Abstract
Background
Birth weight has an impact on adult bone mass. Higher birth weight is associated with greater bone mineral content (BMC) and children born small for gestational age (SGA) are at an increased risk for impaired accrual of bone mass. Our aim was to study whether the impact of birth size or early childhood growth on bone mass is visible already in mid-childhood.
Methods
We studied 49 children born large for gestational age (LGA), 56 children born appropriate for gestational age (AGA), and 23 children born SGA at 5.0–8.7 years of age. Body composition was assessed by whole-body dual-energy X-ray absorptiometry. Fasting blood samples and anthropometric data were collected.
Results
The children born SGA had lower bone mineral density (BMD) Z-score (P<0.001) and age- and sex-adjusted BMD (P<0.005) than the LGA and AGA children. Adjusted BMC, muscle mass, and body fat percentage (%BF) did not differ between the study groups. Muscle mass, BMI SD score (SDS), %BF, and serum dehydroepiandrosterone sulfate (DHEAS) concentration were the strongest predictors of high BMD in mid-childhood.
Conclusion
SGA-born children had lower BMD in mid-childhood compared with AGA- and LGA-born ones. Muscle mass or BMI SDS, %BF, and DHEAS were significant predictors of childhood BMD.
Similar content being viewed by others
Main
Birth weight affects bone mass in adulthood, as higher birth weight is associated with greater bone mineral content (BMC) (1). Intrauterine growth restriction and programming are acknowledged to have an influence on cardiometabolic health in childhood and adulthood (2, 3), and children born small for gestational age (SGA), especially preterm, are at an increased risk for impaired accrual of adult bone mass (1, 4, 5). Children born SGA without catch-up growth have lower total body bone mineral density (BMD) in early adulthood compared with those with catch-up growth (6).
Almost maximal bone mass is achieved during the first two decades of life (7). Nutrition, especially calcium and vitamin D, and mechanical load affect the final accrual (8). The association between BMI and volumetric BMD in children may mostly be determined by lean mass (LM) (9), not body fat mass that has been reported to have a negative association with bone mass in childhood (10, 11).
Vitamin D is essential for bone metabolism (12). Although serum 25-hydroxyvitamin D (25(OH)D) concentrations are recommended to exceed 50 nmol/l in children (13), there is evidence that low 25(OH)D status is common in children (14). The association between birth size and 25(OH)D concentrations has not been widely studied. No differences in serum 25(OH)D concentrations were detected between newborn infants born SGA, appropriate (AGA), and large for gestational age (LGA) (15); however, it is unclear whether the status is similar also in older children. In adults, higher BMI leads to lower 25(OH)D (16) and the association of overweight with low 25(OH)D is seen also in children and adolescents (17).
The purpose of our study was to investigate whether the impact of birth size or early childhood growth on bone mass is visible already in prepubertal children.
Methods
The study cohort included 128 Caucasian children (67 boys) born singleton at term between 2004 and 2007 in Eastern Finland (18, 19). In brief, the children were enrolled according to their birth size and studied at 5.0–8.7 years of age (mean (95% confidence interval (CI), 6.9 (6.8–7.1) years; Table 1). SGA was defined as gender-specific birth weight ≤−2.0 SD score (SDS), LGA as birth weight ≥+2.0 SDS, and AGA as birth weight and length being between −1.0 and +1.0 SDS. Anthropometric data at birth, at the age of 2 years, and at examination were recorded.
BMI was calculated as the body weight divided by the square of the height (kg/m2). Sex- and age-specific SDS for weight, height, and BMI were calculated according to the recently published Finnish growth reference (20). Catch-up or catch-down growth was determined as an increase or decrease in weight or height SDS more than 0.67 during the first 2 years of life, respectively (21).
Areal BMD and BMC, body fat percentage (%BF), and muscle mass were assessed by whole-body dual-energy X-ray absorptiometry, using the Lunar device (Lunar Prodigy Advance; GE-Medical Systems, Madison, WI). BMD Z-scores were calculated using the recently published data by Crabtree et al. (22). LM was defined as a sum of muscle mass and BMC. Total body less head parameters were used for the analyses.
Fasting blood samples were collected for serum analyses of 25(OH)D, ionized calcium, dehydroepiandrosterone sulfate (DHEAS), IGF-1 concentrations, and plasma analysis of alkaline phosphatase (ALP) concentrations. DHEAS Z-scores were created using recently published reference data on Caucasian children (23). Serum 25(OH)D concentrations were assessed using chemiluminescence immunoassay (DiaSorin, Stillwater, MN). Serum-ionized calcium and plasma ALP concentrations were determined with routine automated methods [ion-selective electrode and photometric (The International Federation of Clinical Chemistry and Laboratory Medicine recommendation), respectively] in the laboratory of Kuopio University Hospital. Serum DHEAS concentrations were analyzed using electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). Serum IGF-1 concentrations were determined using an ELISA kit (Mediagnost, Reutlingen, Germany).
A written informed consent was obtained from all parents and participating children aged ≥6 years. The study protocol was approved by the Committee on Research Ethics of the Hospital District of Northern Savo.
Statistical Analyses
Data are presented as mean (95% CI). Analyses were conducted using SPSS statistical software (version 24; SPSS, IBM, Armonk, NY). A significance level of 0.05 was used in all analyses. Skewed data were either logarithm or square root-transformed before parametric analyses, and power-transformed to geometric means for presentation. ANOVA was used for comparisons between the study groups on anthropometric measures and BMD Z-scores. ANCOVA was used for comparisons between the study groups on metabolic and imaging parameters. Following factors were used for adjusting ANCOVA (vary between analyses): month of the blood sampling, age, sex, BMI SDS, weight, and height. Obtained estimated means are presented in Table 1. Linear regression was used to analyze factors predicting BMD. The regression coefficients are expressed as standardized betas. Each model contained sex, age, 25(OH)D, ionized calcium, ALP, DHEAS, and IGF-1, and additional predictors were as follows: Model 1: birth weight SDS, muscle mass, %BF; Model 2: birth length SDS, muscle mass, %BF; Model 3: the change in weight SDS from birth to the age of 2 years, muscle mass, %BF; Model 4: the change in height SDS from birth to the age of 2 years, muscle mass, %BF; Model 5: birth size group, muscle mass, %BF; Model 6: birth weight SDS, BMI SDS.
Results
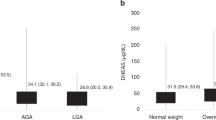
Subject characteristics for the three birth weight groups are shown in Table 1. The SGA children had significantly lower age- and sex-adjusted BMD and BMD Z-scores compared with the LGA and AGA groups (Table 1). The boys in the whole study population had lower BMD than the girls [adjusted for age and height, P=0.028, mean (95% CI) 0.69 (0.68–0.70) and 0.70 (0.69–0.71) g/cm2, respectively]. The boys had higher muscle mass [P=0.006, mean (95% CI) 16.8 (16.5–17.1) kg] and LM [P=0.011, mean (95% CI) 17.4 (17.0–17.7) kg] than the girls [16.1 (15.8–16.5) and 16.7 (16.4–17.1) kg, respectively]. The mean %BF of the boys was lower [P<0.001, mean (95% CI) 17.7 (16.0–19.5)] than that of the girls [23.8 (21.5–26.5)] when adjusted for age and height. There were no statistical differences between the birth weight groups in BMC, muscle mass, LM, or %BF (Table 1). A significant difference was seen in plasma ALP concentrations between the groups (Table 1), but not between the sexes. The girls had significantly higher serum-ionized calcium concentrations than the boys [P=0.001, mean (95% CI) 1.27 (1.26–1.28) and 1.25 (1.25–1.26) mmol/l, respectively]. A trend toward lower 25(OH)D concentrations (adjusted for the month of the blood-sampling time, age, and BMI SDS) was seen in the LGA children compared with the AGA ones [Table 1, the post hoc test (Sidak correction) P=0.086].
Muscle mass or BMI SDS was the strongest weight-related predictor of higher BMD in the whole study population. In addition, %BF was a significant predictor in the whole study population (Table 2). In the boys, being born SGA predicted lower BMD in mid-childhood (beta −0.27, P<0.001). Higher DHEAS concentrations predicted higher BMD in all models in the whole study population (Table 2 and Figure 1) and among the boys (beta 0.22–0.26, P=0.001–0.006), but the association between BMD and DHEAS in girls remained weaker (beta 0.05–0.19, P=0.053–0.548). The other biochemical parameters [25(OH)D, ionized calcium, ALP, IGF-1] did not significantly associate with BMD (Table 2).
Discussion
In this study, we evaluated the effect of birth size on BMD in prepubertal children. Being born SGA predicted lower BMD in prepubertal boys, but not in girls. Being born LGA had no significant impact on BMD. Current weight, especially muscle mass, was the strongest predictor of BMD in mid-childhood, but also birth weight SDS and serum DHEAS concentrations were positively associated with BMD.
There are not many studies on birth size and bone mass in childhood. In a recent cohort study, 6-year-old SGA-born children had lower and LGA-born children had higher BMC, but not BMD compared with AGA-born ones (24). Biosca et al. showed a significant difference in age-, sex-, and weight-adjusted total body BMC between SGA (lowest mean), AGA, and LGA (highest mean) children at the age of 8 years, but when adjusted additionally for height the difference turned nonsignificant. No differences between the birth size groups were found in total body BMD (25). In a previous study on children aged 3–12 years, lumbar spine and femoral neck BMD were lower in SGA than in AGA children (26). In our study, the SGA children had the lowest BMD among the study groups, and lower birth weight SDS in the whole study population and being born SGA in the boys predicted lower BMD in mid-childhood. A positive association between birth weight and adult BMC, but not BMD, at the lumbar spine and hip has been reported previously (1). Children born SGA, especially preterm, are at an increased risk for impaired accrual of adult bone mass (1, 4, 5).
Not only birth size, but also early growth has an impact on future bone mass. Catch-up growth especially during the first 2 years of life reduced the adverse effect of small birth size on childhood bone mass (24). SGA-born children had lower lumbar spine BMD Z-scores than AGA-born children when there was no catch-up growth (26). Children born SGA with no catch-up growth had lower total body BMD in early adulthood compared with children born SGA with catch-up growth (6). Weight at the age of 1 year had a positive association with adult bone mass (1), and catch-up growth in weight but not in height predicted higher BMD in adulthood (6). Our data did not show any significant impact of early catch-up or catch-down growth in weight or height on BMD in mid-childhood.
The association between BMI and volumetric BMD in children may mostly be mediated by LM. It has been debated whether fat mass is protective against fractures in childhood as it is in adults (27). Body fat mass is reported to have both positive and negative associations with bone mass in childhood (10, 11, 28). Our results did not show any differences in muscle mass, LM, or %BF between the birth size groups when adjusted for age, sex, and height. In linear regression analysis both high muscle mass and %BF predicted higher BMD in the whole study population.
Vitamin D is vital for bone metabolism by regulating calcium homeostasis, but it has also a positive effect on cardiometabolism (29), as serum 25(OH)D deficiency associates with cardiovascular disease (30), diabetes (31), and hypertension (32). Interestingly, vitamin D did not have a significant impact on BMD in our study cohort. However, the LGA-born children did have a trend toward lower 25(OH)D concentrations than the AGA-born children. Other studies have showed an association between low vitamin D concentrations and low bone density in children and adolescents (33). In this study vitamin D concentrations were adequate in all groups, which may explain our result. Serum 25(OH)D concentrations are recommended to exceed 50 nmol/l in children for health benefits (13).
DHEAS is an adrenal androgen precursor and its concentrations increase individually through childhood (34). Children with premature adrenarche had higher areal BMD than their controls, but the differences were nonsignificant after adjusting for height SDS (35). We demonstrated recently that LGA children had lowest and SGA children highest DHEAS concentrations in mid-childhood (18). Linear regression analysis in the current study showed that DHEAS concentrations had a positive association with BMD in the whole study population and separately in boys but not in girls. DHEAS is metabolized to a more potent androgen testosterone and to estrone (36). There is evidence that both androgens and estrogens have a positive effect on bone mass accrual (36, 37). Our recent study showed a positive association between DHEAS and IGF-1 concentrations, but no differences in IGF-1 concentration were found between the birth size groups (18). IGF-1 has a significant impact on bone mineral accrual, and it has been positively associated with BMC in prepubertal children (38, 39). In this study IGF-1 did not have any impact on BMD. This is in agreement with a previous report showing that IGF-I is a determinant of cortical bone mass but not cortical bone density (38).
We acknowledge several limitations in this study. First, the sample size was relatively small, thus affecting the power of the analyses. Second, the AGA children were heavier than the Finnish children on average at examination. This might have an impact on the results, and that is why our analyses were adjusted also for body size. Third, the BMD was not measured as volumetric but areal. The International Society for Clinical Densitometry recommends total body less head dual-energy X-ray absorptiometry measurement as one of the methods for performing BMC and areal BMD measurements in pediatric subjects (40). The strengths of our study include the detailed data from birth, early childhood growth, and examination. The data at examination included anthropometric, biochemical, and imaging data. In addition, the study participants were enrolled strictly according to the birth size, and the AGA group was selected to represent the children close to the mean birth weight and length.
In conclusion, children born SGA had lower BMD in mid-childhood compared with children born AGA and LGA. Muscle mass or BMI SDS was the strongest weight-related predictor of childhood BMD, whereas %BF and serum DHEAS as a marker of adrenal androgen secretion were also associated positively with BMD.
References
Baird J, Kurshid MA, Kim M, Harvey N, Dennison E, Cooper C . Does birthweight predict bone mass in adulthood? A systematic review and meta-analysis. Osteoporos Int 2011;22:1323–34.
Saenger P, Czernichow P, Hughes I, Reiter EO . Small for gestational age: short stature and beyond. Endocr Rev 2007;28:219–51.
Risnes KR, Vatten LJ, Baker JL et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 2011;40:647–61.
Buttazzoni C, Rosengren B, Tveit M, Landin L, Nilsson JA, Karlsson M . Preterm children born small for gestational age are at risk for low adult bone mass. Calcif Tissue Int 2016;98:105–13.
Callréus M, McGuigan F, Åkesson K . Birth weight is more important for peak bone mineral content than for bone density: the PEAK-25 study of 1,061 young adult women. Osteoporos Int 2013;24:1347–55.
Leunissen RW, Stijnen T, Boot AM, Hokken-Koelega AC . Influence of birth size and body composition on bone mineral density in early adulthood: the PROGRAM study. Clin Endocrinol 2008;69:386–92.
Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R . Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 1991;73:555–63.
Heaney RP, Abrams S, Dawson-Hughes B et al. Peak bone mass. Osteoporos Int 2000;11:985–1009.
Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB . Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone 2010;46:977–84.
Wey HE, Binkley TL, Beare TM, Wey CL, Specker BL . Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab 2011;96:106–14.
Rocher E, Chappard C, Jaffre C, Benhamou CL, Courteix D . Bone mineral density in prepubertal obese and control children: relation to body weight, lean mass, and fat mass. J Bone Miner Metab 2008;26:73–8.
Koo W, Walyat N . Vitamin D and skeletal growth and development. Curr Osteoporos Rep 2013;11:188–93.
Munns C, Zacharin MR, Rodda CP et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 2006;185:268–72.
Tolppanen A, Fraser A, Fraser WD, Lawlor DA . Risk factors for variation in 25-hydroxyvitamin D3 and D2 concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab 2012;97:1202–10.
Akcakus M, Koklu E, Budak N, Kula M, Kurtoglu S, Koklu S . The relationship between birthweight, 25-hydroxyvitamin D concentrations and bone mineral status in neonates. Ann Trop Paediatr 2006;26:267–75.
Vimaleswaran KS, Berry DJ, Lu C et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med 2013;10:e1001383.
Turer CB, Lin H, Flores G . Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 2013;131:e152–61.
Nordman H, Voutilainen R, Antikainen L, Jääskeläinen J . Prepubertal children born large for gestational age have lower serum DHEAS concentrations than those with a lower birth weight. Pediatr Res 2017;82:285–9.
Nordman H, Voutilainen R, Laitinen T et al. Growth and cardiovascular risk factors in prepubertal children born large or small for gestational age. Horm Res Paediatr 2016;85:11–7.
Saari A, Sankilampi U, Hannila ML, Kiviniemi V, Kesseli K, Dunkel L . New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann Med 2011;43:235–48.
Monteiro PO, Victora CG . Rapid growth in infancy and childhood and obesity in later life - a systematic review. Obes Rev 2005;6:143–54.
Crabtree NJ, Shaw NJ, Bishop NJ et al. Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults - the ALPHABET study. J Bone Miner Res 2017;32:172–80.
Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A . Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin Endocrinol 2015;82:712–8.
Heppe DH, Medina-Gomez C, de Jongste JC et al. Fetal and childhood growth patterns associated with bone mass in school-age children: the Generation R Study. J Bone Miner Res 2014;29:2584–93.
Biosca M, Rodríguez G, Ventura P et al. Central adiposity in children born small and large for gestational age. Nutr Hosp 2011;26:971–6.
Silvano L, Miras M, Pérez A et al. Comparative analysis of clinical, biochemical and genetic aspects associated with bone mineral density in small for gestational age children. J Pediatr Endocrinol Metab 2011;24:511–7.
Dimitri P, Bishop N, Walsh JS, Eastell R . Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone 2012;50:457–66.
Heidemann M, Holst R, Schou AJ et al. The influence of anthropometry and body composition on children's bone health: the childhood health, activity and motor performance school (the CHAMPS) study, Denmark. Calcif Tissue Int 2015;96:97–104.
Golden NH, Abrams SA . Committee on Nutrition. Optimizing bone health in children and adolescents. Pediatrics 2014;134:e1229–43.
Wang L, Song Y, Manson JE et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5:819–29.
Mattila C, Knekt P, Männistö S et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care 2007;30:2569–70.
Forman JP, Giovannucci E, Holmes MD et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 2007;49:1063–9.
Winzenberg T, Jones G . Vitamin D and bone health in childhood and adolescence. Calcif Tissue Int 2013;92:140–50.
Voutilainen R, Jääskeläinen J . Premature adrenarche: etiology, clinical findings, and consequences. J Steroid Biochem Mol Biol 2015;145:226–36.
Utriainen P, Jääskeläinen J, Saarinen A, Vanninen E, Mäkitie O, Voutilainen R . Body composition and bone mineral density in children with premature adrenarche and the association of LRP5 gene polymorphisms with bone mineral density. J Clin Endocrinol Metab 2009;94:4144–51.
Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C . Androgens and bone. Endocr Rev 2004;25:389–425.
Wang Q, Nicholson PH, Suuriniemi M et al. Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab 2004;89:1698–703.
Garnett SP, Högler W, Blades B et al. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr 2004;80:966–72.
Breen ME, Laing EM, Hall DB et al. 25-hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab 2011;96:E89–98.
Gordon CM, Leonard MB, Zemel BS . International Society for Clinical Densitometry. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 2014;17:219–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Statement of Financial Support:
This study was supported by Pediatric Research Foundation, Kuopio University Hospital State Research Funding, and Finnish Cultural Foundation, Päijät-Häme Regional fund. The funders had no role in (i) study design; (ii) the collection, analysis, and interpretation of data; (iii) the writing of the report; and (iv) the decision to submit the paper for publication.
Rights and permissions
About this article
Cite this article
Nordman, H., Voutilainen, R., Laitinen, T. et al. Birth size, body composition, and adrenal androgens as determinants of bone mineral density in mid-childhood. Pediatr Res 83, 993–998 (2018). https://doi.org/10.1038/pr.2018.12
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2018.12
This article is cited by
-
Association between dehydroepiandrosterone sulphate levels at 7 years old and bone mineral density at 10 years old: a prospective cohort study
European Journal of Pediatrics (2022)