Abstract
Background
Maintenance of cholesterol homeostasis is crucial for brain development. Brain cholesterol relies on de novo synthesis and is cleared primarily by conversion to 24S-hydroxycholesterol (24S-HC) with brain-specific cholesterol 24-hydroxylase (CYP46A1). We aimed to investigate the impact of hypoxia–ischemia (HI) on brain cholesterol metabolism in the neonatal mice.
Methods
Postnatal day 9 C57BL/6 pups were subjected to HI using the Vannucci model. CYP46A1 expression was assessed with western blotting and its cellular localization was determined using immunofluorescence staining. The amount of brain cholesterol, 24S-HC in the cortex and in the serum, was measured with enzyme-linked immunosorbent assay (ELISA).
Results
There was a transient cholesterol loss at 6 h after HI. CYP46A1 was significantly upregulated at 6 and 24 h following HI with a concomitant increase of 24S-HC in the ipsilateral cortex and in the serum. The serum levels of 24S-HC correlated with those in the brain, as well as with necrotic and apoptotic cell death evaluated by the expression of spectrin breakdown products and cleaved caspase-3 at 6 and 24 h after HI.
Conclusion
Enhanced cholesterol turnover by activation of CYP46A1 represents disrupted brain cholesterol homeostasis early after neonatal HI. 24S-HC might be a novel blood biomarker for severity of hypoxic–ischemic encephalopathy with potential clinical application.
Similar content being viewed by others
Main
Human brain cholesterol, which constitutes 25% of total body cholesterol, is crucial for brain development because of its importance in membrane integrity, myelination, synaptogenesis, and neurotransmission (1, 2). The majority of brain cholesterol is stored in myelin sheaths, and the rest in the membranes of neurons, glial cells, and other cellular elements. To accommodate rapid brain growth in the neonatal period, the rates of cholesterol biosynthesis and accretion are the highest during this stage (first 3 weeks after birth in the rodents), a critical time for neuroplasticity, and then decline with age to reach a constant cholesterol level at adulthood (3, 4, 5, 6). As cholesterol carried in lipoproteins in the blood cannot cross the blood–brain barrier, brain cholesterol is produced exclusively from de novo synthesis (3, 7) using Acetyl-CoA as the starting material and HMG-CoA reductase (HMGCR) as the rate-limiting enzyme. However, 24S-hydroxycholesterol (24S-HC), its metabolite that is generated through hydroxylation by cholesterol 24-hydroxylase (CYP46A1), is capable of traversing the blood–brain barrier and entering circulation to the liver for excretion (4, 7). CYP46A1 is brain-specific (4, 8); thus, most circulating 24S-HC has a cerebral origin (9) and the serum 24S-HC level could be an indication of brain cholesterol metabolism (10). In fact, plasma 24S-HC has been used as a surrogate marker of neuronal loss and brain atrophy in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (11).
Cholesterol biosynthesis involves multiple enzymatic reactions and is an oxygen-consumptive process that requires 11 oxygen molecules for the conversion of Acetyl-CoA to cholesterol (12). Therefore, cholesterol synthesis is sensitive to reduced O2 availability and is limited in the conditions of HI. This is evidenced by two studies in neonatal HI rats showing chronic loss of brain cholesterol lasting at least 3 days or 3 months following the insults (13, 14). Unfortunately, there are no additional investigations on the changes of cholesterol metabolism in response to HI in the immature brain and the underlying mechanisms. Recent in vitro studies suggested that oxidative stress (6) and endogenous neurotransmitters upregulate CYP46A1, with glutamate eliciting the highest increase in CYP46A1 activity (15). These are well-accepted mechanisms that are associated with brain damage in neonatal HI encephalopathy (HIE), the clinical syndrome of brain dysfunction in the newborns with few tools for diagnosis and intervention (16). Studies on regulation of cholesterol homeostasis in the developing brain, as well as its disturbance after HI at early postnatal stage, would allow for a better understanding of lipid disorders and their involvement in HIE gray and white matter injury.
The present study focused on the responses of cholesterol metabolism after neonatal HI on postnatal day 9 (P9) mice, an age equivalent to full-term human infants. We hypothesized that the brain-specific cholesterol hydroxylase, CYP46A1, is activated following neonatal HI leading to an enhanced production of 24S-HC in the brain, and in the circulation. Herein, we demonstrated an increase and correlation of the 24S-HC levels in the serum and in the brain, and, importantly, a significant positive correlation between serum 24S-HC levels and cortical injury evaluated by the expression of spectrin breakdown products (SBDPs) and cleaved caspase-3, representing activation of necrosis and/or apoptosis, at 6 and 24 h after HI. These findings suggested a possibility of using 24S-HC as a potential blood biomarker for severity of HIE brain injury.
Methods
All animal experiments were approved by the University of California San Francisco institutional animal care and use committee. C57BL/6 mice (Charles River Laboratory, Hollister, CA) with litters were allowed food and water ad libitum. Both sexes were used on P9.
Neonatal Brain HI
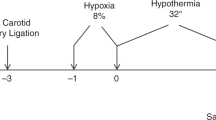
Neonatal HI was performed using the Vannucci model. On P9, the pups underwent left common carotid artery coagulation through a vertical midline neck incision under isoflurane anesthesia (2–3% isoflurane, balanced oxygen) to induce unilateral ischemia. The animals were allowed to recover for 1 h with their dam and then exposed to 60 min of hypoxia in a humidified chamber at 37 °C with 10% oxygen/balanced nitrogen to induce global hypoxia. Sham-operated control animals received isoflurane anesthesia and exposure of the left common carotid artery without coagulation and hypoxia. The animals were dissected immediately after hypoxia (0 h) or returned to their dams until they were killed at 1, 6, 24, 48, or 72 h after the procedure.
Sample Preparation
The cortices were dissected and quickly sliced into small pieces on ice, mixed, and divided into three equal fractions for protein (western blotting), cholesterol, or 24S-HC measurements, respectively. For protein purification, the tissue was homogenized in radioimmunoprecipitation assay buffer (R0278, MilliporeSigma, Temecula, CA) with protease and phosphatase inhibitors, followed by centrifugation at 14,000 r.p.m. for 15 min. The supernatant was saved and the protein concentration was measured with BCA kit (Pierce Biotechnology, Rockford, IL). For 24S-HC assay, the tissue was sonicated with 95% ethanol; after centrifugation at 7,000g for 5 min, the supernatant (A) was collected and the pellet was sonicated again in the extract buffer (ethanol:dichloromethane (1:1; v/v)) followed by centrifugation at 7,000g for 5 min. The resultant supernatant (B) was combined with supernatant A and dried with speed vacuum. The dried pellet was rehydrated by adding 8 μl of 95% ethanol and 242 μl of assay buffer 40 (24S-hydroxycholesterol ELISA (enzyme-linked immunosorbent assay) Kit, ab204530, Abcam, Cambridge, MA). For cholesterol measurement, the tissue was extracted with 200 μl of chloroform:isopropanol:IGEPAL CA-630 (7:11:0.1) in a microhomogenizer and spun at 13,000g for 10 min to remove insoluble material. The organic phase was air-dried at 50 °C for 15 min to remove chloroform. The samples were then dried with speed vacuum and dissolved in 200 μl cholesterol assay buffer (Cholesterol Quantitation Kit, MAK043, MilliporeSigma) for cholesterol quantification.
Serum was collected from the same animals using a BD SST microtainer capillary blood collection tube with serum separator (#365967, BD, Franklin Lakes, NJ).
Western Blotting
An equal amount of protein samples (40 μg) was applied to 4–12% Bis-Tris SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane at 30 V overnight at 4 oC. After blocking with TBS containing 0.05% Tween 20 (TBST) buffer with 5% milk for 1 h, the blots were probed overnight at 4 °C with the following primary antibodies: anti-cholesterol-24-hydroxylase 1A7 (1:500; MAB2259, MilliporeSigma), anti-α-spectrin (1:5,000, MAB1622, MilliporeSigma), anti-cleaved caspase-3 (1:1,000, #9664, Cell Signaling Technology, Danvers, MA), and anti-β-actin (1:5,000, sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA). Appropriate secondary horseradish peroxidase-conjugated antibodies (Santa Cruz Biotechnology) were used. The protein signal was visualized with enhanced chemiluminescence and developed with a radiographic film. Image J software was used to measure the mean optical densities (OD) and areas of protein signal on the film after scanning.
Enzyme-Linked Immunosorbent Assay for 24S-HC Levels in the Cortex and Serum
A competitive ELISA kit (ab204530, Abcam) was used to measure the levels of 24S-HC in the cortex and serum. A fresh set of 24S-HC standard was prepared at a range of 0.39–100 ng/ml and a clear 96-well plate coated with goat anti-rabbit IgG was used. Briefly, 100 μl of standards and samples were pipetted into the appropriate wells. An aliquot of 50 μl of the diluted biotin-labeled 24S-HC conjugate was then added to each well except for the blanks. Fifty microliters of the rabbit 24S-HC antibody that can bind to either 24S-HC in the samples or to the labeled 24S-HC conjugate was added to each well, except for the blanks and the nonspecific binding (NSB) wells. The plate was incubated for 1 h at room temperature (RT). After washing four times with 400 μl of wash buffer, 200 μl of streptavidin–horseradish peroxidase solution was pipetted into each well to bind to biotin-labeled 24S-HC conjugate. The plate was incubated for 30 min at RT. After the second wash, 200 μl of 3,3′,5,5′-Tetramethylbenzidine substrate solution was added and incubated at RT for 30 min. To stop the reaction, 50 μl of stop solution (1 N HCl) was added and the absorbance (OD) was read at 450 nm with a microplate reader (BioTek Synergy, Winooski, VT). The intensity of signal was inversely proportional to the levels of 24S-HC in the samples. For each standard and sample, the net OD=OD−NSB OD. A standard curve was made for the calculation of the levels of 24S-HC. The concentration of cortical 24S-HC was normalized to the wet weight of the starting tissue (ng/mg wet weight). The concentration of serum 24S-HC was normalized to the serum protein concentration (ng/mg protein).
Cholesterol Measurement
A cholesterol quantitation kit (MAK043, MilliporeSigma) was used to measure the concentration of cholesterol in the cortex. The samples prepared above were diluted at 1:10 with assay buffer and seven cholesterol standards (0.02–0.14 μg/μl) were prepared. Hundred microliters of standards and samples were pipetted into the appropriate wells of a clear 96-well plate. Fifty microliters of reaction mix buffer including cholesterol probe, cholesterol enzyme mix, and cholesterol esterase were added to each well. The plate was incubated at 37 °C for 60 min and protected from light. The absorbance was read at 570 nm and the concentration of cholesterol in the samples was calculated using the standard curve. The levels of cortical cholesterol were normalized to the wet weight of the starting tissue (μg/mg wet weight).
Immunofluorescent Staining
At 24 h after HI, the sham and injured mice were perfuse-fixed with 4% paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.4). Brains were dissected and post-fixed in 4% paraformaldehyde overnight at 4 °C, and then transferred to 30% sucrose in 0.1 mol/l phosphate buffer for cryoprotection for 3 days. The brains were embedded in optimal cutting temperature (O.C.T.) compound and cryosections were cut at 16 μm. The sections were defrosted and air-dried at RT for 2 h. After washing with phosphate-buffered saline (PBS), sections were boiled in 0.01 M sodium citrate buffer (pH 6.0, with 0.05% Tween 20) at 90 °C for 30 min to retrieve antigen. After washing with PBS, the sections were blocked with blocking buffer (10% goat serum and 0.1% Triton X-100 in PBS) for 1 h and then incubated overnight at 4 °C with mouse anti-CYP46A1 antibody (1:50, MAB2259, MilliporeSigma), paired with another rabbit antibody that labels the following different cell types: anti-NeuN for neurons (1:500, #ab177487, Abcam), anti-glial fibrillary acidic protein (GFAP) for astrocytes (1:500, #Z0334, Dako, Santa Clara, CA), anti-Iba1 for microglia (1:1,000, #019-19741, Wako Chemicals, Richmond, VA), or anti-myelin basic protein (MBP) for oligodendrocytes (1:200, #78896, Cell Signaling Technology). Antibody against cleaved caspase-3 (1:500, #9664, Cell Signaling Technology) was used for double staining with anti-CYP46A1 to study their colocalization. After washing with 0.025% Tween/PBS for three times, the sections were incubated with goat anti-mouse Alexa Fluor 568 (1:500; #A-11004, Invitrogen, Grand Island, NY) and goat anti-rabbit Alexa Fluor 488 (1:500, #A-11034, Invitrogen) for 1 h at RT and exposed to 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and then cover-slipped with ProLong Diamond antifade reagent (Invitrogen). Images were taken with Leica TCS SP5 Spectral Confocal Microscope.
Statistical Analysis
SAS Wilcoxon–Mann–Whitney test was used to evaluate the OD values of protein expression in western blotting, brain concentrations of cholesterol, and the cortical and serum levels of 24S-HC. Pearson’s correlation coefficient analysis was used to evaluate the correlation between cortical and serum levels of 24S-HC, and between serum 24S-HC and protein levels of SBDP at 145/150 kDa, SBDP at 120 kDa, or cleaved caspase-3. Differences were considered significant at P<0.05.
Results
Upregulation of CYP46A1 with a Concomitant Increase of 24S-HC in the Brain Following Neonatal HI
To investigate how cholesterol metabolism is influenced by neonatal HI, we measured the protein expression of CYP46A1, the enzyme responsible for elimination of excess cholesterol from the brain, at various post-HI time points for up to 3 days. As shown in Figure 1a, CYP46A1 was significantly upregulated at 6 and 24 h after HI compared to the sham animals at the same time points, and declined at 48 and 72 h (sham vs. HI: P=0.0339 at 6 h; P=0.0026 at 24 h; n=5–6 for sham animals, n=6–12 for HI animals at 6–72 h). The increased expression of CYP46A1 was confirmed by immunofluorescent staining with anti-CYP46A1 antibody at 24 h after HI, when the fluorescent intensity was much higher in the ipsilateral cortex compared with that of the sham animals and the contralateral hemisphere (left panels in Figure 1b). It is interesting that the expression of CYP46A1 in the ipsilateral cortex was largely localized to the cells undergoing apoptotic cell death as visualized by the expression of cleaved caspase-3 (Figure 1b).
Upregulation of CYP46A1 with a concomitant increase of 24S-HC in the ipsilateral (ipsi) cortex after neonatal HI. (a) Protein expression of CYP46A1 was measured by western blotting at the indicated time points and was presented as the OD ratio to β-actin and normalized to the values of sham 0 h (graph on the right, sham vs. HI: *P=0.0339 at 6 h; *P=0.0026 at 24 h; n=5–6 for sham animals, n=6–12 for HI animals at 6–72 h). (b) Immunofluorescent staining with anti-CYP46A1 antibody at 24 h after HI showed enhanced expression in the ipsi cortex than that in the contralateral side and in the sham animals (left panels). CYP46A1 staining in the ipsi cortex was largely colocalized with the expression of cleaved caspase-3 (middle panels). (c) Increased production of 24S-HC (ng/mg tissue wet weight) in the ipsi cortex at 6 and 24 h after HI (sham vs. HI: *P=0.0167 at 6 h; *P=0.0085 at 24 h; n=3–6 for sham animals, n=5–7 for HI animals at 6–72 h). The time course of the changes in 24S-HC in the sham-, contra-, and ipsi cortex is shown on the right. At 6 h, 24S-HC in the ipsi cortex was higher than that in the contralateral side (ipsi- vs. contra-: *P=0.0493). #Significant difference between the HI ipsi- and the sham animals only, but not between the HI ipsi- and HI contralateral hemisphere. 24S-HC, 24S-hydroxycholesterol; HI, hypoxia–ischemia; OD, optical density.
The pattern of 24S-HC levels in the ipsilateral cortex was similar to that of CYP46A1 expression with a marked increase at 6 and 24 h post-HI compared with that of the sham controls (Figure 1c, sham vs. HI: P=0.0167 at 6 h; P=0.0085 at 24 h; n=3–6 for sham animals, n=5–7 for HI animals at 6–72 h). At 6 h, 24S-HC in the ipsilateral cortex was higher than that in the contralateral side, too (Figure 1c, HI ipsilateral vs. HI contralateral: P=0.0493 at 6 h). HI-induced overproduction of 24S-HC in the ipsilateral cortex sustained at 48 h, although the difference was not statistically significant compared with that in the sham animals (Figure 1c, sham vs. HI: P=0.0526 at 48 h). There was a trend of more 24S-HC produced in the contralateral cortex (underwent hypoxia) at 6 and 24 h; however, the differences were not significant (Figure 1c, right panel). These results demonstrated that the upregulated CYP46A1 was functional, which led to robust cholesterol turnover early after HI, along with an enhanced production of its metabolic product 24S-HC in the injured cortex, but not in the non-injured contralateral hemisphere.
To determine whether the increased cholesterol metabolism following neonatal HI would diminish brain cholesterol amount, we measured cholesterol levels in the sham and HI-injured ipsi- and contralateral cortex at the same time points. We found a significant, but transient loss of cholesterol at 6 h after HI in the ipsilateral cortex (Figure 2a, sham vs. HI ipsi: P=0.0304 at 6 h; n=7), but not in the contralateral side (sham vs. HI contra: P=0.1736; n=7) compared with the sham animals. These changes represented the net results of de novo cholesterol synthesis and turnover. The enhanced cholesterol hydroxylation/breakdown may, at least in part, contribute to the cholesterol loss at 6 h, whereas the elevated expression of HMGCR, the rate-limiting enzyme for cholesterol biosynthesis, at 24 h (Figure 2b, sham vs. HI: P=0.047 at 24 h, n=4–11) indicates feedback mechanisms for replenishment and re-establishment of cholesterol balance at 24 h and the time points thereafter.
Transient cholesterol loss in the ipsilateral cortex at 6 h after neonatal HI. (a) The time course of the changes in cholesterol amount in the sham-, contra-, and ipsilateral cortex following neonatal HI (sham vs. HI: *P=0.0304 at 6 h; n=4–7 for each time point). (b) Protein expression of HMGCR in the sham and HI-injured cortices was measured by western blotting at the indicated time points. (c) HMGCR expression is presented as the OD ratio to β-actin and is normalized to the values of sham 0 h (sham vs. HI: *P=0.047 at 24 h, n=3–11). 24S-HC, 24S-hydroxycholesterol; HI, hypoxia–ischemia; HMGCR, HMG-CoA reductase; OD, optical density.
Expression of CYP46A1 in Neurons and Oligodendrocytes
CYP46A1 was originally reported to be neuron-specific; however, several recent studies demonstrated its presence in glial cells, including microglia and astrocytes in different injury paradigms (17, 18). To study the cellular localization of CYP46A1 in our neonatal HI model, we stained the brain sections from the sham and injured pups (at 24 h post-HI) with CYP46A1 antibody paired with another antibody specific for neuron (NeuN), astrocyte (GFAP), oligodendrocyte (MBP), or microglia (Iba1). Figure 3 indicates that the enzyme was expressed in neurons (shown in the cortical region) and oligodendrocytes (shown in the corpus callosum) in both sham (Figure 3a) and the injured animals (Figure 3b). Colocalization was not found in the astrocytes and microglia in the sham (not shown) and injured brains (Figure 3b). The CYP46A1 staining was in the cytoplasm.
Expression of CYP46A1 in the neurons and oligodendrocytes. Images of double immunofluorescent staining with CYP46A1 antibody (red, middle panels) paired with another antibody specific for neuron (NeuN), oligodendrocyte (MBP), astrocyte (GFAP), or microglia (Iba1), shown in green on the left panels. The representative images from the sham (a) and HI-injured animals at 24 h after HI (b). 24S-HC, 24S-hydroxycholesterol; HI, hypoxia–ischemia;.
Correlation of the 24S-HC Levels in the Serum and in the Brain after Neonatal HI
As CYP46A1 converts brain cholesterol to the more soluble 24S-HC, which readily crosses blood–brain barrier, and this enzymatically generated oxysterol is specifically made in the brain, we asked whether there is an efflux of 24S-HC from the brain into the periphery circulation after HI. We measured the serum levels of 24S-HC and evaluated its relationship with brain levels. In line with the increased production in the brain, there was a boost of serum 24S-HC levels at 6 h after HI, which sustained for 48 h before returning to the sham values at 72 h (Figure 4a, sham vs. HI, P=0.0102 at 6 h; P=0.0046 at 24 h; P=0.0167 at 48 h; n=3–6 for sham animals, n=5–13 for HI animals at 6–72 h). There was a strong correlation of 24S-HC levels in the serum and in the ipsilateral cortex (Figure 4b, R2=0.521, P<0.0001, n=40), implicating the brain as the major source of circulating 24S-HC (note that the concentrations of 24S-HC in the serum were normalized to serum protein concentrations as ng/mg protein, whereas in the brain they were normalized to the tissue wet weight as ng/mg wet weight).
Correlation of the 24S-HC levels in the serum and in the ipsilateral cortex after neonatal HI. (a) Quantification of the serum levels of 24S-HC (ng/mg serum protein) at the indicated post-HI time points. Sham vs. HI, *P=0.0102 at 6 h, *P=0.0046 at 24 h, *P=0.0167 at 48 h; n=3–6 for sham animals, n=5–13 for HI animals at 6–72 h. (b) Correlation of 24S-HC levels in the serum and in the ipsilateral cortex. The samples used in Pearson’s correlation coefficient analysis were those included in Figure 1c (n=40, R2=0.521, P<0.0001). 24S-HC, 24S-hydroxycholesterol; HI, hypoxia–ischemia;.
Correlation of Serum 24S-HC Levels with Brain Injury at 6 and 24 h After HI
As cholesterol is essential for membrane and myelin integrity, and CYP46A1 upregulation may cause membrane disruption or myelin breakdown leading to cell death and brain injury after HI, we hypothesized that increased serum 24S-HC values could forecast HI-induced cell loss and gray/white matter damage. We assessed acute brain injury within 3 days with the protein expression of SBDP at 145/150 and 120 kD representing activation of necrosis and/or apoptosis, as well as the cleaved caspase-3, indicating apoptotic cell death. The expression of both SBDP at 145/150 and 120 kD considerably increased at 6 and 24 h after HI compared with the sham animals (Figure 5a, for SBDP 145/150 kD, sham vs. HI, P=0.0094 at 6 h, P=0.0013 at 24 h. For SBDP 120 kD, sham vs. HI, P=0.036 at 6 h, P=0.0087 at 24 h, n=4–6 for sham animals, n=6–15 for HI animals from 6 to 72 h). The increase in cleaved caspase-3 levels lasted longer for 48 h and subsided at 72 h (Figure 6a, sham vs. HI, P=0.0112 at 6 h; P=0.0027 at 24 h; P=0.0228 at 72 h, n=5–6 for sham animals, n=7–10 for HI animals from 6 to 72 h). There was a positive correlation between serum 24S-HC levels and SBDP 145/150 kD at 6 and 24 h post-HI (Figure 5b, at 6 h: R2=0.713, P=0.002, n=10; at 24 h: R2=0.656, P=0.003, n=11), and with cleaved caspase-3 at the same time points (Figure 6b, at 6 h: R2=0.738, P=0.002, n=10; at 24 h: R2=0.462, P=0.044, n=9). The correlation between serum levels of 24S-HC and SBDP 120 kD was also significant at 24 h (Figure 5b, R2=0.557, P=0.008, n=11).
Correlation of the serum levels of 24S-HC with the expression of SBDPs at 6 and 24 h after neonatal HI. (a) Protein expression of SBDP145/150 kD and SBDP 120 kD in the cortices was measured by western blotting at the indicated time points and was presented as the OD ratio to β-actin and normalized to the values of sham 0 h (for SBDP 145/150 kD), or an internal control (IC, for SBDP 120 kD). For SBDP 145/150 kD, sham vs. HI, *P=0.0094 at 6 h, *P=0.0013 at 24 h. For SBDP 120 kD, sham vs. HI, *P=0.036 at 6 h, *P=0.0087 at 24 h (n=4–6 for sham animals, n=6–15 for HI animals from 6 to 72 h). (b) Correlation of the serum levels of 24S-HC with the expression of SBDP 145/150 kD at 6 h (top), at 24 h (middle), and with the expression of SBDP 120 kD at 24 h (bottom). Sample number, R2, and P values are shown in the graphs. 24S-HC, 24S-hydroxycholesterol; HI, hypoxia–ischemia; OD, optical density; SBDP, spectrin breakdown product.
Correlation of the serum levels of 24S-HC with the expression of cleaved caspase-3 at 6 and 24 h after neonatal HI. (a) Protein expression of cleaved caspase-3 in the cortices was measured by western blotting at the indicated time points and presented as the OD ratio to β-actin and normalized to an IC. Sham vs. HI, *P=0.0112 at 6 h, *P=0.0027 at 24 h, *P=0.0228 at 72 h (n=5–6 for sham animals, n=7–10 for HI animals from 6 to 72 h). (b) Correlation of the serum levels of 24S-HC with the expression of cleaved caspase-3 at 6 h (top) and at 24 h (bottom). Sample number, R2, and P values are shown in the graphs. 24S-HC, 24S-hydroxycholesterol; HI, hypoxia–ischemia; IC, internal control; OD, optical density.
Discussion
We demonstrated an enhanced brain cholesterol turnover following neonatal HI as evidenced by the upregulation of CYP46A1 in the ipsilateral cortex, leading to an increased production of 24S-HC in the injured hemisphere and simultaneous excretion into the bloodstream. The correction between the levels of serum 24S-HC and brain necrosis/apoptosis at early post-HI stage supports a possible value of plasma level of 24S-HC as a novel and early biomarker for severity of neonatal HI brain damage.
Present mainly in the brain, CYP46A1 catalyzes the hydroxylation of cholesterol into 24S-HC to eliminate surplus cholesterol from the brain, and thereby has an important role in maintenance of cholesterol homeostasis (19, 20). CYP46A1 protein expression is low before 1 week after birth in mice and gradually increases to reach a steady level around 3 weeks (6, 8). In humans, it reaches the adult level at around 3 years of age (8). This allows deposition of nearly all cholesterol at an early age to assist in the rapid expansion of the brain and myelin, and in the removal of redundant cholesterol as the animal matures. We showed an upregulation of CYP46A1 following neonatal HI at P9 sustaining at least 24 or 48 h. It is unknown how this enzyme is activated by HI, whether this response is simply adaptative, or is it involved in processes of brain injury or protection. CYP46A1 promoter activity appears to be resistant to changes of molecules implicated in regulation of cholesterol homeostasis including cholesterol and 24S-HC (6), but can be induced by the Sp-family of transcriptional factors (21) and epigenetic modifications (22). Recent studies have suggested two mechanisms for CYP46A1 activation: oxidative stress (6) and glutamate (15), both of which are associated with cell death in brain ischemia, and with overactivation of the NMDA receptors, the major ionotropic glutamate receptors mediating excitotoxicity in HI brain injury. CYP46A1 enzymatic product 24S-HC was found to be a potent allosteric modulator to enhance the function of NMDA receptors (23, 24). These findings suggest a reciprocal activation of the NMDA receptor pathway and the cholesterol metabolism pathway, which may form a viscous cycle to exacerbate brain damage following neonatal HI.
Cholesterol loss is expected to be the direct consequence of CYP46A1 activation. This might be partially responsible for the reduction in cholesterol in the ipsilateral cortex at 6 h after HI. The changes of the total cholesterol amount found here reflected the balance between de novo synthesis and breakdown by CYP46A1. We did not measure cholesterol that was newly synthesized after neonatal HI; however, elevated generation of 24S-HC could downregulate cholesterol synthesis via blocking the proteolytic activation of transcription factor sterol regulatory element-binding protein 2 (SREBP2), which controls the pathway of cholesterol biosynthesis (25). In connection with excitotoxicity, a Ca2+-dependent cholesterol loss was found in cultured hippocampal neurons exposed to NMDA, which was paralleled by translocation of CYP46A1 from the endoplasmic reticulum, where it normally resides, to the plasma membrane and release of 24S-HC into the medium (26). Apparently, we cannot draw the conclusion that cholesterol loss at 6 h was solely attributable to the action of CYP46A1. The level of HMGCR was unchanged at 6 h, but increased at 24 h after HI, which might prevent further reduction of cholesterol in the injured hemisphere. The other two studies of neonatal HI in P7 Sprague Dawley (SD) rats also reported the diminished cholesterol contents, which lasted longer for 3 days or 3 months following the initial insult (13, 14). Disturbance of cholesterol homeostasis after neonatal HI may have adverse consequences on cell survival and brain development. For example, accelerated cholesterol hydroxylation found in this study produced a high level of 24S-HC, which has been shown to be cytotoxic to neurons by inducing apoptosis, necroptosis (a form of programmed necrosis) (27, 28), inflammatory responses (29), and cognitive impairment (30). The colocalization of increased CYP46A1 with cleaved caspase-3 in the ipsilateral cortex indicates that CYP46A1 may be associated with cell death following HI. Further studies are required to elucidate the functional roles of CYP46A1 and 24S-HC in neonatal brain HI, especially in long-term neurological deficits.
Although primarily confined to neurons in the healthy brain, CYP46A1 has been found in other cell types in different disease conditions, for example, in activated microglia and reactive astrocytes in brain trauma (17, 18), in astrocytes of Alzheimer’s disease patients (31), or in macrophages/microglia in an animal model of multiple sclerosis (32). They were proposed to clear extracellular cholesterol released from the damaged cell membranes, which has not been proved experimentally. We show that CYP46A1 is localized in neurons and oligodendrocytes, but not in astrocytes and microglia. White matter is a common lesion site in rodent neonatal HI (33), and in human infants, especially preterm babies (34), who suffered from HIE. As oligodendrocytes are sensitive to oxidative stress and excitotoxicity in the developing brain (35), if CYP46A1 is upregulated in oligodendrocytes after HI, it may break down cholesterol leading to myelin degradation or limiting its availability for myelin synthesis and white matter development as cholesterol in oligodendrocytes is a rate-limiting factor for brain maturation (2). Targeted inhibition or deletion of CYP46A1 in oligodendrocyte may reveal its precise function in the normal brain and under disease conditions. Different cell populations play distinct roles in regulating cholesterol homeostasis. For example, neurons can utilize external cholesterol transported from glial cells (36, 37). Similarly, brain cells may coordinate for cholesterol clearance because not all brain cells, even not all neurons, express CYP46A1 (8, 20).
Studies with CYP46A1 knockout mice have suggested that this enzyme is responsible for the production of ≈98–99% of brain 24S-HC and ≈60–80% 24S-HC in the serum (9, 38). Our data support the cerebral origin of blood 24S-HC owing to the concomitant increase and correlation of 24S-HC in the serum and in the brain. Although it is unknown whether an acute elevation of serum 24S-HC can predict histological and functional outcomes at later time points, based on its brain specificity, and a strong correlation with necrotic and apoptotic cell death early after HI, this pilot work suggests that blood 24S-HC might be a biologically plausible marker for HIE to assist clinical diagnosis. SBDP can be detected in the serum of human neonates and the amounts are associated with the severity of HIE clinical syndromes with good sensitivity and specificity (39). However, serum SBDP can be derived from extracerebral organs when damaged. Radiological or functional measures are required to explore the feasibility of using blood 24S-HC as a diagnostic tool in HIE patients. Targeted lipid profiling discovered plasma sphingomyelins as potential markers for acute stroke patients (40). Lipids could be better tools for diagnosis than protein markers because of their abundance in the brain, small size for readily crossing the blood–brain barrier, and higher sensitivity to oxidative stress for a possible earlier detection.
Taken together, this is the first study investigating a brain-specific cholesterol turnover pathway following neonatal HI. Activation of CYP46A1 may have more specialized roles than just removing cholesterol from the central nervous system. Future work is required to gain better insight into cholesterol homeostasis in the developing brain, which is subjected to a more sophisticated regulation than that in the adult brain where cholesterol is largely metabolically inert.
References
Mauch DH, Nagler K, Schumacher S et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001;294:1354–7.
Saher G, Brugger B, Lappe-Siefke C et al. High cholesterol level is essential for myelin membrane growth. Nat Neurosci 2005;8:468–75.
Dietschy JM . Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem 2009;390:287–93.
Dietschy JM, Turley SD . Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 2004;45:1375–97.
Quan G, Xie C, Dietschy JM, Turley SD . Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res Dev Brain Res 2003;146:87–98.
Ohyama Y, Meaney S, Heverin M et al. Studies on the transcriptional regulation of cholesterol 24-hydroxylase (CYP46A1): marked insensitivity toward different regulatory axes. J Biol Chem 2006;281:3810–20.
Jurevics H, Morell P . Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem 1995;64:895–901.
Lund EG, Guileyardo JM, Russell DW . cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci U S A 1999;96:7238–43.
Bjorkhem I, Lutjohann D, Diczfalusy U, Stahle L, Ahlborg G, Wahren J . Cholesterol homeostasis in human brain: turnover of 24 S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res 1998;39:1594–600.
Lutjohann D, von Bergmann K . 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry 2003;36 (Suppl 2): S102–6.
Leoni V, Caccia C . 24S-hydroxycholesterol in plasma: a marker of cholesterol turnover in neurodegenerative diseases. Biochimie 2013;95:595–612.
DeBose-Boyd RA . Feedback regulation of cholesterol synthesis: sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res 2008;18:609–21.
Ramirez MR, Muraro F, Zylbersztejn DS et al. Neonatal hypoxia-ischemia reduces ganglioside, phospholipid and cholesterol contents in the rat hippocampus. Neurosci Res 2003;46:339–47.
Yu Z, Li S, Lv SH et al. Hypoxia-ischemia brain damage disrupts brain cholesterol homeostasis in neonatal rats. Neuropediatrics 2009;40:179–85.
Mast N, Anderson KW, Johnson KM, Phan TTN, Guengerich FP, Pikuleva IA . In vitro cytochrome P450 46A1 (CYP46A1) activation by neuroactive compounds. J Biol Chem 2017;292:12934–46.
Millar LJ, Shi L, Hoerder-Suabedissen A, Molnar Z . Neonatal hypoxia ischaemia: mechanisms, models, and therapeutic challenges. Front Cell Neurosci 2017;11:78.
Cartagena CM, Ahmed F, Burns MP et al. Cortical injury increases cholesterol 24 S hydroxylase (Cyp46) levels in the rat brain. J Neurotrauma 2008;25:1087–98.
Smiljanic K, Lavrnja I, Mladenovic Djordjevic A et al. Brain injury induces cholesterol 24-hydroxylase (Cyp46) expression in glial cells in a time-dependent manner. Histochem Cell Biol 2010;134:159–69.
Moutinho M, Nunes MJ, Rodrigues E . Cholesterol 24-hydroxylase: brain cholesterol metabolism and beyond. Biochim Biophys Acta 2016;1861:1911–20.
Russell DW, Halford RW, Ramirez DM, Shah R, Kotti T . Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem 2009;78:1017–40.
Milagre I, Nunes MJ, Gama MJ et al. Transcriptional regulation of the human CYP46A1 brain-specific expression by Sp transcription factors. J Neurochem 2008;106:835–49.
Shafaati M, O'Driscoll R, Bjorkhem I, Meaney S . Transcriptional regulation of cholesterol 24-hydroxylase by histone deacetylase inhibitors. Biochem Biophys Res Commun 2009;378:689–94.
Linsenbardt AJ, Taylor A, Emnett CM et al. Different oxysterols have opposing actions at N-methyl-D-aspartate receptors. Neuropharmacology 2014;85:232–42.
Paul SM, Doherty JJ, Robichaud AJ et al. The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors. J Neurosci 2013;33:17290–300.
Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL . Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA 2007;104:6511–8.
Sodero AO, Vriens J, Ghosh D et al. Cholesterol loss during glutamate-mediated excitotoxicity. EMBO J 2012;31:1764–73.
Noguchi N, Urano Y, Takabe W, Saito Y . New aspects of 24(S)-hydroxycholesterol in modulating neuronal cell death. Free Radic Biol Med 2015;87:366–72.
Yamanaka K, Saito Y, Yamamori T, Urano Y, Noguchi N . 24(S)-hydroxycholesterol induces neuronal cell death through necroptosis, a form of programmed necrosis. J Biol Chem 2011;286:24666–73.
Alexandrov P, Cui JG, Zhao Y, Lukiw WJ . 24S-hydroxycholesterol induces inflammatory gene expression in primary human neural cells. Neuroreport 2005;16:909–13.
Zhao S, Liao W, Xu N et al. Polar metabolite of cholesterol induces rat cognitive dysfunctions. Neuroscience 2009;164:398–403.
Brown J 3rd, Theisler C, Silberman S et al. Differential expression of cholesterol hydroxylases in Alzheimer's disease. J Biol Chem 2004;279:34674–81.
Lavrnja I, Smiljanic K, Savic D et al. Expression profiles of cholesterol metabolism-related genes are altered during development of experimental autoimmune encephalomyelitis in the rat spinal cord. Sci Rep 2017;7:2702.
Liu Y, Silverstein FS, Skoff R, Barks JD . Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr Res 2002;51:25–33.
Back SA . White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 2017;134:331–49.
Silbereis JC, Huang EJ, Back SA, Rowitch DH . Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Dis Model Mech 2010;3:678–88.
Funfschilling U, Jockusch WJ, Sivakumar N et al. Critical time window of neuronal cholesterol synthesis during neurite outgrowth. J Neurosci 2012;32:7632–45.
Pfrieger FW, Ungerer N . Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res 2011;50:357–71.
Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW . Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem 2003;278:22980–8.
Wu H, Li Z, Yang X, Liu J, Wang W, G Liu . SBDPs and Tau proteins for diagnosis and hypothermia therapy in neonatal hypoxic ischemic encephalopathy. Exp Ther Med 2017;13:225–9.
Sheth SA, Iavarone AT, Liebeskind DS, Won SJ, Swanson RA . Targeted lipid profiling discovers plasma biomarkers of acute brain injury. PLoS ONE 2015;10:e0129735.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Statement of Financial Support
This work was supported by the National Institute of Neurological Disorders and Stroke (RO1NS084057 to Dr. Jiang).
Rights and permissions
About this article
Cite this article
Lu, F., Zhu, J., Guo, S. et al. Upregulation of cholesterol 24-hydroxylase following hypoxia–ischemia in neonatal mouse brain. Pediatr Res 83, 1218–1227 (2018). https://doi.org/10.1038/pr.2018.49
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2018.49
This article is cited by
-
Cholesterol metabolism and brain injury in neonatal encephalopathy
Pediatric Research (2021)
-
CYP46A1 Activation by Efavirenz Leads to Behavioral Improvement without Significant Changes in Amyloid Plaque Load in the Brain of 5XFAD Mice
Neurotherapeutics (2019)