Abstract
Translation of mitochondrial-specific DNA is required for proper mitochondrial function and energy production. For this purpose, an elaborate network of dedicated molecular machinery including initiation, elongation and termination factors exists. We describe a patient with an unusual phenotype and a novel homozygous missense variant in TUFM (c.344A>C; p.His115Pro), encoding mtDNA translation elongating factor Tu (EFTu). To date, only four patients have been reported with bi-allelic mutations in TUFM, leading to combined oxidative phosphorylation deficiency 4 (COXPD4) characterized by severe early-onset lactic acidosis and progressive fatal infantile encephalopathy. The patient presented here expands the phenotypic features of TUFM-related disease, exhibiting lactic acidosis and dilated cardiomyopathy without progressive encephalopathy. This warrants the inclusion of TUFM in differential diagnosis of metabolic cardiomyopathy. Cases that further refine genotype-phenotype associations and characterize the molecular basis of mitochondrial disorders allow clinicians to predict disease prognosis, greatly impacting patient care, as well as provide families with reproductive planning options.
Similar content being viewed by others
Introduction
Mitochondrial dysfunction typically leads to a spectrum of clinically heterogeneous often devastating disorders, as the mitochondria produce most of the energy for cell function [1, 2]. Mitochondrial energy production is an intricate process involving the coordinated enzymatic activity of the mitochondrial respiratory chain (MRC), which includes over 85 proteins and is organized into five complexes; additional proteins participate in synthesis, assembly and maintenance of the MRC [1, 3]. The majority of these components are encoded by nuclear DNA, while mitochondrial DNA (mtDNA) encodes only a small portion of the elements involved [1, 2]. The genetic and biochemical complexity of mitochondrial function makes accurate molecular diagnosis of mitochondrial disorders challenging, particularly considering the broad and often overlapping clinical features [4]. Classically, tissues with high energy demands, such as the heart, muscles and central nervous system, are most severely affected [2].
We describe a patient with a novel homozygous missense variant in TUFM, encoding the translation elongation factor Tu (EFTu) required for mtDNA translation [3]. TUFM variants had been previously reported in four patients with autosomal recessive combined oxidative phosphorylation deficiency 4 (COXPD4, MIM #610678). The patient reported here presented with lactic acidosis and dilated cardiomyopathy (DCM), leading to early demise, without the typical neurological findings associated with TUFM variants.
Methods
Participants
The family was clinically evaluated by a multidisciplinary medical team at Rambam Health Care Campus, following the proband’s hospitalization. The study was approved by the institutional Helsinki Committee and informed consent was obtained as customary.
Genetic analysis
Sequencing
We performed trio whole exome sequencing (WES) in collaboration with the Regeneron Genetics Center (RGC). Briefly, 1 µg of high-quality genomic DNA was fragmented using a custom reagent kit from Kapa Biosystems. Targeted captured of protein-coding regions was performed using the IDT xGen capture reagent (Integrated DNA Technologies, Coralville, IA, USA). Paired-end libraries were prepared from captured fragments and sequenced on the Illumina HiSeq2500 platform (Illumina, San Diego, CA, USA) using v4 chemistry. Sequencing was performed to achieve at least 90% of target bases covered at 20x or more.
Filtering
WES data were analyzed using the Genoox Ltd. data analysis platform (Tel Aviv, Israel). Filtering was done on a trio-based paradigm to identify all recessive (homozygous and compound heterozygous), X-linked, and potential de novo variants in the proband. Variants were prioritized based on their effect on the protein (missense, nonsense, frameshift, splice-site) and minor allele frequency below 1% in general population databases, such as gnomAD (http://gnomad.broadinstitute.org/) and 1000Genomes (http://www.internationalgenome.org/), the Greater Middle-East Variome (http://igm.ucsd.edu/gme/), the Rambam Genetics Institute internal database of over 1000 Israeli exomes, and the RGC database. Given the severe phenotype presentation, variants with reported homozygotes or hemizygotes in healthy individuals were filtered out. Because of the reported consanguinity, downstream analysis focused on recessively inherited variants in genes related to mitochondrial function.
Validation and segregation
Validation of the TUFM variant and segregation analysis were done by Sanger sequencing, using the primers: forward 5′-AGCTCTGCCTCTAGCACTGG-3′ and reverse 5′- TTAATCTCCTCCCCACAAGC-3′.
Mutation and protein analyses
The pathogenicity of the amino acid substitution in EF-Tu was assessed by multiple algorithms embedded in Alamut Visual v.2.10 (Interactive Biosoftware, Rouen, France), including SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), MutationTaster (http://www.mutationtaster.org/) and Align GVGD (http://agvgd.hci.utah.edu/). Genomic evolutionary rate profiling (GERP) scores were obtained for each variant [5]. ConSurf was used for additional conservation analysis [6].
The EF-Tu and EF-Ts (encoded by TSFM) proteins were modelled using SWISS-MODEL and I-TASSER, based on the bovine crystal structure of mitochondrial Elongation Factor Tu/Ts complex (PDB #1XB2) [7,8,9], and visualized using UCSF Chimera [10].
Mitochondrial respiratory chain analysis
The enzymatic activities of respiratory chain complexes were measured in isolated muscle mitochondria by spectrophotometric methods as previously described [11, 12]. Briefly, complex I was measured as rotenone sensitive NADH-CoQ reductase monitoring the oxidation of NADH at 340 nm in the presence of coenzyme Q1; complex II was measured as succinate dehydrogenase (SDH) based on the succinate-mediated phenazine methosulfate reduction of dichloroindophenol at 600 nm; complex I + III was measured as rotenone sensitive NADH cytochrome c reductase, following reduction of oxidized cytochrome c at 550 nm; complex IV (cytochrome c oxidase) was measured by following the oxidation of reduced cytochrome c at 550 nm Citrate synthase (CS); a ubiquitous mitochondrial matrix enzyme, serving as a control, was measured in the presence of acetylCoA and oxaloacetate by monitoring the liberation of CoASH coupled to 50, 50-dithiobis (2-nitrobenzoic) acid at 412 nm. All spectrophotometric measurements were carried out using a double beam spectrophotometer (UVIKON 930, Secomam, France).
Mitochondrial DNA sequencing and analysis
The full-length mitochondrial genome was amplified in two pieces from 50 ng of high quality genomic DNA using previously established primer sequences [13] with LA Taq (Takara, Kusatsu, Shiga Prefecture, Japan) using the following PCR conditions: 95 °C for 3 min followed by 30 cycles of 98 °C for 20 s, 68 °C for 15 s, and 72 °C for 10 min. The PCR products were purified using AMPure XP beads (Beckman Coulter, Brea, CA, USA). Both PCR products were quantified by fluorescence (Thermo Fisher, Waltham, MA, USA), pooled equally, and prepared for Illumina sequencing using the NEBNext Ultra II FS kit (New England Biolabs, Ipsqich, MA, USA) according to the manufacturer’s protocol. During PCR dual asymmetric barcodes were added to the library. The sample was sequenced on an Illumina HiSeq2500 rapid run flow-cell using 2 × 125 bp reads. We achieved an overall average coverage of the mitochondrial genome of 7300×. Paired-end sequence files were aligned to the human mitochondrial reference genome sequence NC_012920.1, achieving full coverage of the reference sequence with no deletions identified. Further processing of the sequence to detect possible heteroplasmy was performed using mtDNA-Server [14].
Results
Case description
The proband was the third son of healthy consanguineous parents of Muslim Arab descent with one previous spontaneous miscarriage (Fig. 1a). Pregnancy was notable for intrauterine growth restriction with delivery at 38 weeks, birth weight 2580 g (7th percentile), and head circumference 32.5 cm (6th percentile). The neonatal period was normal and at 45 days he underwent an uneventful surgical correction of a left inguinal hernia. His mother reported delay in motor milestones, including poor head control, inability to roll-over at 6 months, and difficulty in grasping toys; however, he was alert and communicative.
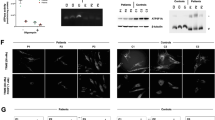
A homozygous TUFM missense variant leads to a mitochondrial cardiomyopathy syndrome. a Pedigree and genotype of TUFM: c.344A>C; p.His115Pro in a family with mitochondrial disorder presenting as dilated cardiomyopathy and lactic acidosis. HOM homozygous, HET heterozygous, WT wild type. b Schematic representation of EF-Tu protein with locations of previously reported pathogenic variants. c EF-Tu protein structure colored by domains. The affected amino acids implicated in mitochondrial disease, as described in this and previous reports of TUFM-patients, are highlighted. d EFTu (colored by domains) forms a complex with EFTs (yellow). Histidine in position 115 is colored red, GDP is colored magenta
The boy presented in critical condition at 6 months, following a two-day history of flu-like symptoms. He was apathetic with cold extremities, prolonged capillary refill, tachycardia (170 beats/min), hypotension (60/20 mmHg), and hypothermia (34 oC). Laboratory results revealed severe metabolic acidosis: pH <6.8 (normal: 7.35–7.45), lactate >20 mmol/l (normal <1.3), unmeasurable bicarbonate and glucose 20 mg/dl (normal: 70–100). The patient was admitted to the pediatric intensive care unit in severe cardiogenic shock, and was put on Veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Initial echocardiogram showed ejection fraction of 20% with severe dilated cardiomyopathy (DCM); there was no evidence of structural anomalies. Metabolic work-up revealed elevated blood alanine and decreased methionine in amino acid analysis, significantly elevated urine ketones and lactate, dicarboxylic aciduria, and 3-hydroxypropionic aciduria, and Krebs cycle metabolites urine organic acid analysis. Initially, CPK was elevated (1500 U/l, normal: 35–300) but normalized over time, and there was evidence of liver dysfunction including hypoalbuminemia and coagulopathy. Brain imaging was not performed. The boy died within a month; the parents declined autopsy.
Exome analysis
Trio WES identified five homozygous, four compound heterozygous and no X-linked recessive or de novo variants (Table 1). Of these, a homozygous missense variant in TUFM (NM_003321.4): c.344A>C; p.His115Pro was the main candidate due to its relation to mtDNA translation. The variant was not observed in any of the publically available or internal variant databases (Table 1), and it was predicted to be damaging by multiple bioinformatics algorithms (Table 1), and co-segregated appropriately with the disease, i.e. the variant was not present in homozygous state in neither of the healthy siblings (Fig. 1a).
Protein analysis
The affected residue, histidine at position 115, is located in domain 1 of the EFTu protein (Fig. 1b–d). This domain which is imperative for GTP/GDP-binding and complex formation with elongation factor Ts (EFTs). His115 is highly conserved across eukaryotes (Table 1, GERP 5.42; Supplementary material Fig. S1, ConSurf score 9) and predicted to be an exposed and functional residue (Supplementary material). Since proline is smaller and more hydrophobic than histidine, the His115Pro substitution may lead to loss of hydrogen bonds and compromise proper protein folding, thus affecting the function of this domain.
Respiratory chain analysis in muscle biopsy
Muscle biopsy showed nonspecific myopathy with multiple large mitochondria. Enzymatic activities of respiratory chain complexes in muscle mitochondria showed decreased activities of respiratory chain complexes I, I + III, and IV (33, 28, 35% residual activity, respectively), while activity of complex II (103% activity) was intact when normalized to citrate synthase (Table 2).
Mitochondrial genome analysis
Given the phenotype of the patient was consistent with mitochondrial dysfunction, in order to rule out possible mitochondrial mutations or deletions that may be contributing to the phenotype, we performed mitochondrial genome sequencing of the proband. We achieved complete coverage of the full mitochondrial genome at an average of ~7300×(Supplementary material Fig. S2). We did not detect any deletions or significant heteroplasmic variant sites in the proband’s mitochondrial genome. Analysis of polymorphic sites detected heteroplasmy ranging from 0.63–2.9%, which is considered within normal and benign mitochondrial heteroplasmy. Identified homoplasmic sites were consistent with variants in the N1b1a mitochondrial haplogroup identified for the proband and found in individuals from West Asia, including the Arabian Peninsula consistent with the reported maternal ancestry [15].
Discussion
Energy production by the mitochondria involves the coordinated activity of a myriad of proteins encoded by both nuclear and mtDNA [1, 2]. Translation of mtDNA occurs within the mitochondria by dedicated molecular machinery including initiation, elongation and termination factors [3]. Pathogenic variants in genes encoding one of the four mitochondrial elongation factors, EFG1, EFG2, TUFM and TSFM, have been recognized as the cause of severe mitochondrial diseases [3].
The TUFM gene encodes mitochondrial elongation factor EF-Tu, a highly conserved GTPase, which forms a ternary complex with mitochondrial aminoacyl-tRNAs and GTP [16]. Active EFTu facilities translation elongation by GTP-mediated binding of aminoacyl-tRNAs to the ribosomal A site, where appropriate codon recognition occurs. The aminoacyl-tRNA is released by EFTu using GTP hydrolyzation. The remaining GDP-bound EFTu is inactive but can be reactivated by EFTs (encoded by TSFM), which mediates the exchange of GDP with GTP [17]. Pathogenic variants in both TUFM and TSFM genes were shown to hamper mtDNA translation, impeding production of multiple essential mitochondrial proteins, and leading to severe phenotypes [17,18,19]. In accord with impaired mtDNA translation in our patient, the activities of respiratory chain complexes containing mtDNA-encoded subunits clearly show a relative decrease, while complex II, which is solely encoded by nuclear DNA remained unaffected (Table 2). To date, only four patients with bi-allelic TUFM variants have been reported; two patients had severe early-onset lactic acidosis and progressive fatal infantile encephalopathy, while the clinical phenotype in the other two patients was sparsely detailed, and neither mentioned cardiac involvement [18,19,20]. Notably, the clinical course in our patient was different from the previously reported patients (Table 3). Cardiac involvement, including DCM with severe left ventricular dysfunction, was the main clinical finding and cause of death in our patient. The patient did not exhibit progressive early-onset encephalopathy or other neurological manifestations. In addition, decompensation with severe lactic acidosis occurred at a later age than previously described. Multiple defects in MRCs were present, and liver involvement was noted, both of which are consistent with previous reports. Although cardiac involvement was not reported in previously-described TUFM patients, cardiomyopathy is a characteristic feature of numerous mitochondrial disorders [2]. In fact, hypertrophic cardiomyopathy was reported in several, but not all, patients with bi-allelic variants in TSFM. This suggests that cardiac and neurological phenotypic variability is inherent to mitochondrial disorders of elongation factor defects [17, 21].
Both previously described patients with an encephalopathy phenotype had TUFM variants that disrupt domain 2 of the protein, predicted to affect aminoacyl-tRNA-binding (Fig. 1b, c) [18, 19]. The novel variant identified in our patient (p.His115Pro) resides in domain 1 of EFTu, in proximity to EFTs (Fig. 1d), and thus may affect Tu/Ts complex stability and EF-Tu reactivation. This is similar to the reported TSFM missense variants that were predicted to affect EFTs binding with EFTu, and consequently GDP-GTP exchange [17]. Additional patient reports are necessary to assess whether a clear genotype-phenotype correlation can be associated with mutations in different TUFM domains.
Previous studies have implicated a tissue-specific expression pattern of elongation factors as an important predictor of the resulting phenotype [22, 23]. Although neither EFTu nor EFTs demonstrate a relatively high expression in the myocardium, previous data described a 1:1 EFTu-to-EFTs ratio in muscle, liver, fibroblasts compared to a 1:6 ratio in the heart [23]. Perhaps, variants that affect EFTu/EFTs binding are more damaging in the heart due to this unfavorable ratio. In-line with this, both over-expression and under-expression of either EFTu or EFTs was previously shown to impede mitochondrial translation, emphasizing the importance of a balanced ratio [23].
In conclusion, we describe a novel TUFM homozygous variant resulting in a cardiomyopathy phenotype without significant neurological involvement, expanding the genotypic and phenotypic spectrums of TUFM pathogenic variants. This case warrants the inclusion of TUFM as a candidate gene for early-onset cardiomyopathy. Refining genotype-phenotype associations for rare diseases is essential for predicting tissue involvement, clinical progression and prognosis, and providing families with comprehensive genetic counseling.
References
Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: What is new and what challenges remain? Science. 2015;349:1494–9.
Koopman WJH, Willems PHGM, Smeitink JAM. Monogenic mitochondrial disorders. N Engl J Med. 2012;366:1132–41.
Pearce S, Nezich CL, Spinazzola A. Mitochondrial diseases: translation matters. Mol Cell Neurosci. 2013;55:1–12.
Brunel-Guitton C, Levtova A, Sasarman F. Mitochondrial diseases and cardiomyopathies. Can J Cardiol. 2015;31:1360–76.
Cooper GM, Stone EA, Asimenos G, Green ED, Batzoglou S, Sidow A. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 2005;15:901–13.
Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44(W1):W344–50.
Jeppesen MG, Navratil T, Spremulli LL, Nyborg J. Crystal structure of the bovine mitochondrial elongation factor Tu-Ts complex. J Biol Chem. 2005;280:5071–81.
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42(W1):1–7.
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat Methods. 2014;12:7–8.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12.
Shufaro Y, Lebovich M, Aizenman E, Miller C, Simon A, Laufer N, et al. Human granulosa luteal cell oxidative phosphorylation function is not affected by age or ovarian response. Fertil Steril. 2012;98:166–72.
Saada A, Shaag A, Elpeleg O. mtDNA depletion myopathy: elucidation of the tissue specificity in the mitochondrial thymidine kinase (TK2) deficiency. Mol Genet Metab. 2003;79:1–5.
Vossen RHAM, Buermans HPJ. Full-length mitochondrial-DNA sequencing on the PacBio RSII. Methods Mol Biol. 2017;1492:179–84.
Weissensteiner H, Forer L, Fuchsberger C, Schöpf B, Kloss-Brandstätter A, Specht G, et al. mtDNA-Server: next-generation sequencing data analysis of human mitochondrial DNA in the cloud. Nucleic Acids Res. 2016;44(W1):W64–9.
Fernandes V, Alshamali F, Alves M, Costa MD, Pereira JB, Silva NM, et al. The Arabian cradle: mitochondrial relicts of the first steps along the southern route out of Africa. Am J Hum Genet. 2012;90:347–55.
Burnett BJ, Altman RB, Ferrao R, Alejo JL, Kaur N, Kanji J, et al. Elongation factor Ts directly facilitates the formation and disassembly of the escherichia coli elongation factor Tu·GTP·aminoacyl-tRNA ternary complex. J Biol Chem. 2013;288:13917–28.
Smeitink JA, Elpeleg O, Antonicka H, Diepstra H, Saada A, Smits P, et al. Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am J Hum Genet. 2006;79:869–77.
Valente L, Tiranti V, Marsano RM, Malfatti E, Fernandez-Vizarra E, Donnini C, et al. Infantile encephalopathy and defective mitochondrial DNA translation in patients with mutations of mitochondrial elongation factors EFG1 and EFTu. Am J Hum Genet. 2007;80:44–58.
Di Nottia M, Montanari A, Verrigni D, Oliva R, Torraco A, Fernandez-Vizarra E, et al. Novel mutation in mitochondrial elongation factor EF-Tu associated to dysplastic leukoencephalopathy and defective mitochondrial DNA translation. Biochim Biophys Acta. 2017;1863:961–7.
Kohda M, Tokuzawa Y, Kishita Y, Nyuzuki H, Moriyama Y, Mizuno Y, et al. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 2016;12:1–31.
Ahola S, Isohanni P, Euro L, Brilhante V, Palotie A, Pihko H, et al. Mitochondrial EFTs defects in juvenile-onset leigh disease, ataxia, neuropathy, and optic atrophy. Neurology. 2014;83:743–51.
Ravn K, Schönewolf-Greulich B, Hansen RM, Bohr AH, Duno M, Wibrand F, et al. Neonatal mitochondrial hepatoencephalopathy caused by novel GFM1 mutations. Mol Genet Metab Rep. 2015;3:5–10.
Antonicka H, Sasarman F, Kennaway NG, Shoubridge EA. The molecular basis for tissue specificity of the oxidative phosphorylation deficiencies in patients with mutations in the mitochondrial translation factor EFG1. Hum Mol Genet. 2006;15:1835–46.
Acknowledgements
We thank the patient’s parents for participating in this study. We are grateful to the medical and nursing staff at the pediatric intensive care unit (PICU), Ruth Rappaport Children’s Hospital, Rambam Health Care Campus for their dedicated work and patient care. Corinne Alban, Department of Genetic and metabolic Diseases Hadassah Medical Center, is acknowledged for technical assistance.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
CG-J, SEW, JDO, and ARS are full-time employees of the Regeneron Genetics Center from Regeneron Pharmaceuticals Inc. and receive stock options as part of compensation. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hershkovitz, T., Kurolap, A., Gonzaga-Jauregui, C. et al. A novel TUFM homozygous variant in a child with mitochondrial cardiomyopathy expands the phenotype of combined oxidative phosphorylation deficiency 4. J Hum Genet 64, 589–595 (2019). https://doi.org/10.1038/s10038-019-0592-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0592-6
This article is cited by
-
GATD3A, a mitochondrial deglycase with evolutionary origins from gammaproteobacteria, restricts the formation of advanced glycation end products
BMC Biology (2022)
-
A functional genomics pipeline identifies pleiotropy and cross-tissue effects within obesity-associated GWAS loci
Nature Communications (2021)