Abstract
Genome editing of the human embryo using CRISPR/Cas9 has the potential to prevent hereditary diseases from being transmitted to the next generation. However, attitudes to this technology have not been examined sufficiently among the genetic professionals who will use it in the near future. We conducted a questionnaire survey of Japanese clinical geneticists and certified genetic counselors. Differences were observed between them in their recognition of this technology and impressions on its difficulty and cost. Both groups worried about misuse of it, with insufficient information and rules. As key elements for such rules, they considered ethics, safety, and purpose. Most disapproved of modifying physical traits as an enhancement, though they hoped for the treatment of severe diseases. At current clinical sites, they tended to adopt a prudent attitude by mentioning only the possibility of genome editing in the future. Academic policies and legislation are required, especially for application in human embryos, through a consensus of professionals and general citizens. Furthermore, professionals should maintain awareness of new developments and regularly reexamine attitudes for the ongoing development of more suitable rules, education systems, and clinical protocols. As preparation for changes, opportunities to address ethical issues and initiate discussions are also required.
Similar content being viewed by others
Introduction
Extensive studies of the pathophysiology of congenital diseases have contributed to their treatment. Enzyme replacement therapy has drastically improved the prognoses of metabolic enzyme deficiencies. However, therapy has to be lifelong. Subsequent gene therapies incorporating a normal gene into the genome of patient's somatic cells might solve this problem [1, 2]. Numerous gene therapies have been developed, and 2930 clinical trials are being conducted around the world as of December 2018 [3]. Some gene therapeutic methods are approved as products against specific diseases in the United States and European Union [4], and two products are almost approved in Japan [5, 6]. When genetic modifications are applied to not only somatic cells but also fertilized eggs, it is theoretically feasible to prevent hereditary diseases from being transmitted to the next generation. Although applications to fertilized eggs remain a controversial subject in both technical and ethical terms [7, 8], mitochondrial replacement against mitochondrial diseases has been reported [9]. In Japan, a clinical trial for infertility treatment using eggs with augmented developmental competence via autologous mitochondrial transfer is registered in the database of the University Hospital Medical Information Network (UMIN000021387).
Therapeutic modification of a gene into an inappropriate locus may cause abnormal transcription or deleterious effects on the function of another gene at that locus. In particular, modification errors in the germline could lead to transmission to the patient’s descendants.
In 2012, Jennifer Doudna reported a novel genome editing technology that made it possible to more accurately modify a gene at an appropriate position using the CRISPR/Cas9 system [10]. Therefore, this technology has been expected to be a new candidate for genetic therapeutic approaches. CRISPR/Cas9-mediated somatic editing has prompted extensive research; 14 clinical trials against cancers, HIV, and leukemia are active or recruiting participants as of March 23, 2019 according ClinicalTrials.gov, a clinical trials registry. The first genome-editing experiment on a human fertilized egg with β-thalassemia was conducted in China in April 2015 [11]. However, this report using developing technology for germline editing was currently published without evaluation of its errors or any consensus of its application. Thus, discussion on the use of genome editing for human fertilized eggs has grown since then.
In December of the same year, an international summit was held in Washington D.C., including scientists, ethical scholars, legal experts, and human rights advocacy groups from more than 20 countries. They proposed that genome editing technology for human embryos should be available only as part of basic research, and clinical applications should be prohibited because of ethical and technological concerns about the effects on individuals and the next generation. In Japan, a similar statement as “A joint proposal from four academic societies on human genome editing” was announced by the Japan Society of Gene and Cell Therapy, The Japan Society of Human Genetics, Japan Society of Obstetrics and Gynecology, and Japan Society for Reproductive Medicine [12].
In February 2017, the United States Academy of Sciences announced the approval of genome editing of human fertilized eggs only in cases of serious diseases [13]. Although this announcement may be a first step to realization of the clinical application of genome editing in human reproductive cells, establishment and improvement of legal and educational systems are required. As a foundation for policymaking, the opinions of both specialists and general citizens are indispensable. Responses to this announcement were issued from major countries and organizations [14]. In September 2017, the Science Council Japan proposed requirements for adequate rules for research and applications, risk evaluation system for products using somatic genome editing, and tentative prohibition of clinical genome editing in germline cells [15].
Taking the potential of genome editing into consideration, discussion for a consensus among general people who will share benefits of the technology is required [16]. Additionally, it is necessary to understand the thoughts and attitudes on this technology of clinicians, especially genetic professionals, who will probably make use of the technology at clinical sites in the near future. Some surveys among general people have been conducted worldwide [17], in western countries [18,19,20], China [21], and Japan [22]. In addition, a survey of medical field personnel was presented at the American Heart Association meeting in 2017 [23]. However, the attitudes of clinical genetic professionals have not been studied sufficiently.
Here we conducted a study to examine the attitudes of Japanese clinical genetic professionals on genome editing within the scope of their current knowledge and experience.
Methods
We conducted a questionnaire survey of Japanese board-certified instructors of Clinical Geneticists (CGs) and Certified Genetic Counselors (CGCs), both of which are certified by the Japan Society of Human Genetics and Japanese Society for Genetic Counseling. This study was approved by the Social, Ethical, and Legal Issues Committee of the Japanese Society for Genetic Counseling. Considering that this study was a questionnaire survey distributed to genetic professionals, institutional review board approval was not required.
Preparation of the questionnaire
The draft of the questionnaire was prepared based on questions used in domestic symposia and previous research [17, 24, 25]. All of the questions were reviewed and discussed by the Social, Ethical and Legal Issues Committee of the Japanese Society for Genetic Counseling, and created as selective questions in a self-answer form. This survey was conducted in Japanese language.
A total of 36 questions were involved in our questionnaire. The questionnaire was divided in to seven main themes: 1. Self-information on the respondent; 2. Knowledge of genome editing technology; 3. The perception of genome editing, and acceptance criteria on the practical application of genome editing; 4. Opinions on genome editing use in basic and clinical research; 5. Factors for making regulations and rules on the use of genome editing; 6. Opinions on therapy options including genome editing and future plans; and 7. Free entry. Individual question IDs were given to each question (Supplementary Table 1). The IDs are shown in the each of the corresponding Results sections.
Questionnaire investigation
We performed a cross-sectional study involving an anonymous, self-administered questionnaire of CGs (n = 285) and CGCs (n = 205) after excluding five individuals whose addresses were unknown (n = 485). The survey period was from August 2017 to October 2017. We mailed the questionnaire to the participants with an instruction to respond through paper-based posting or a Web-based system using free software, LimeSurvey [26].
Statistical analysis
All missing values were excluded, and analysis was performed using descriptive statistics for each question item. For testing the differences between groups, we used Student’s t-test or analysis of variance (ANOVA) for numeric variables, and chi-square test or Fisher’s exact probability test for nominal or ordinal variables. Fisher's exact probability test was applied when an expected frequency less than five existed in an expected frequency table for comparative analysis of the group [27]. The significance level of each test was basically 0.05. In the subsequent post-hoc test, the p value after Bonferroni correction was used. Statistical analyses were conducted using JMP pro 13.0.0 (SAS Institute Inc.) software.
Results
Self-information of respondents [Q1–Q6]
A total of 277 clinical genetic professionals (57.1%, 277/485) participated in the study by completing the questionnaire form. Respondents consisted of 176 of 283 CGs (62.2%), and 101 of 202 CGCs (50.0%). Answers were provided using paper-based mailing and the web-based system by 208 (75.1%) and 69 (24.9%) respondents, respectively. The other self-information including educational and clinical backgrounds are shown in Table 1 [Q1–Q6].
Knowledge of genome editing technology [Q7–Q8]
We gave all clinical genetic professionals basic information on genome editing prior to this study to explain the intentions of the study and what genome editing is. We asked them to answer the questions regarding their existing knowledge before reading the information in order to avoid biased answers from arising.
In the evaluation of their self-evaluation of knowledge on genome editing, we used a seven-point scale from zero to six corresponding to “Understanding all of its characteristics such as principle, purpose, and problems with experience of using the technology”, “Understanding all of the characteristics but without experience”, “Understanding part of the characteristics”, “Some understanding from TV or Web information”, “Not understanding well but interested in the technology”, “Heard once about the technology or not interested in it”, and “Know nothing about the technology” [Q7]. The average score of CGs (3.7) was significantly higher than that of CGCs (2.7) (p < 0.001, t-test) (Supplementary Figure 1A left). Those with a score of three or more were 74.0% (205/277) were regarded as respondents with a good understanding of genome editing. In order to confirm they really understood about genome editing, we tested the respondents in this group using six additional questions: “It is possible to modify genes freely without failure”, “It is a requirement to have a license to perform gene editing”, “Gene editing is endorsed by national authorities”, “Such treatment is covered by insurance”, “Has a Nobel Prize been won for this technology?” and “The correct usage is prescribed in law” [Q7.2]. The average score was 5.71/6 (correct answer rate: 95.2%) among the group who understood genome editing, and there was no significant difference between CGs (average score: 5.73) and CGCs (average score: 5.64) (p = 0.34, t-test) (Supplementary Figure 1A right).
Because it is necessary to consider the ethical aspects of clinical use alongside the technical understanding of genome editing, we also tested on the recognition of the statement proposed by four academic associations of genetics [Q8]. The joint statement in April 2016 was a current concept of genome editing technology in Japan at the time of starting our study.
A total of 74.7% (207/277) of respondents recognized the proposal, although the percentage among CGs (80.1%, 141/176) was higher than that among CGCs (65.3%, 66/101) (Supplementary Figure 1B).
In addition, we tested whether experience in basic research on genetics was related to the level of understanding of genome editing and the joint statement of the four academic associations of genetics [Q6]. Because the experience ratio of CGs (65.9%, 116/176) was higher than that of CGCs (38.6%, 39/101) (Supplementary Figure 1C, Table 1), we evaluated knowledge about genome editing and the statement in every group separated by their experience in basic research. The average score indicating knowledge of genome editing was 5.07 in experienced group, and this was significantly higher than 3.15 in the non-experienced group (t-test, p < 0.001). The ratio of cognition of the statement was 83.2% (129/155) in experienced group and 64.0% (78/122) in non-experienced group, and this difference was also significant (chi-square test, p < 0.001) (Supplementary Fig. 1D).
The perception of genome editing [Q11–Q14]
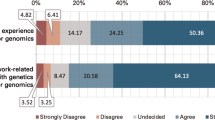
Among genetic professionals, 86.6% (240/277, Fig. 1a) showed concerns about misuse, and 5.78% (16/277, Fig. 1A–g) answered that the rules were adequate. Meanwhile, 48.7% (135/277, Fig. 1a–c) expected early applications for treatments. In addition, CGCs felt the technology was highly complicated and expensive (Fig. 1a–d) [Q11]. Subsequently, we asked about anxiety regarding editing technology and requirements for clinical use, because the anxieties and requirements could influence the perceptions held by genetic professionals. In particular, they had anxieties about “The probability of unexpected side effects due to the modification” (78.7%, 218/277), “Non-medical uses such as a designer baby, and subsequent loss of human diversity” (67.5%, 187/277), and “Having insufficient methods to evaluate the success of the modification” (23.5%, 65/277) (Fig. 1b) [Q14]. In addition, they required “Safety”, “Selection of subjects or targeted diseases for modification”, and “Endorsement by the nation or an organization” when this technology was practically applied to different kinds of cells, including non-human cells, human somatic cells, and human germline cells. (Supplementary Figure 2) [Q12]. Germline cells here include eggs, sperm, fertilized eggs, and early embryos.
The perception of genome editing. [Q9–Q14]. a Indicates the impressions on genome editing technologies. This question permitted multiple selections. The black and white bars indicate the percentages of CGs and CGCs, respectively, selecting the item. The numbers of respondents are indicated. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01). a–g in (a) are indicate as follows: (a) concerns for misuse, (b) highly complicated technology, (c) expectation for early application as a treatment, (d) expensive technology, (e) very safe technology, (f) taking a long time for practical applications, (g) inadequate regulations in place for use of the technology. b Indicates anxieties about genome editing technology. Up to two items could be chosen per respondent. This question had an item of “others” with free entry, but this item was not included in this figure. The black and white bars indicate the percentages of CGs and CGCs, respectively, selecting each item. The numbers of respondents are indicated. a–e in (b) indicate as follows: (a) taking too much time to obtain the necessary knowledge and skills, thus limiting other clinical work, (b) having costs and risks out of proportion to its effect, compared with other treatments, (c) having insufficient methods to evaluate the success of the modification, (d) the probability of unexpected side effects due to the modification, (e) non-medical uses such as a designer baby, and subsequent loss of human diversity
We studied the perceptions of genetic professionals on media reports [Q13]. Because general people often obtain information on genome editing through the mass media. Many respondents answered “Not enough information has been reported” (40.1%, 111/277), and their perceptions on the content of such information in the mass media were “Information has been reported, but it was not necessarily correct” (28.5%, 79/277), or “Information has been reported, but it focused only on positive aspects” (26.4%, 73/277) (Supplementary Figure 3).
Acceptable research and applications of genome editing [Q9–Q10]
Because the majority of clinical genetic professionals (61.0%) were concerned about the safety of genome editing technology (Fig. 1A–e), under the condition that its technological safety was guaranteed, we confirmed opinions on its applications for the eight purposes shown in Fig. 2a. Here we defined safety as ideal conditions with accurate gene editing and without side effects. It was found that most of the clinical genetic professionals opposed modifying physical characteristics through editing of germline cells (93.5%, 259/277, Fig. 2A–f) and of somatic cells (88.1%, 244/274, Fig. 2a(c)). We also found opposition to application for non-serious diseases using germline and somatic cells as well (Fig. 2a(g)). On the other hand, in order to overcome severe hereditary diseases, more than half of the participants accepted applying this technology to somatic cells, and nearly 30% agreed even for germline cells, (Fig. 2a(h)) [Q9]. In addition, we tried to test how much accuracy was required for editing for the same eight purposes. For application to an embryo to treat a non-serious disease, more than 95% of professionals opposed its use or would have agreed only when the success rate was 100%. However, for the treatment of serious disease, the opposition decreased to less than 80%, and more than 15% of professionals agreed to use the technology in embryos if the success rate was more than 75% (Supplementary Table 2) [Q10].
The opinions to the applications of genome editing. [Q15–Q23]. a Indicates acceptance criteria for practical applications of genome editing on the condition that its technological safety is guaranteed. Each question allowed single choice from “agree”, “reservations” and “opposed”, with black, gray, and white bars indicating the percentages of respondents who chose the respective response. Numbers inside the bars indicate the percentages of respondents who chose each response. a–h in (a) are indicated as follows: (a) modification for breeding and appreciation, (b) alteration to develop foods and drugs for human use, (c) modification of the physical characteristics§ of individuals, (d) alteration of the characteristics of individuals related to surmountable diseases§§, (e) prevention or treatment of severe genetic diseases and cancers, (f) alteration of the physical characteristics§ of descendants, (g) alteration of the characteristics of descendants related to surmountable diseases§§, (h) Prevention of severe genetic diseases with serious symptoms, or without appropriate treatments, etc. §Physical characteristics include height and color of skin or eye, for example. §§Surmountable diseases include ones where the disease that can be treated, prevented, or corrected easily, such as hereditary obesity or myopia. b Indicates opinions on research using genome editing in germline cells. This question permitted a single section. The black and white bars indicate the percentages of CGs and CGCs, respectively, selecting the item. The numbers of respondents are indicated. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01). a–e in (b) are indicated as follows: (a) all basic research and clinical applications should be allowed, (b) the use of germline cells in basic research regardless of fertilization process should be allowed, (c) the use of germline cells in basic research without experimental fertilization should be allowed, (d) the use of germline cells should not be allowed in basic or clinical research, (e) Cannot judge
Previous studies reported that a discussion on genome editing increased the acceptability of the technology among general people [22]. However, in this study, while these acceptance criteria on editing of non-human cells were positively affected by a higher understanding level of genome editing, that of human genome editing was not significantly influenced by their knowledge in genetic professionals (Supplementary Figure 4).
Opinions on the applications of genome editing [Q15–Q23]
This technology has been at research stage and has not matured enough for clinical applications yet, except for some clinical trials [28, 29]. Therefore, we investigated contemporary opinions on research [Q15–Q18] and applications [Q19–Q23] about genome editing technology. The genetic professionals agreed with limited use of the technology and restricted to specific issues [Q15] (Supplementary Figure 5A). Moreover, they demanded the establishment of regulations for basic research, by the nation (38.6%, 107/277), government ministries (30.3%, 84/277), or academic associations (24.9%, 69/277). This trend was significantly affected by knowledge on genome editing (p < 0.01, ANOVA) [Q16] (Supplementary Figure 5B, C). Even if genome editing is safe, editing applications to germline cells were not accepted except for the treatment of severe diseases. Subsequently, we asked about opinions on research using genome editing on germline cells (Fig. 2b) [Q17]. More than half of the clinical genetic professionals agreed with its use in basic research but thought that fertilization for experimental purposes should not be done (50.9%, 141/277, Fig. 2B, c). Although both CGs and CGCs agreed with the use of fertilized eggs in basic research, details of the opinions differed between CGs and CGCs. Although CGs agreed with the actual fertilization process with modified germline cells in basic research, CGCs significantly disagreed with the process (corrected p < 0.05, chi-square test, Fig. 2B-b). As another significant difference between them, CGCs chose "Cannot judge" more than CGs did (corrected p < 0.01, Fisher's exact probability test, Fig. 2B–e). These opinions for handling germline cells may be influenced by their thoughts about the start of human dignity. Most professionals were of the opinion that human dignity commenced with “fertilization regardless of in vitro or in vivo” (27.1%, 75/277), “implantation into the uterus” (23.5%, 65/277), and “increased potential of the fetus to survive or grow up” (19.9%, 55/277). This trend was the same in CGs and CGCs. Compared with the previous answers about the criteria for human genome editing techniques [Q17], those who defined “dignity as a human” at an earlier stage than birth near to fertilization [Q18] tended to oppose research using germline cells (p < 0.001, Fisher’s exact probability test) (Supplementary Figure 6).
Subsequently we also asked about current opinions on the clinical applications of genome editing. Although most genetic professionals thought it was “Too early to apply editing in the clinical field” (76.2%, 211/277), they believed the technology would be “Partially accepted as a benefit for patients with certain diseases” (77.3%, 214/277) [Q19, Q20] (Supplementary Figure 7A, B). In addition, more than half of them permitted the clinical use of the technology if it was “Regulated by appropriate laws or rules from academic societies” (55.2%, 153/277), and nearly one-third of them “Would accept it in the future but were opposed at present” (31.9%, 86/277) [Q21] (Supplementary Figure 7C). We tried to determine their opinions separately for the criteria for genome editing using somatic and germline cells, using a six-point scale in the process from basic research to clinical applications [Q22]. For somatic cells, whereas 57.0% (158/277) of professionals would permit “Clinical applications only for serious diseases”, 25.6% (71/277) of them accepted it “Only for basic research” (Supplementary Figure 7D). On the other hand, for human germline cells, only 27.1% (75/277) accepted “Clinical application only for serious diseases” and 31.8% (88/277) accepted it “Only for basic research”. Moreover, 36.1% (100/277) “Did not accept it for use in human germline cells” (Fig. 3a). Among the opponents of human germline cell use, 41.0% (41/100) (Fig. 3A-a) insisted that “Germinal cells including non-human cells must never be used regardless of basic or clinical research” [Q23]. We investigated why the respondents, except for the 41.0% who were strong opponents, would permit the use germline cells even partially, and the most chosen reason was that “Genetic disease occurring in a parent would be prevented from being transmitted to their child” [Q23.2] (76.5% 127/166) (Fig. 3b).
Contemporary opinions about clinical application of genome editing. [Q15–Q23]. a Indicates responses on the permissible use of genome editing for germline cells such as human embryos and gametes (ovum / sperm). This question permitted a single selection. The numbers of respondents are indicated. The sum of respondents who chose a or b (n = 100) were regarded as opponents of clinical applications of genome editing with germline cells. Those who chose c, d, e, and f, framed by a gray dashed square (n = 166), partially or totally accepted the applications. a–f in (a) are indicated as follows: (a) regardless of basic research or clinical application, it should not be allowed at all, (b) basic research using non-human animals may proceed, (cells of human origin cannot be used), (c) basic research on humans should be done, but clinical applications should not be done yet, (d) clinical applications should be permitted only for severe genetic diseases with severe symptoms and no available treatment, (e) clinical applications should be permitted even if it is a therapeutic purpose for a mild disease, (f) clinical applications should be allowed for all purposes, including modifying physical characteristics as well as for disease. b Indicates responses on the reason for accepting the use of germline cells in genome editing. This question was limited to those who chose c, d, e, and f (n = 166) in Fig. 3a. This question permitted multiple choices. The numbers of respondents are indicated. a–f in (b) are indicated as follows: (a) even in case of infertility, it may be possible to increase the possibility of pregnancy or childbirth, (b) a child with a function that his/her parents want can be obtained, (c) genetic disease occurring in a parent would be prevented from being transmitted to their child, (d) it should be permitted for applications to limit serious diseases and subsequent issues, (e) the development of reproductive medicine can be expected to aid Japan’s economic growth, (f) the treatment and prevention of diseases can improve the health conditions of people, leading to a reduction in medical expenditure
Important factors for making regulations and rules on the use of genome editing [Q24–Q26]
It is important to prepare relevant rules and laws for the use of editing technology in a clinical field. Therefore, we examined what factors were required by clinical genetic professionals for developing appropriate rules and laws [Q24]. Most clinical genetic professionals were particularly focused on “Ethical issues” (76.5%, 212/277), “Purpose of use and its target” (67.5%, 187/277), and “Success rate and safety” (65.3%, 181/277) as requirements when establishing rules (Fig. 4). Among the factors, CGCs gave significantly more weight to factors about “Success rate and safety” (p < 0.05, chi-square test, Supplementary Figure 8A). In addition, we asked for opinions about contemporary regulations [Q26] and social systems on genome editing [Q25]. Most professionals did not know the current status of regulations and systems in Japan (44.8%, 124/277, Supplementary Figure 8B-a).
Important factors for making regulations and rules on use of genome editing. [Q24–Q26]. Figure indicates responses on rules related to genome editing technology and factors considered to be important for improving laws. This question permitted up to three choices. The numbers of respondents are indicated. a ethical issues. b Purpose of use and its target. c Success rate and safety. d Level of knowledge and expertise of policy makers. e Level of understanding of general people. f Quality or accessibility of the technology. g Religion and cultural background of the population. h Cost
From the above result, we focused on the aspects of current regulations that are inadequate. Overall, 49.7% (76/153) of professionals who knew the status of current regulations regarded them as inappropriate (Supplementary Figure 8B-b, c, d). As a regulator, professionals thought that “Government” (48.4%, 134/277) or “Academic societies” (39.0, 108/277) were best placed to establish appropriate rules. On the other hand, those who desired citizen-based regulation were in a minority (6%, 17/277) (Supplementary Figure 8C).
Opinions on the therapeutic options including genome editing and future plans [Q27–Q29]
The last section of this survey was on clinical experience in genome editing. Among the respondents, a total of 96.0% (266/277) had experience in the clinical field, and 24.8% (66/266) had been involved in a “Consultation on genome editing” (Supplementary Figure 9A) [Q27]. Main consultation topics were about “Gene therapy” (74.2%, 49/66) or the “Influence on the next generation” (32.9%, 35/66) (Supplementary Figure 9B) [Q27.2]. In addition, a proportion of the responders (31.4%, 87/277) had discussed the subject with their co-workers or friends (Supplementary Figure 9C) [Q28]. Subsequently, we asked how they would answer questions, about treatment using genome editing technology [Q29]. In most cases, we obtained the answer as “It is impossible at present, but there is a possibility for treatment in the future” (122/277, Supplementary Figure 10C), followed by “Without mentioning whether treatment is possible or impossible, only provide a consultation by giving information” (21.7%, 60/277, Supplementary Figure 10D). The later standpoint of the consultation was seen significantly more often with CGCs than CGs (corrected p < 0.05, chi-square test).
Discussion
This study was conducted to investigate the current opinions of clinical genetic professionals on genome editing using a self-reported questionnaire. Overall, 62.2 and 50.0% of CGs and CGCs, respectively, in Japan participated in this study. First, 74.0% of professionals (CGs: 83.5%, CGCs: 57.4%) considered they understood genome editing well (Supplementary Figure 1A left). Although the self-assessment by CGCs was low, the knowledge level (Supplementary Figure 1A right) of CGCs was similar to that of CGs in the group who understood genome editing. A total of 74.7% of respondents recognized the joint statement on the technology of genome editing in Japan. The percentage of CGs who recognized the statement was higher than that of CGCs (80.1 vs. 65.3%) (Supplementary Figure 1B). Knowledge on genome editing and the statement were related to their experience in basic research on genetics (Supplementary Figure 1D). CGs had more experience in basic research than CGCs (65.9 vs. 38.6%) (Supplementary Figure 1C, Table 1). Taking these results into consideration, more-effective education systems for CGCs may enhance the general understanding level of genetic professionals on genome editing. From the investigation on perceptions on genome editing, though professionals hoped for early clinical applications for the technology, they were concerned about misuse, including applications for designer babies in conditions with inadequate rules. In addition, they mentioned problems about a lack of information and biased or incorrect information on genome editing. They felt the media emphasized only the positive aspects and did not report the risks of genome editing. Because press reports are the only source of information for most general people, fair knowledge including the risks of genome editing may not be understood. These deficiencies may prevent genetic professionals from making a consensus with general citizens for clinical applications of genome editing in the future. For clinical applications, clinical genetic professionals required not only proper rules to be established by government or academic associations, but also criteria for target diseases and the safety of the technology. Recently, a report has indicated that genome editing technology is more inaccurate than previous expectations [30], which has made professionals doubt the current safety standards of the technology. These aspects indicate the importance of sharing the latest information among professionals, its correct interpretation for judging the trends of the technology, and subsequent proper distribution of the information to both of professionals and general people.
Even under the condition of promised technical safety, professionals had less support for the use of this technology for purposes of enhancement regardless of use on somatic cells or fertilized eggs. On the other hand, because research and clinical applications have been relatively accepted with respect to life-threatening hereditary diseases, professionals had expectations for treating serious diseases using genome editing technology. A similar trend has reported in general people; if the target disease is more serious, genome editing is more acceptable [21, 31]. Although some reports have shown that general people estimated the risk of genome editing to be much higher due to a lack of knowledge [22]. Therefore, unlike general people, CGCs with much more knowledge on genome editing might hesitate to clarify their opinion and then select “Cannot judge”. A probable reason for this was a difference in their roles as genetic professionals between CGs and CGCs. In comparison with CGs, a physician who focuses on overcoming a disease in a clinical situation, CGCs tend to consider the disease as a variant and strive to support the client's autonomous decision.
Although both CGs and CGCs similarly recognized the potential benefits of genome editing to overcome serious diseases, they required laws and guidelines to remove the concern about the safety and misuse of genome editing. Indeed, when laws and guidelines were properly established, combined with current proponents, around 70% of participants agreed with the partial or total gene editing of fertilized eggs (Supplementary Figure 7C, a, b). The preparation of a new guideline on genome editing of germline cells in Japan has started. In September 2018, a draft of the new guideline was proposed by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare at an expert meeting in Japan [32]. The guideline seems to satisfy the factors that professionals regarded most highly for making rules in our study. For example, only surplus fertilized eggs from artificial reproductive technology for the treatment of infertility could be used in research on genome editing. In addition, the genetically modified eggs are prohibited from being transferred to the uterus because the safety and success rate are still unclear. The genome editing of a fertilized egg is permitted only for basic research on reproductive medicine, and the purpose must be strictly evaluated by the government. Furthermore, this guideline requires proper informed consent obtained from donors of the fertilized eggs and interactive ethical reviews from both the agency that provides the egg and the one that conducts the research. Subsequent investigation by professionals should be continued to obtain novel requirements for genome editing, because their thoughts may evolve with progress in this technology and the results of research. A requirement could limit the technology as countermeasures against unethical, illegal, or chancy editing applications. Recently, a Chinese researcher announced the birth of twin babies using genome editing technology of fertilized eggs [33]. A barrage of criticisms has been lodged against this as premature use of the technology without any academic consensus, which will be fraught with ethical and social problems. In response to the announcement, the American Society of Human Genetics immediately insisted again on the importance of scientific and ethical discussions on the use this technology in a statement to reaffirm its position about use of the technology with human fertilized eggs [34]. In addition, the four academic societies of genetics in Japan proposed a "Statement of four related academic societies on clinical application of human embryo genome editing" in December 2018 [35]. Professionals have to catch up with the latest trends in technology, because a problem, besides insufficient guidelines, identified from our study was that around half of respondents did not know the current guidelines on genome editing. This indicates it has to be considered how the established rules including a draft are transmitted to genetic professionals effectively. Sufficient interaction with citizens is also required because citizens are the actual beneficiaries from the technology, although professionals regarded government or academic associations as the most appropriate formulator of the rules. Therefore, occasion to discuss with citizens should be provided in the process of developing laws and ethical guidelines for clinical applications.
Finally, one-quarter of respondents working in clinical sites had already been involved in consultations about genome editing and its effect to their offspring. Because it has not been established how professionals should respond to these kinds of inquiries from patients, the candidate answers depended on the concerns held by genetic professionals. In the current study, the most-frequently chosen answers were “It is impossible at present, but there is a possibility for treatment in the future” (44.0%) and “Without mentioning whether treatment is possible or impossible, only provide consultation by giving information” (21.7%). The second response was chosen significantly more often by CGCs than CGs. The reason for this result might be attributed to the role of CGCs as a specialist for counseling. As information on genome editing has matured and increasingly spread to general people, these kinds of inquiries from patients are expected to increase. It is also required to prepare a guideline on how to respond to patients properly before genome editing technologies are applied to genetic therapies.
As a conclusion, the current study enabled us to investigate the opinions of genetic professionals who will probably use genome editing technology in their future clinical setting. They had concerns about the safety and ethical aspects of the technology, as well as fears over inappropriate applications of it. On the other hand, they also had expectations for its therapeutic application, including genome editing of embryos against serious and difficult-to-treat hereditary diseases. Genetic professionals, however, tended to adopt a prudent attitude to the applications of genome editing because of inadequate rules. There is substantial need for academic policies and legislation for using the technology, especially when using human embryos, through a consensus of professionals and citizens based on the specific circumstances found in Japan. Genome editing technology at present has been developing, and there are promising tools for future medical treatments of contemporary serious diseases, but it is not a panacea. Thus, it is required not only to establish proper policies but also to improve them flexibly with developments in the technology as they happen. The flexibility might be realized through arranging effective education and discussion, and would be created by formulators of the policies and users of the technologies in the genetic medical field, namely clinical genetic professionals. They have to obtain the latest information about technologies and medical circumstances, understand it correctly, and judge whether it can be applied to rules and medicine at that moment. Furthermore, genetic professionals as communicators should disseminate reliable information to general people in order to develop a consensus with them because they or their offspring may receive the benefits of the technology.
In progressing the status of genome editing technology using embryos in medical treatments, our study shows current factors for major groups of genetic professionals, CGs and CGCs, to judge what is required and how they harness their own specialty to understand this controversial technology. We have to pursue alterations in circumstance and subsequent changes in mindset to establish a proper environment including constructive rules, education systems, and clinical protocols.
References
Anderson WF. Human gene therapy. Science. 1992;256:808–13.
Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, et al. Replacement Therapy for Inherited Enzyme Deficiency — Macrophage-Targeted Glucocerebrosidase for Gaucher’s Disease. New Engl J Med. 1991;324:1464–70.
Gene Therapy Clinical Trials Worldwide. http://www.abedia.com/wiley/countries.php. Accessed 05 Jan 2019.
Seoane-Vazquez E, Shukla V, Rodriguez-Monguio R. Innovation and competition in advanced therapy medicinal products. EMBO Mol Med. 2019;11:e9992.
Grupp SA, Laetsch TW, Buechner J, Bittencourt H, Maude SL, Verneris MR, et al. Analysis of a global registration trial of the efficacy and safety of ctl019 in pediatric and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL). Blood. 2016;128:221.
Suda H, Murakami A, Kaga T, Tomioka H, Morishita R. Beperminogene perplasmid for the treatment of critical limb ischemia. Expert Rev Cardiovasc Ther. 2014;12:1145–56.
Kang E, Wu J, Gutierrez NM, Koski A, Tippner-Hedges R, Agaronyan K, et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–5.
Baylis F. The ethics of creating children with three genetic parents. Reprod Biomed Online. 2013;26:531–4.
Zhang J, Liu H, Luo S, Chavez-Badiola A, Liu Z, Yang M, et al. First live birth using human oocytes reconstituted by spindle nuclear transfer for mitochondrial DNA mutation causing Leigh syndrome. Fertil Steril. 2016;106:e375–6.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–72.
Japan Society of Gene and Cell Therapy, Japan Society of Human Genetics, Japan Society of Obstetrics and Gynecology, and Japan Society for Reproductive Medicine. Recommendation on human genome editing. 2016. http://www.jsrm.or.jp/guideline-statem/statement_2016_01.pdf. Accessed 05 Jan 2019.
National Academies of Sciences, Engineering, and Medicine, National Academy of Medicine, National Academy of Sciences, Committee on Human Gene Editing: Scientific, Medical, and Ethical Considerations. Human genome editing: science, ethics, and governance. Washington (DC): National Academies Press; 2017.
Brokowski C. Do CRISPR germline ethics statements cut it? CRISPR J. 2018;1:115–25.
Science Council of Japan. Genome editing technology in medical sciences and clinical applications in Japan. http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-23-t251-1-en.pdf. Accessed 05 Jan 2019.
Ishii T. Germline genome-editing research and its socioethical implications. Trends Mol Med. 2015;21:473–81.
McCaughey T, Sanfilippo PG, Gooden GEC, Budden DM, Fan L, Fenwick E, et al. A Global Social Media Survey of Attitudes to Human Genome Editing. Cell Stem Cell. 2016;18:569–72.
Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, Hardy BW. U.S. attitudes on human genome editing. Science. 2017;357:553–4.
Gaskell G, Bard I, Allansdottir A, da Cunha RV, Eduard P, Hampel J, et al. Public views on gene editing and its uses. Nat Biotechnol. 2017;35:1021–3.
Whitman D. U.S. Public opinion and interest on human enhancements technology. AARP Res. 2018. https://doi.org/10.26419/res.00192.001.
Wang J-H, Wang R, Lee JH, Iao TWU, Hu X, Wang Y-M, et al. Public attitudes toward gene therapy in China. Mol Ther - Methods Clin Dev. 2017;6:40–42.
Uchiyama M, Nagai A, Muto K. Survey on the perception of germline genome editing among the general public in Japan. J Hum Genet. 2018;63:745.
Musunuru Kiran, Lagor William R, Miano Joseph M. What do we really think about human germline genome editing, and what does it mean for medicine? Circ: Cardiovasc Genet. 2017;10:e001910.
Science Council of Japan. Recommendation genome editing technology in medical sciences and clinical appliacations in Japan. September 27, 2017. http://www.scj.go.jp/ja/info/kohyo/pdf/kohyo-23-t251-1-en.pdf. Accessed 05 Jan 2019.
Funk C, Kennedy B, Sciupac E. U.S. public wary about use of biomedical technology for human enhancement. 2016. http://www.pewinternet.org/2016/07/26/u-s-public-wary-of-biomedical-technologies-to-enhance-human-abilities/. Accessed 05 Jan 2019.
Engard NC LimeSurvey http://limesurvey.org. Public Services Q. 2009. https://doi.org/10.1080/15228950903288728.
Cochran WG. Some methods for strengthening the common χ2 Tests. Biometrics. 1954;10:417–51.
Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nat News. 2016;539:479.
Reardon S. First CRISPR clinical trial gets green light from US panel. Nat News. https://doi.org/10.1038/nature.2016.20137.
Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765.
Funk C, Hefferon M. Public views of gene editing for babies depend on how it would be used. 2018. http://www.pewinternet.org/2018/07/26/public-views-of-gene-editing-for-babies-depend-on-how-it-would-be-used/. Accessed 05 Jan 2019.
Cyranoski D. Japan set to allow gene editing in human embryos. Nature. 2018. https://doi.org/10.1038/d41586-018-06847-7.
Cyranoski D, Ledford H. Genome-edited baby claim provokes international outcry. Nature. 2018. https://doi.org/10.1038/d41586-018-07545-0. https://www.nature.com/articles/d41586-018-07545-0. Accessed 05 Jan 2019.
ASHG Reaffirms 2017. Position statement on germline genome editing report from china, if confirmed, would be at odds with field consensus that germline editing is not ready for human use. 2018. http://www.ashg.org/press/201811-genome-editing.shtml. Accessed 05 Jan 2019.
Japan Society of Gene and Cell Therapy, Japan Society of Human Genetics, Japan Society of Obstetrics and Gynecology, and Japan Society for Reproductive Medicine. Statement of four related academic societies on clinical application of human embryo genome editing. 2018. http://jshg.jp/news/2212/. Accessed 05 Jan 2019.
Acknowledgements
We would like to thank all of those who have assisted in the questionnaire survey and have received the guidance and support of clinical geneticists and certified genetic counselors. In addition, the authors wish to acknowledge Dr. Kazuya Setoh, Center for Genomic Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan, for help in the statistical analysis of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taguchi, I., Yamada, T., Akaishi, R. et al. Attitudes of clinical geneticists and certified genetic counselors to genome editing and its clinical applications: A nation-wide questionnaire survey in Japan. J Hum Genet 64, 945–954 (2019). https://doi.org/10.1038/s10038-019-0635-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0635-z
This article is cited by
-
Fundamental knowledge taught in compulsory education for effective genetic counseling: a qualitative study of descriptions in textbooks
Journal of Community Genetics (2023)
-
Public attitudes in the clinical application of genome editing on human embryos in Japan: a cross-sectional survey across multiple stakeholders
Journal of Human Genetics (2022)
-
Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public
Journal of Human Genetics (2020)