. Severe hypophosphatasia: characterization of fifteen novel mutations in the ALPL gene. Hum Mutat. 2003;22:105–6.
Zankl A, Mornet E, Wong S. Specific ultrasonographic features of perinatal lethal hypophosphatasia. Am J Med Genet A. 2008;146A:1200–4.
Sergi C, Mornet E, Troeger J, Voigtlaender T. Perinatal hypophosphatasia: radiology, pathology and molecular biology studies in a family harboring a splicing mutation (648+1A) and a novel missense mutation (N400S) in the tissue-nonspecific alkaline phosphatase (TNSALP) gene. Am J Med Genet. 2001;103:235–40.
Tabara Y, Ikezoe T, Yamanaka M, Setoh K, Segawa H, Kawaguchi T, et al. Nagahama Study Group. Advanced glycation end product accumulation is associated with low skeletal muscle mass, weak muscle strength, and reduced bone density: the Nagahama study. J Gerontol A Biol Sci Med Sci. 2019. https://doi.org/10.1093/gerona/gly233.
Setoh K, Terao C, Muro S, Kawaguchi T, Tabara Y, Takahashi M, et al. Three missense variants of metabolic syndrome-related genes are associated with alpha-1 antitrypsin levels. Nat Commun. 2015;6:7754.
Hosoda Y, Yoshikawa M, Miyake M, Tabara Y, Ahn J, Woo SJ, et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc Natl Acad Sci USA. 2018;115:6261–6.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164.
Otani T, Fukunaga M, Yoh K, Miki T, Yamazaki K, Kishimoto H, et al. Attempt at standardization of bone quantitative ultrasound in Japan. J Med Ultrason. 2001;45:3–13. 2018.
Morita R, Yamamoto I, Yuu I, et al. Quantitative ultrasound for the assessment of bone status. Osteoporos Int. 1997;7:S128–34.
Nakano N, Eto A, Chikaura Y, Matsubara T, Koga K, Kawada K. Measurement of enzyme activity of control materials containing human enzymes by IFCC reference methods. Rinsho Byori. 1991;39:981–93. [in Japanese].
Harris H. The human alkaline phosphatases: what we know and what we don't know. Clin Chim Acta. 1990;186:133–50.
Yasuda J, Katsuoka F, Danjoh I, Kawai Y, Kojima K, Nagasaki M, et al. Regional genetic differences among Japanese populations and performance of genotype imputation using whole-genome reference panel of the Tohoku Medical Megabank Project. BMC Genomics. 2018;19:551.
Orimo H, Goseki-Sone M, Hosoi T, Shimada T. Functional assay of the mutant tissue-nonspecific alkaline phosphatase gene using U2OS osteoblast-like cells. Mol Genet Metab. 2008;94:375–81.
Nguyen HH, van de Laarschot DM, Verkerk AJMH, Milat F, Zillikens MC, Ebeling PR. Genetic risk factors for atypical femoral fractures (AFFs): a systematic review. JBMR. 2018;2:1–11.
Nielson CM, Zmuda JM, Carlos AS, Wagoner WJ, Larson EA, Orwoll ES, et al. Rare coding variants in ALPL are associated with low serum alkaline phosphatase and low bone mineral density. J Bone Min Res. 2012;27:93–103.
Flöter M, Bittar CK, Zabeu JL, Carneiro AC. Review of comparative studies between bone densitometry and quantitative ultrasound of the calcaneus in osteoporosis. Acta Reumatol Port. 2011;36:327–35.
Linglart A, Biosse-Duplan M. Hypophosphatasia. Curr Osteoporos Rep. 2016;14:95–105.
Ozono K, Yamagata M, Michigami T, Nakajima S, Sakai N, Cai G, et al. Identification of novel missense mutations (Phe310Leu and Gly439Arg) in a neonatal case of hypophosphatasia. J Clin Endocrinol Metab. 1996;81:4458–61.
Komaru K, Ishida Y, Amaya Y, Goseki-Sone M, Orimo H, Oda K. Novel aggregate formation of a frame-shift mutant protein of tissue-nonspecific alkaline phosphatase is ascribed to three cysteine residues in the C-terminal extension. Retarded Secret Proteasomal Degrad FEBS J. 2005;272:1704–17.
Acknowledgements
We are extremely grateful to the Nagahama City Office and the non-profit organization Zeroji Club for their assistance in analyzing the Nagahama Study. The Nagahama Study was supported by a University grant, the Center of Innovation Program, the Global University Project, and Grant-in-Aid for Scientific Research (25293141, 26670313, 26293198, 17H04182, 17H04126, 17H04123, and 18K18450) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Practical Research Project for Rare/Intractable Diseases (ek0109070, ek0109070, ek0109196, and ek0109348), the Comprehensive Research on Aging and Health Science Research Grants for Dementia R&D (dk0207006, dk0207027), the Program for an Integrated Database of Clinical and Genomic Information (kk0205008), the Practical Research Project for Lifestyle-related Diseases including Cardiovascular Diseases and Diabetes Mellitus (ek0210066, ek0210096, and ek0210116), and the Research Program for Health Behavior Modification by Utilizing IoT (le0110005) from Japan Agency for Medical Research and Development (AMED); the Takeda Medical Research Foundation; Mitsubishi Foundation; Daiwa Securities Health Foundation; and Sumitomo Foundation. This study was also supported by Research on Rare and Intractable Diseases, Health, Labor and Welfare Sciences Research Grants [H28-Nanchitou (Nan)-Ippan-017 and 19FC1006].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagata, M., Setoh, K., Takahashi, M. et al. Association of ALPL variants with serum alkaline phosphatase and bone traits in the general Japanese population: The Nagahama Study. J Hum Genet 65, 337–343 (2020). https://doi.org/10.1038/s10038-019-0712-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0712-3