Abstract
Background
Traumatic brain injury (TBI) is the leading cause of injury-related death in children, with boys and children under 4 years of age having particularly poor outcomes. Cerebral autoregulation is often impaired after TBI, contributing to poor outcome. In prior studies on newborn pigs, phenylephrine (Phe) preferentially protected cerebral autoregulation in female but not in male subjects after TBI. We hypothesized that, in contrast to the newborn, Phe prevents impairment of autoregulation and tissue injury following TBI in both sexes of older pigs.
Methods
Cerebral autoregulation, cerebrospinal fluid (CSF) extracellular signal-related kinase (ERK) and endothelin, and histopathology were determined after moderate fluid percussion brain injury (FPI) in male and female juvenile pigs after Phe.
Results
Autoregulation was more impaired in male than in female subjects. Phe protects autoregulation in both sexes after FPI, blocks ERK and endothelin, and decreases the number of necrotic neurons in male and female subjects after FPI.
Conclusions
These data indicate that Phe protects autoregulation and limits neuronal necrosis via blockage of ERK and endothelin after FPI in male and female subjects. Together with prior observations in newborn pigs where Phe protected autoregulation in female but not in male subjects, these data suggest that use of Phe to improve outcomes after TBI is both sex- and age-dependent.
Similar content being viewed by others
Main
Traumatic brain injury (TBI) is the leading cause of injury-related death in children and young adults (1), with boys and children under 4 years having particularly poor outcomes (2). Cerebral perfusion pressure (CPP) is defined as mean arterial pressure (MAP) minus intracranial pressure. CPP is low after TBI, causing cerebral ischemia and impaired cerebral autoregulation (1, 2, 3). The 2012 Pediatric Guidelines direct clinicians to maintain CPP above 40 mm Hg in children after TBI (4). However, strategies that use employment of vasoactive agents to increase MAP and thereby augment CPP following TBI, such as norepinephrine (NE), phenylephrine (Phe), epinephrine, and dopamine (DA; (refs 5, 6, 7)), have not been rigorously conducted so as to compare their relative effects on protection of cerebral autoregulation and improvement of ultimate outcome post insult. The cerebral effects of these commonly used drugs in the clinical care of TBI patients are not known.
By definition, cerebral autoregulation is a means by which to maintain constant CBF over a range of blood pressures. Cerebral autoregulation has been studied earlier by us in a newborn pig model of TBI, fluid percussion injury (FPI) (8). Pigs have a gyrencephalic brain containing a white/gray ratio more similar to that of humans. The latter is important because white matter is more vulnerable to injury following TBI. Previous studies showed that cerebral autoregulation is more impaired in male and young pigs than in female and older pigs after TBI, which parallels the clinical experience (8, 9, 10, 11, 12). From a mechanistic standpoint, our earlier studies have noted a more augmented increase in the phosphorylated form of the extracellular signal-related kinase (ERK) isoform of mitogen-activated protein kinase (MAPK) in male compared with female subjects following FPI, which contributed to the equally noted greater impairment of cerebral autoregulation in the former compared with the latter (12).
Prior studies have investigated the possibility that choice of vasoactive agent may contribute to differences in outcome in male and female newborn pigs following FPI. For example, Phe protected cerebral autoregulation in newborn female pigs by blockade of ERK phosphorylation and release of spasmogen endothelin-1 (ET-1), but it augmented the release of these mediators in newborn male pigs following FPI (13, 14). However, DA equally prevented impairment of cerebral autoregulation, which was associated with blockade of ERK phosphorylation in both sexes following FPI (14).
In clinical studies, impairment of neurovascular unit-mediated autoregulation following TBI appears linked to the Glascow Coma Scale (GCS), with greater autoregulatory impairment associated with worse GCS (3). Because we had noted earlier that autoregulation was more impaired in newborn compared with juvenile pigs (8), we were interested in determining whether the sex-based protection of cerebral hemodynamics after FPI+Phe treatment was the same or different in the older juvenile pigs, and how that related to brain tissue injury post injury. In the present study we therefore investigated whether Phe protects autoregulation and limits histopathology after FPI in male and female juvenile pigs, and the role of ERK and ET-1 in that outcome.
Results
Phe Protects Autoregulation in Female and Male Juvenile Pigs after FPI
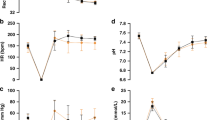
The level of injury was equal in male and female juvenile pigs (1.9±0.1 vs. 2.0±0.1 atm). We chose CPP based on the 2012 Pediatric Guidelines to determine the dose of the intravenous (i.v.) infusion (in μg/kg/min) of Phe. The infusion of Phe began when CPP dropped below 45 mm Hg. CPP values for sham, FPI, and FPI+Phe were, respectively, 70±7, 45±4, and 66±3 mm Hg in male subjects and 71±7, 45±5, and 70±2 mm Hg in female subjects. Intracranial pressure increased after TBI; however, such elevations were blunted by Phe, resulting in normalized (elevated) CPP.
In sham piglets, the THRR was similar in male and female juvenile pigs (Figure1). During unilateral and bilateral carotid artery compression, THRR decreased following FPI to a modestly greater level in male compared with female juvenile pigs (Figure1). Decreases in THRR values were prevented by Phe following FPI in both male and female pigs (Figure1).
THRR during unilateral and bilateral carotid artery compression in (a) juvenile male and (b) female pigs before (sham), after FPI, and after FPI+Phe treatments, n=5. *P < 0.05 compared to corresponding sham value; + P < 0.05 compared to corresponding FPI alone value; and # P < 0.05 compared to corresponding female value. FPI, fluid percussion brain injury; Phe, phenylephrine; THRR, transient hyperemic response ratio.
Moderate and severe hypotension (24±1% and 45±2% decrease in MAP, respectively) produced reproducible increases in pial artery diameter in sham pigs. Pial artery dilation in response to hypotension was similar in male and female juvenile pigs (Figure2). However, pial artery dilation in response to hypotension was impaired in male and female pigs, but the amount of impairment was significantly larger in male compared with female pigs (Figure2). Phe administered following FPI protected pial artery dilatation in response to hypotension in male and female subjects after FPI (Figure2). Papaverine (10−8 and 10−6M) produced pial artery dilation that was not affected by FPI and Phe (Figure3), indicating that alteration of vascular reactivity after FPI was not an epiphenomenon.
Influence of FPI on pial artery diameter during hypotension (moderate, severe) in (a) juvenile male and (b) juvenile female pigs. Conditions are before (sham control), after FPI, and after FPI+Phe treatments, n=5. *P < 0.05 compared to corresponding sham value, + P < 0.05 compared to corresponding FPI alone value, and # P < 0.05 compared to corresponding female value. FPI, fluid percussion brain injury; Phe, phenylephrine.
Phe Blocked Elevation of CSF ET-1 and ERK MAPK in Juvenile Male and Female Pigs after FPI
CSF ET-1 and phosphorylated ERK MAPK concentrations were increased more in juvenile male pigs than in female pigs following FPI (Figure 4). Phe blocked such elevations in CSF ET-1 and ERK MAPK concentrations in both male and female pigs (Figure4).
Influence of FPI and FPI+Phe on ET-1 and phosphorylated ERK MAPK (pg/ml) before (0 time) and 4 h after FPI in (a,b) juvenile male and (c,d) juvenile female pigs, n=5. *P<0.05 compared to corresponding 0 time value, + P < 0.05 compared to corresponding FPI alone value, # P<0.05 compared to corresponding female value. ERK, extracellular signal-related kinase; FPI, fluid percussion brain injury; MAPK, mitogen-activated protein kinase; Phe, phenylephrine.
Phe Prevented Loss of Neurons in CA1 and CA3 Hippocampi in Juvenile Male and Female pigs after FPI
The quantity of necrotic neurons in CA1 and CA3 hippocampi was elevated following FPI, which was blocked by Phe in both juvenile male and female pigs (Figure5). We observed more necrotic neurons in male pigs than in female pigs following FPI (Figure5).
Histopathology after FPI or FPI +Phe. (a) Low-magnification (× 40) typical juvenile male sham control showing both CA1 (#1) and CA3 (#2) hippocampal regions. (b) Higher-magnification (× 100) typical juvenile male sham control CA3 hippocampus. (c) Typical juvenile male FPI CA3 hippocampus (original magnification × 100). (d) Typical FPI+Phe juvenile male CA3 (× 100). (e) Typical juvenile female FPI CA3 hippocampus (original magnification × 100). (f) Typical FPI+Phe juvenile female CA3 hippocampus (× 100). (g) High-magnification (× 600) typical viable sham control male neuron #3, with intact cytoplasm and darkly stained nucleus. (h) High-magnification (× 600) typical male necrotic neurons, showing #4 pyknotic nucleus of small neuron, accompanied by neuronal cytoplasm shrinkage (#5) and granulated eosinophilic characteristics (“red dead” neuron; #6) associated with cell death. Summary data for the mean number of necrotic neurons (i) in CA1 and CA3 hippocampi of juvenile male and female pigs under conditions of sham control, FPI, and FPI+Phe, n=5. *P < 0.05 compared to corresponding sham control value, + P < 0.05 compared to corresponding FPI alone value, # P < 0.05 compared to corresponding female value. FPI, fluid percussion brain injury; Phe, phenylephrine.
Blood Chemistry and Temperature
There were no statistical differences in blood chemistry values among the groups. For example, values of 7.44±0.05, 35±4, and 91±11, and 7.43±0.06, 39±5, and 97±12 mm Hg for pH, pCO2, and pO2 were obtained in sham controls. Values of 7.45±0.04, 33±6, and 91±12, and 7.44±0.03, 36±6, and 94±13 mm Hg were obtained in FPI-treated animals. These values were obtained at the beginning and at the end of these experiments.
Discussion
Results of the present study show that Phe prevents impairment of cerebral autoregulation and limits necrosis of hippocampal CA1 and CA neurons following FPI in both male and female juvenile pigs. These observations are distinctly different from those determined in the younger population of pigs, wherein Phe prevented impairment of cerebral autoregulation only in newborn female but not in newborn male piglets following FPI (13). Taken together, the present study is the first to demonstrate that there are both sex- and age-related differences in outcome, with the use of Phe to normalize CPP after TBI. Using parameters such as brain water content, suture closure, and others, brain growth curves have been constructed for several species to allow for approximations of human age (9). From the latter approach, newborn and juvenile pigs may approximate the human neonate (6 months to 2 years of age) and child (8–10 years of age), respectively (9). The present data therefore advocate for the use of precision medicine approaches in the treatment of younger and older boys and girls following TBI. Although we noted some modest differences in outcome between juvenile male and female subjects in this study, such data are insignificant in contrast to the newborn male after TBI, where Phe had no ability to limit the impairment of cerebral autoregulation post injury.
Results of the present study also noted a relationship between ET-1, ERK MAPK, and outcome after FPI. For example, impairment of cerebral autoregulation is associated with elevation of the CSF concentration of the spasmogen ET-1 and ERK MAPK. Administration of Phe blocked such upregulation and correspondingly prevented impairment of cerebral autoregulation, suggesting that upregulation of both ET-1 and ERK were causally related to impaired cerebral hemodynamics in juvenile pigs. Prior studies conducted in newborn pigs after FPI using pharmacological antagonists of ET-1 and ERK more rigorously demonstrated this cause–effect mechanistic relationship (14). In particular, these studies showed that ET-1 upregulation in the setting of TBI causes release of superoxide, which, in turn, increases the amount of phosphorylated ERK MAPK (14). During hypotension, pial arteries vasodilate to maintain constant CBF (autoregulation) and such vasodilation is dependent on opening of K channels (particularly Katp and Kca; (ref. 14)). However, the ERK released after TBI impairs K channel function, thereby impairing cerebral autoregulation and producing cerebral ischemia (14). In the present study, by preventing upregulation of ET-1 and ERK MAPK, Phe prevents impairment of cerebral autoregulation presumably by protecting K channel-mediated vasodilation.
A third important observation in the present study relates to the ability of Phe to prevent histopathology in both male and female juvenile pigs. In clinical studies, impairment of autoregulation following TBI is linked to worsening of the GCS (3). Therefore, a provocative conclusion from the present study is that interventions post TBI that are designed to preserve autoregulation might have the value-added benefit of also improving cognitive outcome. However, we caution that cognition depends on more than the hippocampus, and cognitive testing was not performed in the present studies. In addition, histology was assessed only at an early time point (4 h post injury). Therefore, additional studies will be needed to determine whether prevention of loss of neurovascular unit integrity durably improves cerebral hemodynamics and cognitive function after pediatric TBI.
Results of the present study finally extend prior work supportive of the hypothesis that choice of vasoactive agent has important consequence in determining outcome as a function of sex and age after TBI. Thus far, we have described the actions of the following three different vasoactive agents: Phe, DA, and NE. The effects of NE on outcome after TBI have been studied previously in two ages. The contribution of ERK MAPK to impairment of cerebral hemodynamics was investigated in all studies. We used Phe in the first study (13) because it is often chosen in the treatment of TBI in young children because of its longer duration of action and peak elevation of MAP (10). We observed that both Phe and NE selectively protected cerebral autoregulation in the newborn female though blockade of ERK MAPK phosphorylation. Newborn male subjects were not protected, and these pressors actually potentiated phosphorylation of ERK MAPK following FPI (13, 15). New data show that Phe prevented impairment of autoregulation in juvenile male and female pigs because of blockade of ERK MAPK phosphorylation, indicating that for this pressor both age and sex will determine outcome. A similar pattern of age and sex dependency in outcome was observed for NE (15, 16). However, DA protected cerebral autoregulation equivalently in both male and female newborn pigs because of equal blockade of ERK MAPK phosphorylation (17). These data suggest that use of DA might be preferable in the treatment of newborns, and either NE or Phe for older children.
There are several limitations to this study. ERK MAPK was assayed in CSF and was used as an indirect index of what may happen to the cellular concentration within brain parenchyma. We do not feel that this reflects damage or pathology because we have reproducibly detected MAPK in CSF under control conditions and monitored its change with a range of stimuli (13). Changes in CSF concentration therefore reflect intracellular events.
Conclusions
There are no evidence-based guidelines or recommendations regarding the choice of vasoactive agent after adult or pediatric TBI. Choice of vasoactive agent across medical centers is variable, and may be related to outcome. The ongoing multiple medical therapy project (18) will provide 3-, 6-, and 12-month outcome for patients given various pressors for CPP support. However, this project will not be able to answer cerebral autoregulation or mechanistic questions. Therefore, results of this study inform the downstream interpretation of cerebral hemodynamic findings observed in the multiple medical therapy project. In conclusion, Phe preserves cerebral autoregulation and limits histopathology after TBI through blockade of ERK MAPK and ET-1 in an age- and sex-dependent manner.
Methods
Anesthetic Regimen, Fluid Percussion Brain Injury, and Visualization of Pial Arteries
All animal protocols were approved by the University of Pennsylvania Animal Care and Use Committee. Juvenile pigs (4 weeks of age, 6.0–7.0 kg) of either sex were studied. The anesthetic regimen consisted of the following: pre-medication with dexmedetomidine (20 μg/kg intramuscularly), induction with isoflurane (2–3%), isoflurane taper to 0% after start of total intravenous anesthesia with midazolam (1 mg/kg/h), fentanyl (100 μg/kg/h), propofol (2–10 mg/kg/h), dexmedetomidine (2 μg/kg/h), and saline (2 ml/kg/h). Blood pressure was monitored via a catheter placed in the femoral artery. The pigs were intubated and ventilated with room air. Temperature was maintained in the normothermic range (37–39 °C) and monitored rectally.
The closed cranial window technique was used for measurement of pial artery diameter and collection of CSF for enzyme-linked immunosorbent assay (11, 12, 13). Intracranial pressure was determined with an Integra Camino monitor. A laser Doppler probe was used to measure CBF qualitatively. CBF was measured quantitatively in the cerebral cortex and hippocampus using radioactively labeled microspheres (13). The method used to induce moderate (2 atm) brain FPI has been described previously (13).
Protocol
Thirty pigs were randomized to one of the following experimental intervention groups (all n=5): (i) sham control (craniotomy but no injury), (ii) FPI, and (iii) FPI post-treated with Phe. CPP was targeted (65–70 mm Hg as per the 2012 Pediatric Guidelines) to determine the dose of the i.v. infusion (typically 0.8–1.3 μg/kg/min i.v.) of Phe, and the Phe treatment was started when CPP decreased below 45 mm Hg. Animals in which pial artery reactivity and histopathology were determined were the same, allowing for within-animal comparison of outcome. These animals were already being given an infusion of saline (to accommodate for loss during ventilation), and in prior studies an infusion of saline elevated above the latter did not make a significant difference in support of CPP over the prolonged time period of the protocol (4 h post FPI; (refs 15, 16)).
Cerebral autoregulation was tested via two techniques. The first quantified the THRR (16, 19). In the second, hypotension was used as the stimulus and was produced by the rapid withdrawal of either 5–8 or 10–15 ml blood/kg, yielding moderate or severe hypotension (decreases in MAP of 25% and 45%, respectively). The decrements in blood pressure were maintained constant for 10 min by either an additional blood withdrawal or a blood reinfusion. The vehicle for all agents was 0.9% saline. In sham control animals, responses to THRR, hypotension (moderate and severe), and papaverine (10−8 and 10−6 M) were obtained twice: once and then again 1 h later. In drug-post-treated animals, drugs were administered after FPI, and responses to THRR, hypotension, and papaverine as well as CSF samples were collected at 1 h post insult. The order of agonist administration was randomized within the animal groups. We waited for 20 min in between rounds of stimuli, to normalize hemodynamic and biochemical conditions.
Enzyme-Linked Immunosorbent Assay
Commercially available enzyme-linked immunosorbent assay kits were used to quantity CSF ERK MAPK and ET-1 (Assay Designs, Farmingdale, NY; Phoenix, Belmont, CA) concentration.
Histologic Preparation
The brains were prepared for histopathology at 4 h post FPI using previously published methods (16). We determined the mean number of necrotic neurons (±SEM) in CA1 and CA3 hippocampi in vehicle control, FPI-, and FPI+Phe-treated pigs, with data displayed for the side of the brain contralateral to the site of injury (the side where pial artery reactivity was investigated). Morphologic criteria for a necrotic neuron are as follows: (i) pyknosis, (ii) granulation of the cytoplasm, and (iii) the emergence of an unstained area between the nucleus and the cytoplasm. The investigator was blinded to the treatment group. Neuronal pathology scoring was described on the basis of damaged neurons per 1.2 mm2 of a specific anatomic region as either mild (1–5), moderate (6–15), or severe (>15).
Statistical Analysis
Values for pial artery diameter and CSF biochemical analyses were analyzed using ANOVA for repeated measures. If the value was significant, the data were analyzed by Fisher’s protected least significant difference test. An α-level of P < 0.05 was considered significant in all statistical tests. Values are represented as mean±SEM of the absolute value or as percentage changes from control value. Using power analysis, we determined that a sample size of 5 yielded statistical significance at the P <0.05 level, with a power of 0.84 for hemodynamic data. Power analysis for histopathology and biochemical indices had powers of 0.82 and 0.85, respectively.
Change history
17 August 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41390-022-02248-9
References
Langlois JA, Rutland-Brown W, Thomas KE . The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil 2005;20:229–38.
Newacheck PW, Inkelas M, Kim SE . Heath services use and health care expenditures for children with disabilities. Pediatrics 2004;114:79–85.
Freeman SS, Udomphorn Y, Armstead WM et al, Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology 2008;108:588–95.
Kochanek PM, Carney N, Adelson PD et al, Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-Second Edition. Pediatr Crit Care Med 2012;13 (Suppl 1): S24–9.
Ishikawa S, Ito H, Yokoyama K et al, Phenylephrine ameliorates cerebral cytotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid occlusion with severe hypotension. Anesth Analg 2009;108:1631–7.
Sookplung P, Siriussawakul A, Malakouti A et al, Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. NeuroCrit Care 2011;15:46–54.
Steiner LA, Johnston AJ, Czosnyka M et al, Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head injured patients. Crit Care Med 2004;32:1049–54.
Armstead WM . Age dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation 2000;7:225–35.
Dobbing J The later development of the brain and its vulnerability. In: JA Davis, J Dobbing, eds. Scientific Foundations of Pediatrics. London: Heineman Medical, 1981: 744–759.
Digennaro JL, Mack CD, Malakouti A et al, Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci 2011;32:420–30.
Armstead WM, Vavilala MS . Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J Cereb Blood Flow Metab 2007;27:1702–9.
Armstead WM, Kiessling JW, Bdeir K et al, Adrenomedullin prevents sex dependent impairment of cerebal autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J Neurotrauma 2010;27:391–402.
Armstead WM, Kiessling JW, Kofke WA et al, Impaired cerebral blood flow autoregulation during post traumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by ERK MAPK upregulation. Crit Care Med 2010;38:1868–74.
Armstead WM, Vavilala MS, Lok J, Ning MM . Age and sex differences in hemodynamics in a large animal model of Brain Trauma. In: Eng Lo, Lok J, Ning MM eds. Vascular Mechanisms in CNS Trauma. New York: Springer, 2014: 269–284.
Armstead WM, Riley J, Vavilala MS . Preferential protection of cerebral autoregulation and reduction of hippocampal necrosis with norepinephrine after traumatic brain injury in female piglets. Pediatr Crit Care Med 2016;17:e130–7.
Armstead WM, Riley J, Vavilala MS . Norepinephrine protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury via block of ERK MAPK and IL-6 in juvenile pigs. J Neurotrauma 2016;33:1761–7.
Armstead WM, Riley J, Vavilala MS . Dopamine prevents impairment of autoregulation after TBI in the newborn pig through inhibition of upregulation of ET-1 and ERK MAPK. Ped Crit Care Med 2013;14:e103–11.
Bell MJ, Adelson PD, Hutchison JS et al, Differences in medical therapy goals for children with severe traumatic brain injury—an International Study. Pediatr Crit Care Med 2013;14:811–8.
Girling KJ, Cavill G, Mahajan RP . The effects of nitrous oxide and oxygen consumption on transient hyperemic response in human volunteers. Anesth Analg 1999;89:175–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
STATEMENT OF FINANCIAL SUPPORT
This work was supported by NIH R01 NS090998.
About this article
Cite this article
Curvello, V., Hekierski, H., Riley, J. et al. RETRACTED ARTICLE: Sex and age differences in phenylephrine mechanisms and outcomes after piglet brain injury. Pediatr Res 82, 108–113 (2017). https://doi.org/10.1038/pr.2017.83
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.83
This article is cited by
-
Pre-clinical models in pediatric traumatic brain injury—challenges and lessons learned
Child's Nervous System (2017)