Abstract
Previous studies have reported differences in muscle function and metabolism between patients with cystic fibrosis (CF) and healthy controls (HC), but it is currently unknown whether these abnormalities are specific to CF or also seen in other airway diseases. In this study, we used magnetic resonance spectroscopy (MRS) during exercise to assess muscle metabolism in CF patients. Twenty patients with CF and 20 age, gender, and habitual activity-matched HCs and a respiratory disease comparison group with primary ciliary dyskinesia (PCD; n = 10) were studied. 31Phosphorus MRS (31P-MRS) was used to characterize muscle bioenergetic metabolism at rest and after high-, moderate-, and low-intensity exercise. CF patients exhibited lower resting ATP/phosphocreatine (PCr) ratio and significantly higher end-exercise pH values compared with both HC and PCD patients. Both CF and PCD patients demonstrated significantly slower PCr recovery time constants after high-intensity exercise. Our results suggest that not only there are specific abnormalities of muscle metabolism in CF patients but also there is a nonspecific impact of respiratory disease on muscle function.
Similar content being viewed by others
Main
It has been suggested that exercise capacity and muscle function are impaired in patients with cystic fibrosis (CF), but debate remains whether exercise limitation in CF is because of the cardiorespiratory system's inability to meet metabolic demands or intrinsic abnormalities in the muscle itself. Inefficient aerobic oxidative metabolism in CF patients has been described, but it remains unclear whether this is due to impaired oxygen delivery (1,2) or to intrinsic abnormalities in muscle function (3,4). It has been suggested that exercise capacity and muscle function may be impaired in patients with CF and researchers have identified CF transmembrane conductance regulator (CFTR) mRNA expression in rodent skeletal muscle (5). A report has shown that an intrinsic alteration of function is linked to the absence of CFTR from skeletal muscle, leading to dysregulated calcium homeostasis, augmented inflammatory or atrophic gene expression signatures, and increased diaphragm muscle weakness (6). Our research team has recently demonstrated the expression of CFTR in human skeletal muscle (7). In addition, evidence suggests that impaired anaerobic performance (8), abnormal anaerobic metabolism (9), and decreased maximal muscle strength and power (10–12) occurs in CF patients. Recently, [31P]phosphorus magnetic resonance spectroscopy (31P-MRS) to assess muscle metabolism has been used in vivo (13,14) to assess the function of creatine kinase, oxidative phosphorylation, and anaerobic glycolysis pathways during exercise and recovery. 31P-MRS, in conjunction with specifically designed exercise protocols, may allow for the analysis of metabolism in patients with CF (15).
The objective of this study was to identify and quantify muscle metabolic abnormalities in patients with CF, compared with a respiratory disease control group with similar patterns of inflammation, infection, and bronchiectasis (16,17) [primary ciliary dyskinesia (PCD)] and matched healthy control (HC) participants. This may help to differentiate the specific impact of CF on muscle metabolism versus inflammatory factors associated with lung disease in general. We hypothesized that the children with CF would have abnormalities in muscle metabolism but not present in HC or in patients with PCD.
METHODS
Participants.
We recruited 20 adolescent patients with CF (10 female) and 10 patients with PCD (four female) from clinics at the Hospital for Sick Children as well as 20 age, gender, and habitual physical activity-matched HC participants. The inclusion criteria for CF and PCD were clinical stability, a forced expiratory volume in 1 s (FEV1) >70% predicted, good nutritional status (BMI, Z score −2 ± 2), and a confirmed diagnosis of these two entities. CF patients had to be free of a recent pulmonary exacerbation in the 3 mo preceding recruitment and have normal oral glucose tolerance tests near the time of the MRS testing. The study was approved by the Research Ethics Board at the Hospital for Sick Children, and all participants and/or their parents signed informed consent.
Parameters of assessment.
Height and weight (model 555; SR Instruments, Tonawanda, NY) were measured, and lean body mass was calculated based on body composition from MRI analysis (18). Pulmonary function (VMax20 Pulmonary Spirometry Instrument; SensorMedics, Yorba Linda, CA) was determined according to standard spirometric techniques (19), expressed as a percentage of predicted value for height and gender (20). Subjects performed an incremental cycling test to determine maximal aerobic capacity (21) and completed the Habitual Activity Estimation Scale questionnaire (22) as an estimation of activity levels as previously described and validated in this population (23,24).
MRI and spectroscopy tests.
MRIs and 31P-MRS data were collected on a General Electric Twin Speed EXCITE II 12.0 1.5-Tesla imaging and spectroscopy system (GE Healthcare, Milwaukee, WI) at the Hospital for Sick Children, Toronto. Participants lay supine in the MR apparatus and performed exercises on a calibrated nonmagnetic up-down ergometer (Lode AEI Technologies). The lower extremities of the subjects were at the center of the magnet bore of the MRI scanner. The nondominant leg was used for testing. It was secured to the cycle ergometer at the knee and at the ankle to minimize movement during exercise. Motion due to movement of muscle in relation to the coil was minimized by securing the coil in a fixed position midway between the hip and the knee with Velcro straps. The ergometer automatically controlled power output by adjusting resistance in relationship to the participants' freely chosen movement frequency. In this way, exercise was controlled for power output, as relative work rate is an important factor in the determination of the metabolic pathways used for ATP generation during exercise and recovery. Watts and repetitions per minute (rpm) of the ergometer were recorded every 5 s during exercise.
Representative measurements were taken from the vastus lateralis muscle (although the ergometer movement required the use of vastus lateralis, medialis, intermedius, and rectus femoris muscles). The data collection involved MRI followed by shimming (calibration) and 31P-MRS spectroscopy data acquisition. Adequate recovery after exercise was ensured by monitoring the phosphocreatine (PCr) and inorganic phosphate (Pi) peaks to ensure that they had returned to resting levels.
Muscle cross-sectional area was determined from the average results of eight images of the thigh (Fig. 1). For spectroscopy measurements, sequential 31P-MRS spectra were obtained under partially saturated conditions with the following parameters: FID-acquire sequence, hard pulse, 30° flip angle, TR 1000 ms, 3500 Hz spectral width, 1024 data points, and 2 NEX (total acquisition time = 8 s per spectrum). Spectral analyses were performed using commercial software (SAGE 7 Dev2005.3; GE Healthcare). Figure 2 shows a typical spectrum acquired at rest, and pre- and postexercise changes are shown in Figure 3. Figures 2 and 3 are single spectra derived from four pulses as described in the methods mentioned above. Resting data that are presented in the article are based on the average metabolite values determined from eight resting scans.
A curve using nonlinear least squares analysis, based on Gaussian line shapes, was used to calculate the areas under the Pi, PCr, and β-ATP peaks (25). 31P metabolite concentrations were calculated by normalizing total muscle phosphate to 41.3 mmol/L (26). Intracellular pH was calculated for each spectrum based on the chemical shift difference between PCr and Pi (27). The cytosolic [Mg2+] was calculated from the chemical shift of β-ATP measured from the resonance of PCr, and this information was used to correct calculated pH for changes in [Mg2+] (28). The time constant of the recovery rate of PCr was calculated during recovery after each exercise bout using an exponential curve fit and was considered to be an index of aerobic metabolic function.
MRS exercise protocols.
Three different exercise protocols were used to probe bioenergetic function during exercise bouts that are typical of children's activities. Participants performed a 30-s bout of maximal exercise to evaluate the physiological response to short bursts of intense activity (29). Average wattage produced was recorded and used to establish the intensity of subsequent exercise. The subjects recovered for 5 min before performing a 90-s exercise bout to evaluate the response to sustained intense exercise at a work rate equivalent to 85% of the mean watts achieved during the 30-s bout. After recovery, participants performed 10 bouts of 30 s exercise, separated by 15 s of rest, at 65% of the work rate during the 30-s exercise bout to evaluate the metabolic response to repeated bouts of moderate intensity activity.
Statistical analysis.
To evaluate the effect of diagnosis (disease group versus control group), comparisons between groups were made using univariable linear regression models with the group (HC versus CF versus PCD) as the independent variable and test results as dependent variables. Analysis models including three-way comparison were used to evaluate the differences between groups with a maximum likelihood algorithm for parameter estimation. A one-way repeated measures ANOVA analysis (factor: group) was used to assess the 5-min exercise test results as 10 samples per patient were collected during this test. Statistical significance was set at p < 0.05.
RESULTS
Participants.
All subjects completed the study protocol. Baseline characteristics for the study population are shown in Table 1. There were no significant differences between CF, PCD, and HC groups at baseline except for shorter height in the PCD group (p = 0.001). Habitual physical activity (22) was similar among the three groups, and there were no significant differences being “very active” (activities that make you breath hard and sweat) or “somewhat active” (walking around activities) during weekdays or weekends. Maximal aerobic capacities were similar in all groups. There were no significant differences between CF, PCD, and HC in muscle cross-sectional area of the thigh determined by MRI. PCD patients had lower cross-sectional bone area than CF and HC (p < 0.001) and lower total leg area than HC (p = 0.03; Table 2).
31P-MRS resting measures results.
31P-MRS variables were assessed at rest, and the results were analyzed as the average of 16 resting spectra collected sequentially. There were no differences between groups in resting levels of PCr or Pi or Pi/PCr ratio. However, the resting ATP:PCr ratio exhibited a trend toward being lower in CF patients compared with PCD and HC (CF: 0.257 ± 0.048 versus PCD: 0.286 ± 0.048 versus HC: 0.288 ± 0.05; p = 0.05 in CF versus HC only, CF versus PCD; p = 0.09 in CF versus PCD, mean ± SD; Table 3). On further analysis, resting ATP levels were lower in CF patients compared with PCD and HC (CF: 7.8 ± 1.2 mmol/L versus PCD: 8.6 mmol/L ± 1.2 versus HC: 8.5 ± 1.0 mmol/L; p = 0.04, mean ± SD).
31P-MRS results after 30 s and 90 s exercise.
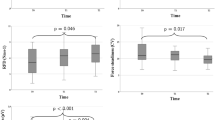
31P-MRS results were collected immediately before exercise and during recovery after the 30- and 90-s exercise bouts (Table 4). There was no significant difference among the three groups in the ratio of Pi:PCr after both exercise bout. CF patients demonstrated a reduced change in pH from rest to end exercise after 90 s of intense exercise compared with PCD and HC groups (p = 0.02; Fig. 4). Similarly, the time constant of PCr recovery after exercise was increased in the CF and PCD groups after the 90 s exercise bout compared with HC (p = 0.02; Fig. 5). There were no significant differences between work achieved by the CF and HC groups during exercise. Patients with PCD had lower work outputs during the 30- and 90-s exercise bouts (p < 0.001) although the relative metabolic stress (Pi:PCr ratio) was equivalent in all groups.
PCr recovery after exercise. Note significantly slower postexercise PCr recovery time constant in patients with CF and PCD. Vertical arrows indicate half time of recovery. (A) PCr recovery for HC (half time of recovery 19.3 + 7.7 s), significant difference vs CF and PCD. (B) PCr recovery for CF patients (half time of recovery 27.2 + 11.7 s). (C) PCr recovery for PCD patients (halftime of recovery 29.6 + 13.5 s).
Exercise data were normalized by determining the power output (watts) per unit of cross-sectional muscle area for each group. There were no differences between CF and HC groups in this measure; however, patients with PCD demonstrated significantly lower watts/cross-sectional area than CF or HC (p = 0.01). Changes in pH postexercise were also evaluated after normalization for watts/cross-sectional area, and PCD patients were found to have a greater change in pH than CF or HC (p < 0.01).
31P-MRS results during 5 min exercise.
31P-MRS results were collected immediately before exercise, after each of ten 30-s exercise bouts and during recovery (Table 4). There were no significant differences in work rates achieved during exercise between the CF and HC groups, but PCD patients exhibited lower watts during the exercise bout (p < 0.001). The relative metabolic stress (Pi:PCr ratio) was equivalent in all groups. A two-way repeated measures ANOVA (factors: group and time) analysis did not reveal any differences between groups in ATP production rates via the three bioenergetic pathways during this lower intensity exercise test.
DISCUSSION
Our results suggest abnormalities in muscle bioenergetic pathways both during rest (lower resting ATP concentrations) and short bouts of high-intensity exercise (higher end-exercise pH values) in patients with CF that are not observed in respiratory disease controls and HC. Both CF and PCD patients demonstrated delayed postexercise PCr recovery times when compared with HC, suggesting a nonspecific effect of respiratory disease on skeletal muscle oxidative function. However, CF patients were capable of achieving the same workload despite these findings. No significant differences were observed between CF, PCD, and control subjects during aerobic exercise, which is in keeping with well-preserved exercise capacity during an incremental cycling test.
The CF and PCD patients were relatively healthy as evidenced by their near-normal FEV1, good nutritional status, activity levels, and exercise testing results compared with HC. MRI cross-sectional muscle area in the thigh was similar for the three groups. The MRI technique, coupled with careful matching of our controls to patients with CF, has allowed us to accurately assess muscle metabolism, while controlling for general fitness, activity level, and muscle mass—a limitation in previous research (1). Furthermore, our results were observed under conditions where end-exercise Pi:PCr ratios were statistically equivalent in the groups, suggesting that the metabolic demands of the activities were similar. The lower work outputs achieved by the PCD group despite having similar muscle cross-sectional area and metabolic stress (Pi:PCr ratio) suggest that patients with PCD may experience greater functional impairment than patients with CF, but this finding requires further evaluation in a larger sample size of PCD patients.
31P-MRS measurements obtained at rest demonstrated lower intramuscular ATP:PCr ratio and ATP concentrations in the CF group compared with PCD and HC groups. Resting muscle ATP values have not been previously reported in the CF or PCD populations. Whether the decreased ATP levels we have observed is primary (6,7,30,31) or secondary to a defect in CFTR in the muscles of patients with CF cannot be directly determined from the current data. It is important to note that the biological consequence of this relatively small difference is unclear. Future research using direct chemical measurement methods should be performed to confirm these findings.
CF patients also demonstrated a higher end-exercise pH, in other words, reduced end-exercise intramuscular acidosis, compared with HC and patients with PCD. Although patients with CF have altered bicarbonate transport (32–34), this could be considered a physiological benefit as higher intracellular bicarbonate concentration could lead to less metabolic acidosis during high-intensity exercise. The significant results related to mitochondrial function (PCr recovery) and glycolytic metabolism (end-exercise pH) were noted on the 90-s exercise bout. This is expected, as 90 s of intense exercise places a greater demand on the anaerobic system during exercise (increased lactic acid accumulation and decreases in pH levels) and the aerobic system during recovery (to deal with the increased levels of lactate and pyruvate that accumulate) than either the 30 s of intense exercise, in which there is not enough time for the anaerobic system to become fully activated, or the 5 min of moderate exercise, which is not as physiologically stressful at the intramyocellular level.
After normalizing the data for power output per cross-sectional muscle area, PCD patients were found to have a larger change in pH postexercise than CF patients or HC. This occurred despite also producing lower watts per cross-sectional muscle area. It is possible to interpret this as the PCD group being less fit, hence lower muscular power and higher anaerobic stress. However, the CF muscle seems to work quite differently with preserved function and a lower change in pH with exercise than the HC group. This finding may have implications for patient care. More specifically, exercise is considered an important part of physical therapy in CF. In contrast, although chest therapy has been emphasized in the PCD population, little effort has been made to promote exercise in the PCD population. Our current results suggest that exercise, nutrition, and habitual physical activity may be an important adjunct to current clinical approaches for patients with PCD and CF.
The slower PCr recovery time constant after 90 s of intense exercise suggests impaired muscle aerobic oxidative metabolism in adolescent patients with CF and PCD as recovery rate of PCr is largely determined by aerobic oxidative metabolism (35). The observed impairment in mitochondrial oxidative metabolism may be because of a reduced antioxidant capacity arising from systemic inflammation and oxidative damage that lowers the efficiency of mitochondria in patients with CF (36–38) and PCD (39). Interestingly, we observed differences in muscle metabolism using 31P-MRS, which allows for measurement of bioenergetics in the muscle tissue itself, but all groups had similar results on maximal aerobic capacity testing based on gas analysis at the mouth during incremental cycling. Although this might seem to be contradictory, we suggest that although intramyocellular dysfunction exists in the patients in this study, there may have been adaptations that compensate for this dysfunction in the oxygen transport pathway. Direct in vivo measurement of muscle oxidative function in humans with CF and PCD has not been previously reported, although investigators have reached similar conclusions based on results from pulmonary gas exchange in CF patients with more advanced disease than those in this cohort (3,36,37). The CF and PCD patients in this study were relatively healthy (FEV1 >75%), and it is unlikely that gas exchange was limited as this degree of mild airflow limitation has not been associated with maximal exercise limitation. It is important to note that the primary differences between patients with respiratory disease and HC were observed after the high-intensity 90-s exercise bout. This is the level of exercise that is most physiologically demanding, with significant activation of the anaerobic system during exercise and causing the accumulation of lactate in the muscle, which would present a physiological challenge to mitochondrial aerobic metabolism during recovery. This accumulation of waste products would likely not have occurred to the same extent in 30 s of exercise or in repeated bouts of lower intensity exercise. Therefore, it is not surprising that differences were noted at this intensity level, and not during shorter intervals where the anaerobic system is not stressed or during longer duration exercise of moderate stress where differences may not become apparent.
Abbreviations
- CF:
-
cystic fibrosis
- CFTR:
-
cystic fibrosis transmembrane regulator
- FEV1:
-
forced expiratory volume in 1 s
- HC:
-
healthy control
- PCD:
-
primary ciliary dyskinesia
- PCr:
-
phosphocreatine
- Pi:
-
inorganic phosphorus
- 31P-MRS:
-
31phosphorus magnetic resonance spectroscopy
References
Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM 2000 Muscle size and cardiorespiratory response to exercise in cystic fibrosis. Am J Respir Crit Care Med 162: 1823–1827
Klijn PH, van der Net J, Kimpen JL, Helders PJ, van der Ent CK 2003 Longitudinal determinants of peak aerobic performance in children with cystic fibrosis. Chest 124: 2215–2219
Kusenbach G, Wieching R, Barker M, Hoffmann U, Essfeld D 1999 Effects of hyperoxia on oxygen uptake kinetics in cystic fibrosis patients as determined by pseudo-random binary sequence exercise. Eur J Appl Physiol Occup Physiol 79: 192–196
Rosenthal M, Narang I, Edwards L, Bush A 2009 Non-invasive assessment of exercise performance in children with cystic fibrosis (CF) and non-cystic fibrosis bronchiectasis: is there a CF specific muscle defect?. Pediatr Pulmonol 44: 222–230
Fiedler MA, Nemecz ZK, Shull GE 1992 Cloning and sequence analysis of rat cystic fibrosis transmembrane conductance regulator. Am J Physiol 262: L779–L784
Divangahi M, Balghi H, Danialou G, Comtois AS, Demoule A, Ernest S, Haston C, Robert R, Hanrahan JW, Radzioch D, Petrof BJ 2009 Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet 5: e1000586
Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I 2010 Cystic fibrosis transmembrane conductance regulator in human muscle. Dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol 67: 802–808
Shah AR, Gozal D, Keens TG 1998 Determinants of aerobic and anaerobic exercise performance in cystic fibrosis. Am J Respir Crit Care Med 157: 1145–1150
Selvadurai HC, Allen J, Sachinwalla T, Macauley J, Blimkie CJ, Van Asperen PP 2003 Muscle function and resting energy expenditure in female athletes with cystic fibrosis. Am J Respir Crit Care Med 168: 1476–1480
Ionescu AA, Chatham K, Davies CA, Nixon LS, Enright S, Shale DJ 1998 Inspiratory muscle function and body composition in cystic fibrosis. Am J Respir Crit Care Med 158: 1271–1276
de Meer K, Gulmans VA, van Der Laag J 1999 Peripheral muscle weakness and exercise capacity in children with cystic fibrosis. Am J Respir Crit Care Med 159: 748–754
Sahlberg ME, Svantesson U, Thomas EM, Strandvik B 2005 Muscular strength and function in patients with cystic fibrosis. Chest 127: 1587–1592
Taylor DJ 2000 Clinical utility of muscle MR spectroscopy. Semin Musculoskelet Radiol 4: 481–502
Newcomer BR, Boska MD 1997 Adenosine triphosphate production rates metabolic economy calculations pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 20: 336–346
Mattei JP, Bendahan D, Cozzone P 2004 P-31 magnetic resonance spectroscopy. A tool for diagnostic purposes and pathophysiological insights in muscle diseases. Reumatismo 56: 9–14
Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR 2004 Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 169: 459–467
Bush A, Chodhari R, Collins N, Copeland F, Hall P, Harcourt J, Hariri M, Hogg C, Lucas J, Mitchison HM, O'Callaghan C, Phillips G 2007 Primary ciliary dyskinesia: current state of the art. Arch Dis Child 92: 1136–1140
Wells GD, Heale L, Schneiderman JE, Wilkes DL, Atenafu E, Coates AL, Ratjen F 2008 Assessment of body composition in pediatric patients with cystic fibrosis. Pediatr Pulmonol 43: 1025–1032
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J 2005 Standardisation of spirometry. Eur Respir J 26: 319–338
Corey M, Levison H, Crozier D 1976 Five- to seven-year course of pulmonary function in cystic fibrosis. Am Rev Respir Dis 114: 1085–1092
Godfrey S, Davies CT, Wozniak E, Barnes CA 1971 Cardio-respiratory response to exercise in normal children. Clin Sci 40: 419–431
Hay JA, Cairney J 2006 Development of the habitual activity estimation scale for clinical research: a systematic approach. Pediatr Exerc Sci 18: 193–202
Schneiderman-Walker J, Wilkes DL, Strug L, Lands LC, Pollock SL, Selvadurai HC, Hay J, Coates AL, Corey M 2005 Sex differences in habitual physical activity and lung function decline in children with cystic fibrosis. J Pediatr 147: 321–326
Wells GD, Wilkes DL, Schneiderman-Walker J, Elmi M, Tullis E, Lands LC, Ratjen F, Coates AL 2008 Reliability and validity of the habitual activity estimation scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol 43: 345–353
Zanconato S, Buchthal S, Barstow TJ, Cooper DM 1993 31P-magnetic resonance spectroscopy of leg muscle metabolism during exercise in children and adults. J Appl Physiol 74: 2214–2218
Boska M 1994 ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med 32: 1–10
Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG 1985 Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology 35: 781–788
Iotti S, Frassineti C, Alderighi L, Sabatini A, Vacca A, Barbiroli B 2000 In vivo (31)P-MRS assessment of cytosolic [Mg(2+)] in the human skeletal muscle in different metabolic conditions. Magn Reson Imaging 18: 607–614
Inbar O, Bar-Or O 1986 Anaerobic characteristics in male children and adolescents. Med Sci Sports Exerc 18: 264–269
Gadsby DC, Dousmanis AG, Nairn AC 1998 ATP hydrolysis cycles and the gating of CFTR Cl- channels. Acta Physiol Scand Suppl 643: 247–256
Hanrahan JW, Wioland MA 2004 Revisiting cystic fibrosis transmembrane conductance regulator structure and function. Proc Am Thorac Soc 1: 17–21
Quinton PM 2001 The neglected ion: HCO3−. Nat Med 7: 292–293
Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S 2001 Aberrant CFTR-dependent HCO3− transport in mutations associated with cystic fibrosis. Nature 410: 94–97
Reddy MM, Quinton PM 2003 Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature 423: 756–760
Bendahan D, Mattei JP, Guis S, Kozak-Ribbens G, Cozzone PJ 2006 [Non-invasive investigation of muscle function using 31P magnetic resonance spectroscopy and 1H MR imaging]. Rev Neurol (Paris) 162: 467–484
Hebestreit H, Hebestreit A, Trusen A, Hughson RL 2005 Oxygen uptake kinetics are slowed in cystic fibrosis. Med Sci Sports Exerc 37: 10–17
Moorcroft AJ, Dodd ME, Morris J, Webb AK 2005 Symptoms, lactate and exercise limitation at peak cycle ergometry in adults with cystic fibrosis. Eur Respir J 25: 1050–1056
Fischer R, Simmerlein R, Huber RM, Schiffl H, Lang SM 2007 Lung disease severity, chronic inflammation, iron deficiency, and erythropoietin response in adults with cystic fibrosis. Pediatr Pulmonol 42: 1193–1197
Zihlif N, Paraskakis E, Tripoli C, Lex C, Bush A 2006 Markers of airway inflammation in primary ciliary dyskinesia studied using exhaled breath condensate. Pediatr Pulmonol 41: 509–514
Acknowledgements
We thank the volunteers from the Hospital for Sick Children, Crescent School, and Branksome Hall for participating in the research. Cedric Manlhiot assisted with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was funded by a grant from the Canadian Cystic Fibrosis Foundation and the Lynn and Arnold Irwin Foundation at the Hospital for Sick Children. G.D.W. and J.E.S. were supported by the Hospital for Sick Children Research Training Competition Fellowship.
Rights and permissions
About this article
Cite this article
Wells, G., Wilkes, D., Schneiderman, J. et al. Skeletal Muscle Metabolism in Cystic Fibrosis and Primary Ciliary Dyskinesia. Pediatr Res 69, 40–45 (2011). https://doi.org/10.1203/PDR.0b013e3181fff35f
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181fff35f
This article is cited by
-
Does the effect of comprehensive respiratory physiotherapy home-program differ in children with cystic fibrosis and non-cystic fibrosis bronchiectasis?
European Journal of Pediatrics (2022)
-
Active video gaming in primary ciliary dyskinesia: a randomized controlled trial
European Journal of Pediatrics (2022)
-
Physical activity for children with chronic disease; a narrative review and practical applications
BMC Pediatrics (2019)
-
Size-adjusted muscle power and muscle metabolism in patients with cystic fibrosis are equal to healthy controls – a case control study
BMC Pulmonary Medicine (2019)
-
The regulation of skeletal muscle fiber-type composition by betaine is associated with NFATc1/MyoD
Journal of Molecular Medicine (2018)