Abstract
Background
To determine the predictors of achieving independent walking at 2 and 6 months after onset of weakness in children with Guillain-Barre syndrome (GBS).
Methods
Children with GBS admitted to the Tabriz Children’s Hospital were studied prospectively. All patients had frequent clinical evaluations until achieving independent walking. Unaided walking at 2 and 6 months and factors influencing these outcomes were determined using both univariate and multiple analyses.
Results
Between 2003 and 2014, 324 children (mean age: 5.3±3.66 years) were admitted. The mean duration to independent walking was 2.97±3.02 months; 90.5% of patients could walk independently at 6 months. In the univariate analysis, disability score of >3 (P=0.03), autonomic nerve involvement (P=0.003), cranial nerve involvement (P=0.008), and absent compound muscle action potential (CMAP; P=0.048) were found to be significantly associated with poor walking outcome at 6 months. In the multivariate analysis, cranial nerve involvement (P=0.008) and absence of CMAP (P=0.022) were independently associated with poor functional outcome.
Conclusion
Disability score >3, cranial and autonomic nerve involvement, and absence of CMAP were predictors of independent walking in childhood GBS in this study; early rehabilitation program may prevent further impairments secondary to immobility in these patients.
Similar content being viewed by others
Main
Guillain-Barre syndrome (GBS) is an acute, inflammatory polyneuropathy that presents most commonly with progressive weakness and reduced or diminished deep tendon reflexes. Because there has been a significant reduction in poliomyelitis in the last two decades, GBS is the most common cause of flaccid paralysis among children (1, 2, 3). Although it is an autoimmune disease, there have been associations with infections such as Campylobacter jejuni, Cytomegalovirus, Epstein–Barr virus, Mycoplasma pneumonia, and swine flu vaccination (4). There have been few studies on pediatric GBS in Iran; however, one study estimated a GBS prevalence of 2 per 100,000 children in northwestern Iran (5).
Although motor weakness is the main manifestation of GBS, sensory symptoms—mostly pain—in children have also been reported. Other less common manifestations include cranial nerve and autonomic nervous system involvement (1, 2).
GBS is divided into the following categories based on clinical and electrodiagnostic (EDX) findings in the literature (6): (i) acute inflammatory demyelinating polyneuropathy (AIDP), the most common form of GBS, (ii) acute motor and sensory axonal neuropathy (AMSAN), (iii) acute motor axonal neuropathy (AMAN), and (iv) Miller-Fisher.
The frequency of different electrophysiologic subtypes of GBS varies across regions (7). Moreover, the electrophysiologic pattern is one of the outcome predictors in GBS. There have been few reports of childhood GBS prognosis in previous studies (8, 9, 10). In adult patients older than 40 years, preceding diarrhea and a high disability score were reported as predictors of poor outcome (2). To our knowledge, there are limited large prospective studies on long-term outcome in GBS and predictors of functional outcome among children in the literature. Thus, we decided to perform a comprehensive evaluation of long-term outcome and predictors of independent walking among affected children in northwestern Iran.
Methods
In this prospective, observational study, 324 children diagnosed with GBS who had been admitted to the Tabriz Children’s Hospital from June 2003 to January 2014 were evaluated. This hospital is the largest medical center for children in northwestern Iran. Informed consent was obtained from the parents. This research was approved by the institutional ethics committee. The diagnosis of GBS was defined clinically as per the criteria proposed by Asbury and Cornblath (11). Polio virus infection was excluded after the examination of two stool samples.
Neurological examinations were performed by a pediatric neurologist. History of pre-existing illness, clinical and diagnostic details including weakness, pain, cranial and autonomic nerve involvement, CSF analysis, electrophysiologic findings, duration of mechanical ventilation, and motor recovery were recorded. Disability score was evaluated based on the criteria by Hughes et al. (12). In this classification scheme, 0 represents normal, 1 means mild weakness, 2 means ability to walk 5 m independently but unable to run, 3 means walking with assistance, 4 means chair- or bed-bound, 5 means mechanical ventilation, and finally 6 is equal to death. Evaluation of sensory symptoms was not reliable because of the patients’ young ages. Autonomic dysfunction was evaluated by bedside clinical examination. Patients who developed fluctuation in blood pressure and heart rate, arrhythmia, pupillary abnormality, abnormal sweating, and gastrointestinal and urinary dysfunction were considered to have autonomic dysfunction.
Classification of patients as per an EDX study was based on the criteria introduced by Cornblath (13). On the basis of EDX findings, patients were categorized into AIDP, AMSAN, AMAN, Miller-Fisher, and unclassified subgroups.
Patients were classified as having AIDP if the following changes were observed in at least two nerves: prolongation of distal latency (>125% of normal upper limit), slowed nerve conduction velocity (<80% of normal lower limit), f-wave prolongation (>120% of normal upper limit), conduction block (>20% reduction in peak-to-peak amplitude), and temporal dispersion (>15% increase in a negative phase duration). When CMAP amplitudes were reduced without evidence of demyelination based on the above-cited criteria, the patients were considered as AMAN. When there were reduced CMAP and sensory nerve action potential amplitudes, the patient was classified as AMSAN. Those with low-amplitude sensory nerve action potentials and the clinical triad of ataxia, ophthalmoplegia, and areflexia were classified as the Miller-Fisher variant (13).
A pediatric electromyographer was responsible for EDX studies. A Medelec Synergy electromyography device was used to study nerve conduction. Sensory nerve conduction of the sural and median nerves in addition to the tibial, peroneal, median, and ulnar motor nerves was studied for all admitted patients with GBS. The amplitude of the negative phase was measured for CMAP and sensory nerve action potential. F-wave distal latency and nerve conduction velocity were also obtained. We compared the obtained data with normal values reported by Parano et al. (14).
All patients had at least one EDX evaluation, CSF analysis, and frequent clinical evaluations during hospital admission. Patients were followed up after discharge by outpatient visits until they achieved independent walking (up to 18 months).
Patients were treated with intravenous immunoglobin at a dose of 400 mg/kg/day for 5 consecutive days during their hospitalization.
Data Analysis
In an initial descriptive analysis, data were presented as central tendency and dispersion measurements for qualitative variables and simple and relative frequencies (percentages) for quantitative variables. The mean time to independent walking was compared in categories of some of the dependent variables using independent samples t-test and ANOVAs.
Unaided walking at 2 and 6 months was considered as outcomes of GBS, and factors influencing these outcomes were determined using both univariate and multivariate analyses. In the univariate analysis, to explore the association between dichotomous outcomes (walking after 2 and 6 months) and predictive variables, Pearson’s χ2-test (or exact test) was used and simple logistic regression analysis was conducted to report odds ratios for outcome risk factors.
In a multiple analysis, the variables that were significantly associated with GBS outcome in a univariate analysis were considered in the multiple logistic regression model to see the impact of factors influencing the outcome. Bayes adjustment was performed to estimate the odds when there was a zero-cell frequency.
The Kaplan–Meier method was used to estimate the probability of walking after 2 and 6 months for those important risk factors in multivariate analyses. Statistical analyses were performed using the SPSS 16.0 software, and results were considered significant at P<0.05.
Results
From 2003 to 2014, 324 patients (175 male and 149 female patients) admitted to the Tabriz Children Hospital with GBS diagnosis were recruited into this study. The mean age was 5.13±3.66 years (6 months to 16 years of age). Well over half (63.9%) of our patients were under 5 years of age (Table 1). In all, 184 (56.8%) patients had a previous history of infection 1–3 weeks before weakness manifestation, of which 134 (41.3%) had upper respiratory tract infection and 50 (15.5%) were reported to suffer from gastrointestinal infection. For rare causes, such as vaccination, trauma, hepatitis, and mumps, there were just 11 cases in this study. Other patients had no history of previous illness (Table 1).
Clinical signs and symptoms of all patients and disability score based on the Hughes scale are provided in Table 1. One hundred twenty-seven patients (39.4%) had cranial nerve involvement during hospital stay. Autonomic dysfunction, including abnormalities in heart rate, rhythm, and blood pressure; constipation; abnormal sweating; and urinary dysfunction, was noted in 53 patients (16.4%).
Cerebrospinal fluid (CSF) analysis was performed in the first week after admission; protein levels ⩾40 mg/dl were noted in 75.2% of patients, normal studies performed in the first week of disease progression (Table 1).
EDX evaluation was performed during the hospital stay period between 3 and 21 days after onset of weakness (5.85±12.71 days on average). Table 1 illustrates electrophysiologic patterns in detail. All normal studies were performed in the first week of disease progression.
Patients received intravenous immunoglobin treatment, and 36 patients had methyl prednisolone as an adjunct therapy. Twenty-six individuals (8.3%) with disability grades 1 and 2 received no treatment at all. The reported outcomes are depicted in Table 2.
Outcomes
Thirty-one (9.6%) patients were eventually mechanically ventilated, the mean duration of which was 20.75±21.25 days, and five (1.5%) patients expired. Relapse occurred in six (1.8%) patients (Table 2).
The mean time to achieve independent walking was 2.97±3.02 months, and 96% of patients could walk without assistance within 1 year (Table 2).
For evaluation of clinical and electrophysiologic factors associated with outcome, patients were assessed into two groups based on walking ability at 2 and 6 months.
The mean time analysis revealed that the average time for independent walking differed significantly based on disability score, autonomic and cranial nerve involvement, electrophysiologic pattern, and compound muscle action potential (CMAP) amplitude (Table 3).
Walking Ability at 2 Months
Patients <5 years were more likely than older patients to need a recovery time of >2 months to walk independently (P=0.005; Table 4).
Although there was no significant difference in the mean walking time between groups based on pre-illness (Table 3), walking outcome at 2 months showed a significant difference between the groups (P=0.001; Table 4).
Furthermore, those with a history of pre-illness needed more time to start unaided walking than those without pre-existing illness (Table 3).
Children with autonomic nervous system involvement (OR: 3.07, 95% CI: 1.42–6.64, P=0.003) and cranial nerve involvement (OR: 2.69, 95% CI: 1.52–4.74, P=0.001) needed more time to start walking without assistance. In general, 51.6% of patients with an axonal EMG pattern required >2 months to walk independently compared with 33.0% of patients with a demyelinating pattern (OR: 2.5, 95% CI: 1.01–7.06, P=0.048). In addition, disability grade >3 (OR: 20.65, 95% CI: 6.19–68.95, P<0.001) and absence of CMAP (OR: 18.0, 95% CI: 3.40–95.20, P<0.001) emerged as important risk factors for delayed walking (Table 4).
Applying multiple logistic regression to assess the simultaneous effects of the significant variables in the univariate analysis revealed pre-existing gastroenteritis (OR: 3.61, 95% CI: 1.23–10.50, P=0.019), disability score >3 (OR: 11.63, 95% CI: 2.19–61.84, P=0.004), cranial nerve involvement (OR: 3.67, 95% CI: 1.42–9.46, P=0.007), and absence of CMAP (OR: 68.38, 95% CI: 2.17–215.67, P=0.016) as the factors that associated independently with poor outcome at 2 months.
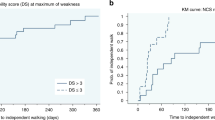
The Kaplan–Meier curve in Figure 1 illustrates the probability of independent walking among groups based on CMAP amplitude. The estimated mean time for unaided walking was 5.27 months in patients with low-amplitude CMAP and 6.11 months in patients with absence of CMAP. The average time for unaided walking was 4.00 and 5.62 months for disability scores ⩽3 and >3, respectively (Figure 2).
Walking Ability at 6 Months
For children with GBS, having a disability score of >3 (OR: 23.28, 95% CI: 1.24–431.77, P=0.03), having autonomic nerve involvement (OR: 4.45, 95% CI: 1.66–11.97, P=0.003), cranial nerve involvement (OR: 3.89, 95% CI: 1.43–10.57, P=0.008), and absence of CMAP (OR: 8.67, 95% CI: 1.09–76.86, P=0.048) were found to be significantly associated with poor walking outcome at 6 months (Table 4). In multivariate analyses, cranial nerve involvement (OR: 1.87, 95% CI: 1.07–2.61, P=0.008) and absence of CMAP (OR: 2.04, 95% CI: 1.09–2.28, P=0.022) were found to be important and independent poor predictors of unaided walking.
The Kaplan–Meier curve indicates that patients with cranial nerve involvement were more likely to experience delayed walking. The estimated mean time to walk unaided was 12.17 months for children with cranial nerve involvement and 9.50 months for those without cranial nerve involvement. Children with absence of CMAP amplitude could walk independently in 10 months on average, if they could not walk in 6 months.
Discussion
This study reports outcomes and predictors of independent walking in children suffering fromGBS in Iran. The strengths of this study are the inclusion of a large number of patients and the prospective nature of the data collection. The reported mortality rate varied among different studies (5–10%; ref. (15)). Approximately 10–30% of patients with GBS may require mechanical ventilation (2, 8, 16). In this study, 9.6% of patients were eventually ventilated mechanically during the hospital stay period, and the mortality rate was 1.5%. Differences in the mortality rate and use of mechanical ventilation could be due to differences in the disease spectrum, study population, supportive care, monitoring, and decision to start artificial ventilation (8).
Long-term functional recovery in children has been better than that in adults; this may be due to adults sustaining more severe injuries or the shorter nerve length or better nerve regeneration in the pediatric population (17). In a prospective study in adults, independent walking occurred in 75% of patients at 3-year follow-up (18). Although the results of the current study showed a significant correlation between age and time to walking, 96% of patients were eventually able to walk independently over a period of 1 year. In this study, patients of a younger age had poor outcomes for walking at 2 months; this may be due to immature nerve fibers in young children. Less myelinated fibers are more vulnerable to nerve injury in GBS (10). In a cross-sectional study by Vajsar et al. (10), younger age and rapid disease progression were reported to be poor predictors for walking in childhood GBS.
There is a paucity of prospective studies addressing long-term recovery in childhood GBS. In a study by Korinthenberg et al. (19), 95 children were evaluated prospectively, and 96% of their patients recovered well within 12 months with minimal functional deficit. The results of this study are comparable to those of the current study.
There is a clinical prognostic scoring system for GBS in adults; in this system, the following three factors are reported as poor outcome predictors at 6 months: age >40 years, history of diarrhea, and high disability score (2, 20). In the current study, in univariate analysis, patients with poor outcome at 2 months were those with the age of <5 years, history of gastroenteritis (GE), disability score >3, autonomic and cranial nerve involvement, axonal electrophysiologic pattern, and absence of CMAP; however, at 6 months, disability score >3, dysautonomia, cranial nerve involvement, and absence of CMAP were poor outcome predictors. However, in multivariate analysis, there was a significant association between poor functional outcome at 6 months with cranial nerve involvement and absence of CMAP.
In a retrospective study, Varkal et al. (21) evaluated 40 children with GBS. Similar to the current study, they suggested axonal motor involvement and acute gastrointestinal infection as predictors of a longer recovery period.
Clinical manifestations of GBS in children are different from those in adults: pain and bulbar dysfunction are more frequent in these patients (22). In our study, 39.4% had cranial nerve involvement, 45.9% had pain, and 16.4% had autonomic dysfunction. Autonomic dysfunction can be seen in 23–66% of GBS cases (2, 19, 23); lower rate of dysautonomia in this study may be because of methods of evaluation. Autonomic dysfunction was evaluated based on bedside clinical examination without any particular testing. In this study, patients with autonomic dysfunction and cranial nerve involvement had significant correlation with time to achieve independent walking; this is comparable to the results of studies conducted in adults (23).
Axonal pattern and absence of or unobtainable CMAP in electrophysiologic study, which that could be due to axonal degeneration or conduction block, is one of the predictors of poor outcome in this and other studies (23, 24, 25). Incidence of the AIDP and AMAN forms of GBS varies in different regions, which may be due to genetic background and inciting pathogens. The frequency of AMAN in our region is higher than that in Western countries (7). In a cross-sectional study on 37 children followed up for up to 11 years, there was no significant association between electrophysiologic pattern and outcome (9). There is a significant correlation between axonal electrophysiologic pattern and CMAP amplitude with outcome at 2 months; however, at 6 months, only CMAP amplitude had a significant association with functional outcome in this study. The analysis of long-term recovery in children with GBS is an under-investigated area in Iran. Recently, Salehiomran et al. (26) have studied 17 children diagnosed with GBS in Iran, and have suggested a good clinical outcome with no long-term disability.
On the basis of the findings of this study, children with a disability score >3, autonomic and cranial nerve involvement, and absence of CMAP had poor outcomes with respect to achieving independent walking at 6 months. Therefore, in patients who had these predictors, early-rehabilitation program has a role in preventing further side effects and deconditioning secondary to immobility.
References
Asbury AK, Cornblath DR . New concepts of GBS. J Child Neurol 2000;15:183–91.
Willison H J, Jacobs BC, Van Doom PA . Guillain Barre syndrome. Lancet 2016;388:717–27.
Paradiso G, Tripoli J, Galicchio S, Fejerman N . Epidermiological,clinical, and electrodiagnostic finding in childhood Guillain - Barre syndrome:a reappraisal. Ann Neurol 1999;46:701–707.
Hughes RA, Rees JH . Clinical and epidemiologic feature of GBS. Clin Infect Dis 1997;176:592–8.
Barzegar M, Davari Farid S, Dastgerdi S, Malekian A, Toopchizadeh V . Childhood Guillian-Bare syndrome in the Iran's East Azerbaijan province: 2001-2005. Iran J child Neurol 2008;2:25–31.
Hadden RD, Cornblath DR, Hughes RA et al, Electrodiagnostical classification Guillain-Barré syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neural 1998;44:780–8.
Toopchizadeh V, Barzegar M . Electrophysiologic features of childhood Guillain-Bare syndrome in Iran. J Pediatr Neurol 2008;6:11–6.
Kalra V, Sankhyan N, Sharma S, Gulati S, Choudhry R, Dhawan B . Outcome in childhood Guillain-Bare syndrome. Indian J Pediatr 2009;76:795–9.
Roodbol J, de Wit Mc, Aarsen FK, Catsman-Berrevoets CE, Jacobs BC . Long term outcome of Guillain Barre Syndrome in children. J Peripher Nerv Syst 2014;19:121–6.
Vajsar J, Fehlings D, Stephens D . Long-term outcome in children with Guillain Barre syndrome. J Pediatr 2003;142:305–9.
Ausbury AK, Cornblath DR . Assessment of current diagnostic criteria for Guillain-Bare syndrome. Ann Neurol 1990;27:21–4.
Hughes RA, Newsom-Davis JM, Perkin GD, Pierces JM . Controlled trial prednisolone in acute polyneuropathy. Lancet 1978;7:750–3.
Cornblath DR . Electrophysiology in Guillain-Bare syndrome. Ann Neurol 1990;27:17–20.
Parano E, Uncini A, De Vivo DC, Lovelace RE . Electrophysiologic correlates of peripheral nervous system maturation in infancy and childhood. J Child Neurol 1993;8:336–8.
Lee JH, Sung LY, Rew IS . Clinical presentation and prognosis of childhood Guillain Barre syndrome. J Paediatr Child Health 2008;441:449–54.
Aggarwal AN, Gupta D, Lai V, Behera D, Jindal SK, Prabhakar S . Ventilatory management of respiratory failure in patients with severe GBS. Neurol India 2003;51:203–5.
Delanoe C, Sabire G, Landrieu P, Huault G, Metral S . Acute inflammatory demyelinating polyneuropathy in children, clinical and electrodiagnostic studies. Ann Neurol 1998;44:350–6.
Dhar R, Stitt L, Hahn AF . The morbidity and outcome of patients with Guillain- Barre syndrome admitted to the intensive care unit. J Neurol Sci 2008;264:121–8.
Korinthenberg R, Schessl J, Kirschner J . Clinical presentation and course of childhood Guillain Barre syndrome: a prospective multicenter study. Neuropediatrics 2007;38:10–7.
Van Koningsveld R, Steyeberg EW, Hughes RA, Swan AV, Van Doom PA, Jacobs BC . A clinical prognostic scoring system for Guillain Barre syndrome. Lancet Neurol 2007;6:589–94.
Varkal MA, Uzunhan TA, Aydınlı N, Ekici B, Çalışkan M, Özmen M . Pediatric Guillain-Barré syndrome: indicators for a severe course. Ann Indian Acad Neurol 2015;18:24–8.
Wu X, Shen D, Li T et al, Distinct clinical characteristics of pediatric Guillain Barre Syndrome: a comparative study between children and adults in Northeast China. PLOS ONE 2016;11:1–12.
Verma R, Chaudhari TS, Raut TP, Garg RK . Clinico-electrophysiological profile and predictors of functional outcome in Guillain Barre syndrome. J Neurol Sci 2013;335:105–11.
Berciano J, Garcia A et al, Fulminant Guillain-barre syndrome with universal inexcitability of peripheral nerves: a clinicopatological study. Muscle Nerve 1997;20:846–57.
Ortiz CF . Factors affecting prognosis in childhood Guillain-Barre syndrome. Rev Neurol 2004;38:518–23.
Salehiomran MR, Nikkhah A, Mahdavi M . Prognosis of Guillain-Barré Syndrome in children. Iran J Child Neurol 2016;10:38–41.
Acknowledgements
This research was supported by the Pediatric Health Research Center and Physical Medicine and Rehabilitation Research Center in Tabriz Medical Science University. We are especially grateful to the patients and their parents for participation and cooperation in this research. We take responsibility for the integrity and accuracy of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
This study was financially supported and conducted under the supervision of “Pediatric Health Research Center” and “Physical Medicine and Rehabilitation Research Center” of Tabriz University of Medical Sciences, related to government.
Rights and permissions
About this article
Cite this article
Barzegar, M., Toopchizadeh, V., Maher, M. et al. Predictive factors for achieving independent walking in children with Guillain-Barre syndrome. Pediatr Res 82, 333–339 (2017). https://doi.org/10.1038/pr.2017.67
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.67
This article is cited by
-
Clinical and Electrophysiological Factors Predicting Prolonged Recovery in Children with Guillain–Barré Syndrome
Indian Journal of Pediatrics (2022)