Abstract
Background
To validate the findings of a single-center pilot study showing elevated urinary N-terminal B-type natriuretic peptide (NTproBNP) concentrations in preterm infants subsequently developing severe retinopathy of prematurity (ROP) in a multicenter setting across eight European and Middle East countries.
Methods
Prospective observational study in 967 preterm infants <30 weeks’ gestational age assessing the capacity of urinary NTproBNP on days of life (DOLs) 14 and 28 to predict ROP requiring treatment.
Results
Urinary NTproBNP concentrations were markedly elevated in infants who developed ROP requiring treatment (n=94) compared with survivors without ROP treatment (n=837), at both time points (median (interquartile range) DOL14: 8,950 (1,925–23,783) vs. 3,083 (1,193-17,393) vs. 816 (290-3,078) pg/ml, P<0.001) and DOL28 (2,203 (611–4,063) vs. 1,671 (254–11,340) vs. 408 (162–1,126) pg/ml, P<0.001). C-statistic of NTproBNP for treated ROP or death was 0.731 (95% confidence interval 0.654–0.774) for DOL14 and 0.683 (0.622–0.745) for DOL28 (P<0.001). Threshold scores were calculated, potentially enabling around 20% of infants with low NTproBNP scores never to be screened with ophthalmoscopy.
Conclusion
There is a strong association between early urinary NTproBNP and subsequent ROP development, which can be used to further refine subgroups of patients with high or low risk of severe ROP.
Similar content being viewed by others
Main
Retinopathy of prematurity (ROP) is responsible for a large proportion of potentially preventable childhood blindness worldwide, with approximately two-thirds of preterm infants displaying ROP-mediated visual impairment being born in middle-income countries (1). This proliferative disorder of blood vessels in the immature retina develops gradually after birth. Therefore, a window exists to screen, detect, and treat in a timely manner where indicated. Screening by indirect ophthalmoscopy is supposed to start at a corrected gestational age of 31 weeks and a chronological age of >4 weeks. Numbers of ophthalmologists who are able and willing to perform ROP screening are often limited by liability concerns, reimbursement issues, and lack of training opportunities both in high- and middle-income countries (2, 3, 4). Besides being an expensive and limited resource, ophthalmoscopy is distressing to families to observe and potentially destabilizing infants. Adverse events related to the procedure or eye drops used include life-threatening apnea, bradycardia, hypoxia, tachycardia, emesis, and retinal hemorrhages (5, 6, 7, 8, 9, 10), whereas the pain and stress response to ophthalmoscopy is poorly blunted by oral sucrose (11).
Although a considerable proportion of very preterm infants develop some degree of ROP, only a small fraction progresses to severe ROP requiring treatment (laser surgery or intravitreal pharmacological therapy). Thus, biomarkers that define infants at high or low risk of ROP requiring intervention would have wide implications for many infants, families, and health economies. Such biomarkers would ideally have high sensitivity and specificity and should be used on noninvasive samples by local laboratories.
In response to increased preload or afterload, cardiac myocytes release B-type natriuretic peptide (BNP) into the circulation. BNP acts on the heart (accelerated myocardial relaxation), the vasculature (vasodilation), and the kidneys (natriuresis). It is generated by proteolytic cleavage of a precursor protein, yielding the biologically active BNP and an inert N-terminal fragment, N-terminal B-type natriuretic peptide (NTproBNP, amino acids 1–76). Measuring NTproBNP concentrations is widely being used for the assessment of cardiac failure in adults, children, and neonates (12, 13, 14). NTproBNP concentrations in blood parallel those determined in urine (15). In preterm infants, elevated NTproBNP concentrations measured in blood or urine have been shown to herald incipient bronchopulmonary dysplasia and are indicative of a hemodynamically significant patent ductus arteriosus (16).
In a single-center pilot study, we found that urinary NTproBNP/creatinine ratios (UNBCR) during the first month were significantly elevated in preterm infants who developed severe ROP, compared with controls (17). To further assess the predictive power of urinary NTproBNP concentrations and UNBCR during the first month of life to allow early identification of infants at high or low risk of severe ROP, we conducted a prospective observational study (REDEXAM, REDucing Eye EXAMinations in preterm infants) in neonatal intensive units in eight European and Middle East countries.
Methods
Study Setting
Neonatal intensive care units are located in the United Kingdom, The Netherlands, Belgium, Norway, Germany, Austria, Turkey, and Israel after approval of the ethical committees of each site.
Patients and Measurements
Preterm infants with a gestational age below 30 who completed weeks alive at 10 days were eligible (recruitment periods varied by site). After written informed parental consent, spot urine samples were collected on DOL (day of life) 14 and DOL28 (or as close as possible). Urine was collected by each center by their usual method, and included using cotton wool balls or pads in the nappy, bags, or clean catches by staff or parents at routine nappy changes, and stored at −80 °C until analysis. Urinary NTproBNP concentrations were determined at each site in batches by standard hospital laboratory-automated commercial chemiluminescent sandwich immunoassays (Roche Modula P E170 run on a cobas e 411 analyzer, Roche Diagnostics International, Rotkreuz, Switzerland) as described previously (17), whereas urinary creatinine concentrations were determined enzymatically or by the Jaffé method. Further data recorded were maternal age and the infant’s weight on DOL14 and DOL28. Proportional weight gain was calculated by dividing the gain of the infant’s weight since birth on DOL14 or DOL28 by birth weight.
ROP Outcomes
ROP-screening examinations using serial binocular indirect ophthalmoscopy were carried out by experienced local ophthalmologists according to local and national guidelines and were not influenced in any way by this study. The ophthalmologists staged ROP according to the International Classification of Retinopathy of Prematurity (18) and allocated treatment according to the Early Treatment for Retinopathy of Prematurity criteria (19). They were unaware of urinary NTproBNP concentrations.
Statistical Analysis
NTproBNP results for infants with known ROP outcome and at least one urinary NTproBNP concentration measured were analyzed using SPSS 23.0 (SPSS, Chicago, IL). As NTproBNP results and other variables lacked a normal distribution, data are presented as median and interquartile range. Dichotomous values are presented as numbers (percentages). We assessed the strength of association between continuous variables by Spearman rank order coefficients. Differences in the distribution of continuous variables were assessed by the Mann–Whitney U-test (for two groups) or the Kruskal–Wallis test (more than two groups, followed by post hoc analysis employing the Mann–Whitney U-test). For paired samples, the Wilcoxon test was used. Continuous variables found to be significantly associated with a dichotomous variable by univariate analysis were entered into multivariate linear regression analysis with backward elimination for P>0.1. Predictive values were estimated by the areas under receiver operating characteristic (ROC) curves (C-statistic).
Results
Patient Characteristics
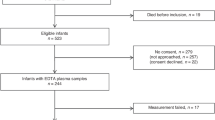
A total of 1,000 infants were recruited between January 2012 and July 2015 (exact recruitment periods varied by site). After exclusion of 33 infants (gestational age ≥30 weeks, n=13; failed urine collection both DOL14 and DOL28, n=20), 967 infants were included in the final analysis (UK, n=265; Germany, n=163; Turkey, n=118; the Netherlands, n=106; Austria, n=171; Israel, n=71; Belgium, n=49; Norway, n=24). The cohort consisted of 517 boys (53.5%) and 319 multiples (33.0%; 280 twins, 35 triplets, and 4 quadruplets). Median (interquartile range) gestational age was 273/7 (26 1/7−286/7) weeks and birth weight was 945 (780–1,165) g. The actual age at which urine was collected for DOL14 samples (n=904) was 14.35±1.37 days (mean±SD) and 28.33±1.68 days for DOL28 samples (n=839). In all, 36 infants died before discharge. Among the survivors, 550 infants were without ROP, 133 with ROP stage 1, 150 with ROP stage 2, and 98 with ROP stage 3. Treatment for ROP was performed in 94 infants (laser ablative surgery, n=76; intravitreal bevacizumab injections, n=18). Survivors with ROP treatment compared with survivors without ROP treatment had lower gestational age (254/7 (245/7−266/7) weeks vs. 275/7 (262/7–290/7) weeks, P<0.001), lower birth weight (755 (640–905) g vs. 980 (815–1,180) g, P<0.001), and lower proportional weight gain between birth and DOL28 (31.4 (19.0–41.9)% vs. 36.1 (24.8–48.1)%, P=0.007) but not between birth and DOL14 (P=0.080). The sex ratio, multiple vs. singleton pregnancy, and maternal age did not differ between survivors with or without ROP treatment (P>0.1). Patient characteristics by country are given in Table 1.
Urinary NTproBNP Concentrations and UNBCR
Baseline data
Urinary NTproBNP concentrations declined from median 992 (330–4,082) pg/ml on DOL14 to 470 (170–1,465) pg/ml on DOL28 (P<0.001). The same pattern was observed for UNBCR (DOL14: 92 (30–413), DOL28: 39 (15–137) pg/ml, P<0.001).
Associations with patient characteristics
Urinary NTproBNP concentrations were inversely related to birth weight (DOL14: Rs=−0.475, DOL28: Rs=−0.428, P<0.001) and gestational age (DOL14: Rs=−0.502, DOL28: Rs=−0.447, P<0.001). UNBCR showed similar relationships with birth weight (DOL14: Rs=−0.489, DOL28: Rs=−0.439, P<0.001) and gestational age (DOL14: Rs=−0.501, DOL28: Rs=-0.455, P<0.001).
Urinary NTproBNP concentrations and UNBCR were slightly higher in boys than in girls on DOL14 (NTproBNP: 1,146 (387–4,436) vs. 801 (272–3,527) pg/ml, P=0.010; UNBCR: 103 (36–441) vs. 79 (24–386)×10−4, P=0.018) but not on DOL28 of life (NTproBNP: 468 (197–1,575) vs. 438 (151–1,329) pg/ml, P=0.142; UNBCR: 41(17–122) vs. 37 (12–137), P=0.195).
Associations with survival, ROP stages, and ROP treatment
Urinary NTproBNP concentrations and UNBCR were markedly elevated in infants who subsequently died (n=36) and in survivors with severe ROP requiring intervention (laser or intravitreal therapy; n=94), as compared with survivors without ROP intervention (n=837; Table 2).
Survivors with no/little ROP (stage 0 or 1) had lower urinary NTproBNP concentrations and UNBCRs than infants with ROP stage 2 or ROP stage 3 (Table 3). There was no significant difference between infants with no ROP and infants with ROP stage 1. In contrast, median NTproBNP levels were increased almost threefold in infants with ROP stage 2 and approximately fivefold in infants with ROP stage 3, as compared with the levels in infants without ROP.
Prediction of survival without treated ROP
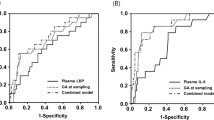
The capacity of NTproBNP and UNBCR to predict survival without ROP treatment was assessed by the areas under ROC curves (Table 4). ROC areas of NTproBNP or UNBCR to predict survival without ROP treatment were larger on DOL14 than on DOL28, whereas those of UNBCR were only slightly larger than plain NTproBNP at both time points. For urinary NTproBNP DOL14, the areas under the ROC curve to predict survival without ROP treatment were very high in participating units in Turkey (0.872), Austria (0.818), and Germany (0.817), high in Belgium (0.780), Israel (0.767), and the Netherlands (0.736) but lower in the UK (0.685) and not significantly different from chance in Norway (0.432).
The three variables that differed significantly between infants who did and those who did not survive without ROP treatment and that were known on DOL14 (gestational age, birth weight, and NTproBNP DOL14) were entered into multivariate linear regression analyses aimed at survival without ROP treatment as dependent variable and using backward elimination if P>0.1. This was repeated for the five variables known on DOL28 (gestational age, birth weight, NTproBNP DOL14, NTproBNP DOL28, and proportional weight gain DOL28). All factors were retained in the final equations. Linear coefficients (ß) were then applied as weighted values to create scores predictive of survival without ROP treatment. These scores were calculated using the formula DOL14 (300−0.156 × gestational age (weeks)−0.118 × birth weight (g)+0.146 × NTproBNP DOL14 (pg/ml)) and DOL28 (300−0.118 × gestational age (weeks)−0.131 × birth weight (g)+0.067 × NTproBNP DOL14 (pg/ml)+0.180 × NTproBNP DOL28 (pg/ml)−0.110 × proportional weight gain birth to DOL28 (fraction)). Scores on DOL14 were 300 (208–636) vs. 656 (341–2,753) vs. 1,539 (464–3,627) in survivors without ROP treatment, survivors with ROP treatment, and infants who died, respectively (P<0.001), and scores on DOL28 were 212 (315–666) vs. 851 (353–3,930) vs. 1,247 (482–2,511), respectively (P<0.001).
No infant with a score of DOL14<197, and no infant with a score DOL28<189, developed ROP requiring treatment (thresholds for 100% specificity and 100% positive predictive value for survival without ROP requiring treatment). In this cohort, 20.6% of infants were below the DOL14 threshold and 15.3% below the DOL28 threshold ROP, translating into sensitivities of 20.6%/15.3% and negative predictive values of 16.4%/14.3%, respectively.
Discussion
This multinational study confirms that urinary NTproBNP concentrations during the first month of life are markedly elevated in preterm infants <30 weeks’ gestation who, many weeks later, develop severe ROP requiring intervention. An even larger elevation of urinary NTproBNP concentrations was observed in infants who subsequently died, linking circulatory stress in the first month of life to mortality and morbidity of very preterm infants.
Although immaturity and oxygen exposure are pivotal for the development of ROP, the risk of severe ROP is further increased by poor postnatal growth. This link is thought to be mediated by low levels of insulin-like growth factor 1 and serves as a base for ROP prediction models such as WINROP (20) or CHOPROP (21). Furthermore, severe ROP is more common in infants with conditions that increase myocardial pressure or volume load, such as sepsis (22, 23), blood transfusions (23, 24), pulmonary hypertension treated with inhaled nitric oxide (25), patent ductus arteriosus (23), or inotrope use (22, 26, 27, 28, 29). Such conditions lead to BNP release and have been associated in several groups of patients with increased NTproBNP (30, 31, 32, 33). Thus, in addition to slow weight gain and low insulin-like growth factor 1 levels, we hypothesize that circulatory compromise and increased BNP release during the first month of life have a role in ROP development.
The potential physiological role of BNP itself in the development of ROP is not yet understood. Relative hypoxia triggers BNP secretion from cultured human retinal pigment epithelium (34), whereas retinal vessels express receptors for BNP (35). BNP, however, has so far not been investigated in animal models of oxygen-induced proliferative retinopathy. In adults without overt cardiovascular compromise, higher levels of NTproBNP were found to be associated with microvascular damage in the retina (36, 37) and other body parts (36, 38). Thus, it remains up to future experimental investigations to explore and characterize the role of cardiovascular compromise and natriuretic peptides in the pathophysiology of ROP.
Previous investigations have used UNBCR rather than plain urinary NTproBNP concentrations to estimate the hemodynamic significance of a patent ductus arteriosus and to assess the risk of ROP or bronchopulmonary dysplasia. The power to predict survival without ROP intervention of UNBCR was only marginally better than that of plain NTproBNP, probably reflecting steady-state kidney function at the age of 2–4 weeks in the infants studied. Diagnostic patterns of UNBCR and plain NTproBNP were highly similar, limiting the merits of additionally determining creatinine concentrations in urine samples. Thus, there is no need to correct for creatinine when using NTproBNP as a screening aid for ROP detection, increasing its potential in low-income settings.
This study has several limitations, including the lack of standardization of ROP outcomes by central review of ophthalmoloscopic findings. Local thresholds for ROP treatment have been shown to differ markedly by country (39) and may have affected the performance of NTproBNP. A fraction of infants with stage 3 ROP in this study were actually not treated. However, the approach taken was highly pragmatic, and we have analyzed by treatment received, a clearly defined end point. This issue has also large multicenter prospective randomized controlled trials including those investigating the effect of different oxygen target saturations on morbidity and mortality of very preterm infants. Participating neonatal intensive care units also had different strategies of fluid management, inotropes, transfusion thresholds, and treatment of a patent ductus arteriosus, which may also affect the relative performance of NTproBNP. In our study, NTproBNP concentrations were determined at prespecified time points, not adjusted for potential peaks associated with particular events such as sepsis or surgery. Although overall predictive power of NTproBNP measurements was lower than that in the pilot study (17), the results also differed markedly by country. Urinary NTproBNP DOL14 below 372 pg/ml (a threshold met by more than 26% of all infants studied) was able to identify all infants who did not require ROP treatment in Turkey, Austria, Germany, Belgium, Israel, and the Netherlands, but not in the UK and Norway.
NTproBNP and NTproBNP-based scores share the problem of limited generalizability with proportional weight gain (20, 21) or risk factor-based models (22, 24). In contrast to the expectations underlying this multinational endeavor, the results of this study do not allow for a change in current clinical practice in infants <30 weeks. However, elevated NTproBNP measured during the first month of life may be helpful to identify infants at greatly increased risk for severe ROP, allowing for early targeted intervention in future trials. NTproBNP may also be promising in identifying more mature infants with increased risk of ROP, who currently may be screened only at the request of the attending neonatologist.
Conclusions
Elevated urinary NTproBNP concentrations during the first month of life are strongly associated with subsequent development of severe ROP, whereas low levels are associated with low risk of severe ROP. The overlapping ranges of NTproBNP concentrations of infants with and without severe ROP, however, preclude to harness these substantial differences into an algorithm to reduce eye examination in very preterm infants. The roles of cardiovascular compromise and natriuretic peptides in the pathophysiology of ROP remain to be determined.
References
Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C . Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013;74 (Suppl 1): 35–49.
Fierson WM, Capone A Jr . American Academy of Pediatrics Section on Opthalmology, American Academy of Ophthalmology. Telemedicine for evaluation of retinopathy of prematurity. Pediatrics 2015;135:e238–54.
Zepeda-Romero LC, Gilbert C . Limitations in ROP programs in 32 neonatal intensive care units in five states in Mexico. Biomed Res Int 2015;2015:712624.
Vinekar A, Jayadev C, Kumar S et al. Impact of improved neonatal care on the profile of retinopathy of prematurity in rural neonatal centers in India over a 4-year period. Eye Brain 2016;8:45–53.
Rush R, Rush S, Nicolau J, Chapman K, Naqvi M . Systemic manifestations in response to mydriasis and physical examination during screening for retinopathy of prematurity. Retina 2004;24:242–5.
Dhaliwal CA, Wright E, McIntosh N, Dhaliwal K, Fleck BW . Pain in neonates during screening for retinopathy of prematurity using binocular indirect ophthalmoscopy and wide-field digital retinal imaging: a randomised comparison. Arch Dis Child Fetal Neonatal Ed 2010;95:F146–8.
Jensen AK, Forbes BJ, Wilson LB, Prieto D, Binenbaum G . Widespread retinal hemorrhages after retinopathy of prematurity screening with scleral depression. J AAPOS 2011;15:609–11.
Cohen AM, Cook N, Harris MC, Ying GS, Binenbaum G . The pain response to mydriatic eyedrops in preterm infants. J Perinatol 2013;33:462–5.
Wade KC, Pistilli M, Baumritter A et al. Safety of retinopathy of prematurity examination and imaging in premature infants. J Pediatr 2015;167:e1002.
Agrawal Y, Patri S, Kalavakunta JK, Gupta V . Retinopathy of prematurity screening leading to cardiopulmonary arrest: fatal complication of a benign procedure. Br Med J 2016;2016 pii: bcr2016216594.
Grabska J, Walden P, Lerer T et al. Can oral sucrose reduce the pain and distress associated with screening for retinopathy of prematurity? J Perinatol 2005;25:33–5.
Nir A, Nasser N . Clinical value of NT-ProBNP and BNP in pediatric cardiology. J Card Fail 2005;11:S76–80.
Sugimoto M, Manabe H, Nakau K et al. The role of N-terminal pro-B-type natriuretic peptide in the diagnosis of congestive heart failure in children. - Correlation with the heart failure score and comparison with B-type natriuretic peptide. Circ J 2010;74:998–1005.
Lechner E, Wiesinger-Eidenberger G, Wagner O et al. Amino terminal pro B-type natriuretic peptide levels are elevated in the cord blood of neonates with congenital heart defect. Pediatr Res 2009;66:466–9.
Kurihara N, Miwa M, Matsuzaki Y et al. Usefulness of measurement of urinary N-terminal pro-brain natriuretic peptide in neonatal period. Pediatr Int 2011;53:608.
Kulkarni M, Gokulakrishnan G, Price J, Fernandes CJ, Leeflang M, Pammi M . Diagnosing significant PDA using natriuretic peptides in preterm neonates: a systematic review. Pediatrics 2015;135:e510–25.
Czernik C, Metze B, Müller C, Müller B, Bührer C . Urinary N-terminal B-type natriuretic peptide predicts severe retinopathy of prematurity. Pediatrics 2011;128:e545–9.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991–999.
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684–94.
Jung JL, Wagner BD, McCourt EA et al. Validation of WINROP for detecting retinopathy of prematurity in a North American cohort of preterm infants. J AAPOS 2017;21:229–33.
Gurwin J, Tomlinson LA, Quinn GE et al. A tiered approach to retinopathy of prematurity acreening (TARP) using a weight gain predictive model and a telemedicine system. JAMA Ophthalmol 2017;135:131–136.
van Sorge AJ, Schalij-Delfos NE, Kerkhoff FT et al. Reduction in screening for retinopathy of prematurity through risk factor adjusted inclusion criteria. Br J Ophthalmol 2013;97:1143–7.
Thomas K, Shah PS, Canning R, Harrison A, Lee SK, Dow KE . Retinopathy of prematurity: risk factors and variability in Canadian neonatal intensive care units. J Neonatal Perinatal Med 2015;8:207–14.
Slidsborg C, Jensen A, Forman JL et al. Neonatal risk factors for treatment-demanding retinopathy of prematurity: a Danish national study. Ophthalmology 2016;123:796–803.
van Sorge AJ, Termote JU, Kerkhoff FT et al. Nationwide inventory of risk factors for retinopathy of prematurity in the Netherlands. J Pediatr 2014;164:494–8.
Allegaert K, Cossey V, Naulaers G, Vanhole C, Devlieger H, Casteels I . Dopamine is an indicator but not an independent risk factor for grade 3 retinopathy of prematurity in extreme low birthweight infants. Br J Ophthalmol 2004;88:309–10.
Catenacci M, Miyagi S, Wickremasinghe AC et al. Dopamine-resistant hypotension and severe retinopathy of prematurity. J Pediatr 2013;163:400–5.
Perez-Muñuzuri A, Couce-Pico ML, Baña-Souto A et al. Preclinical screening for retinopathy of prematurity risk using IGF1 levels at 3 weeks post-partum. PLoS ONE 2014;9:e88781.
Hussein MA, Coats DK, Khan H et al. Evaluating the association of autonomic drug use to the development and severity of retinopathy of prematurity. J AAPOS 2014;18:332–7.
Brueckmann M, Huhle G, Lang S et al. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation 2005;112:527–34.
Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettila V . Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med 2007;35:1277–83.
van Albada ME, Loot FG, Fokkema R, Roofthooft MT, Berger RM . Biological serum markers in the management of pediatric pulmonary arterial hypertension. Pediatr Res 2008;63:321–7.
Tobian AA, Sokoll LJ, Tisch DJ, Ness PM, Shan H . N-terminal pro-brain natriuretic peptide is a useful diagnostic marker for transfusion-associated circulatory overload. Transfusion 2008;48:1143–50.
Aaltonen V, Kinnunen K, Jouhilahti EM et al. Hypoxic conditions stimulate the release of B-type natriuretic peptide from human retinal pigment epithelium cell culture. Acta Ophthalmol 2014;92:740–4.
Kozulin P, Natoli R, Bumsted O'Brien KM, Madigan MC, Provis JM . The cellular expression of antiangiogenic factors in fetal primate macula. Invest Ophthalmol Vis Sci 2010;51:4298–306.
Welsh P, Woodward M, Hillis GS et al. Do cardiac biomarkers NT-proBNP and hsTnT predict microvascular events in patients with type 2 diabetes? Results from the ADVANCE trial. Diabetes Care 2014;37:2202–10.
Mutlu U, Ikram MA, Hofman A, de Jong PT, Klaver CC, Ikram MK . N-terminal pro-B-type natriuretic peptide is related to retinal microvascular damage: the Rotterdam study. Arterioscler Thromb Vasc Biol 2016;36:1698–702.
Hamano K, Nakadaira I, Suzuki J, Gonai M . N-terminal fragment of probrain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes. Vasc Health Risk Manag 2014;10:585–9.
Darlow BA, Lui K, Kusuda S et al. International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol 2017 (e-pub ahead of print 7 March 2017; doi:10.1136/bjophthalmol-2016-310041).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
REDEXAM study group
Sinan Abu-Leil12, Benjamin Bausenhardt13, Christoph Czernik14, Uğur Dilmen15,21, Nicholas Embleton16, Zivanit Ergaz-Shaltiel12, Lara Garabedian17, Irene Lok18, Tone Nordvik19, Wes Onland18, Øivind Ringen19, Thomas Skeath16, Oliver Wagner20
12Hadassah Medical Center, Jerusalem, Israel; 13Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria; 14Charité Universitätsmedizin Berlin, Berlin, Germany; 15Zekai Tahir Burak Maternity Hospital, Ankara, Turkey; 16Royal Victoria Infirmary, Newcastle upon Tyne, UK; 17Department of Development and Regeneration, Catholic University of Leuven, Leuven, Belgium; 18Emma Children’s Hospital, Academic Medical Center Amsterdam, Amsterdam, The Netherlands; 19Oslo University Hospital, Oslo, Norway; 20Kepler University Medical Center, Linz, Austria.
21Deceased.
Statement of Financial Support
The study has received financial support from the BLISS innovation grant (London, UK) and intramural funding.
Rights and permissions
About this article
Cite this article
Bührer, C., Erdeve, Ö., van Kaam, A. et al. N-terminal B-type natriuretic peptide urinary concentrations and retinopathy of prematurity. Pediatr Res 82, 958–963 (2017). https://doi.org/10.1038/pr.2017.179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.179
This article is cited by
-
Vasoactive peptides as biomarkers for the prediction of retinopathy of prematurity
Pediatric Research (2024)
-
Diseases associated with prematurity in correlation with N-terminal pro-brain natriuretic peptide levels during the early postnatal life
European Journal of Pediatrics (2023)
-
Reference values for N-terminal Pro-brain natriuretic peptide in premature infants during their first weeks of life
European Journal of Pediatrics (2021)