Abstract
The fetus does not reside in a sterile intrauterine environment and is exposed to commensal bacteria from the maternal gut/blood stream that cross the placenta and enter the amniotic fluid. This intestinal exposure to colonizing bacteria continues at birth and during the first year of life and has a profound influence on lifelong health. Why is this important? Intestinal crosstalk with colonizing bacteria in the developing intestine affects the infant’s adaptation to extrauterine life (immune homeostasis) and provides protection against disease expression (allergy, autoimmune disease, obesity, etc.) later in life. Colonizing intestinal bacteria are critical to the normal development of host defense. Disrupted colonization (dysbiosis) due to maternal dysbiosis, cesarean section delivery, use of perinatal antibiotics, or premature delivery may adversely affect the gut development of host defense and predispose to inflammation rather than to homeostasis, leading to increased susceptibility to disease later in life. Babies born by cesarean section have a higher incidence of allergy, type 1 diabetes, and obesity. Infants given repeated antibiotic regimens during the first year of life are more likely to have asthma as adolescents. This research breakthrough helps to explain the shift in disease paradigms from infections to immune-mediated in children from developed countries. This review will develop this research breakthrough.
Similar content being viewed by others
Main
Over the last decade, scientists and clinicians alike have recognized the importance of bacteria, particularly bacteria colonizing the gastrointestinal tract, in host metabolic and protective function (1). In fact, the microbiome of the mature human intestine is now considered an ancillary organ of the host, providing important contributions to the individual’s health and well-being (2). Evidence for this statement includes the observations that the intestinal microbiome weighs one and a half kilograms, the number of bacterial cells that reside in the intestine exceeds the number of cells in the body by tenfold, the number of genes in the intestinal microbiome is 100-fold greater than the total number of genes in the host, and that the intestinal microbiome is also more metabolically active than the most active body organ, the liver. Accordingly, a better appreciation for how the intestinal microbiome and its metabolites interact with its host is important to our understanding of its role in health and disease.
This principle is particularly important with initial colonization of the infant’s gut. The initial colonization process occurs during gestation and continues until approximately between 18 months and 3 years postpartum when a unique signature of bacteria exist within the distal small intestine and colon, representing the mature microbiome (details of this process will be discussed later). This neonatal period of postpartum life represents the period of adjustment for metabolic and immunologic development of the infant to its extrauterine environment (3, 4). It also represents a time when epigenetic changes in these host functions are very important to the long-term health of the child (5). “Epigenetic changes are mechanisms that alter the phenotypic expression without changing the underlying DNA sequence” (6). These changes occur because of environmental factors (nutrients and microorganisms) that influence human genes during development (gestation and the neonatal period). Examples of human disease are obesity and metabolic syndrome occurring in children with inadequate nutrition during gestation, as occurred in the Dutch famine in World War II (7), and an altered TLR4 expression on intestinal enterocytes in inflammatory bowel disease (8, 9). These changes can affect methylation or acetylation of histones, leading to altered transcriptional events and phenotypic expression of disease that can be passed to subsequent generations. It is now clear that initial intestinal bacterial colonization has an important impact on the development of these functions in the newborn and during infancy (10).
This review will consider the process of normal bacterial colonization of the intestine (symbiosis) and its effect on newborn adaptation to the extrauterine environment. It will also underscore the impact of appropriate colonization on the infant’s development of host defense and metabolic function, which represent major determinants of health throughout infancy and childhood and into adulthood (11). The impact of disrupted intestinal colonization (dysbiosis) in utero and during infancy on the expression of immune and metabolically mediated disease during childhood and later in life will also be considered. Finally, strategies to rectify dysbiosis and prevent disease expression later in life will be considered. These strategies may either decrease or mitigate the risk for disease later in life.
Initial intestinal colonization
The composition of the intestinal microbiome is dependent on many factors including diet, infection, and lifestyle changes. Over the last half century, with improvement in public health, we have reduced the incidence of infectious diseases in developed countries. Improvement in health (better sanitation, vaccinations, use of antibiotics, etc.) has affected the exposure to pathogens, resulting in less-infectious diseases (12). However, these measures along with major changes in diet as part of the western lifestyle have resulted in a paradigm shift in the disease burden to immune-mediated diseases (autoimmunity, allergy, etc.), reflecting less adequate exposure to balanced colonizing bacteria that can influence the development of protective immunologic functions (13). The original “hygiene hypothesis” suggests that increased exposure to microorganisms in large families with multiple siblings and increased infectious diseases resulted in less allergy in younger siblings (14). The hypothesis has evolved to suggest that a modified microbial antigenic exposure early in life as a result of improved public health measures including increased sanitation, vaccination, and antibiotics has changed the way infants adjust immunologically to the extrauterine environment, leading to immune dysfunction and increased inflammatory diseases (15, 16). This observation represents the latest iteration of the so-called “Hygiene Hypothesis” (17). This section outlines what constitutes the ideal circumstances for the appropriate initial colonization process. Ideal conditions for initial colonization results in a symbiotic relationship between the colonizing bacteria and intestinal epithelial and lymphoid tissues (Table 1) and immune and metabolic homeostasis in the infant.
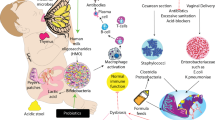
It is now known that the fetus does not reside in a sterile environment. Evidence exists that under normal gestational conditions bacteria from the maternal gut pass into the mother’s blood stream and can ultimately either reside in the placenta or pass through the placenta and enter the amniotic fluid (18, 19). These organisms, far fewer in number than those that inhabit the newborn intestine, can interact with the fetal intestine as the fetus swallows amniotic fluid. Further evidence for this conclusion is that maternal gut organisms have been identified in both meconium and cord blood (20, 21). At present we can only say that microorganisms exist in some normal full-term infants (19, 20) as large prospective studies have not yet been carried out. In addition, their presence is an association and no functional data exist to show cause and effect (21). Data suggesting that intrauterine bacteria interact with the developing fetus have been provided by germ-free animal studies (22) in which immune function at birth differed from that in conventional newborn animals. Additional studies in humans are necessary before a stronger claim can be made. Therefore, bacterial–intestinal “crosstalk” begins in utero and represents the new phase one of crosstalk (e.g., pre-colonization). Unfortunately, there is a lack of studies determining the importance of this process in overall initial colonization and its impact on gut development. Very little information exists regarding bacterial epithelial crosstalk in utero. However, strong evidence exists that initial colonization (phase two) of normal colonization influences immune function in the newborn intestine (23, 24). These observations principally occur in animal studies using germ-free animals. A classic observation made by a Japanese investigator suggests that germ-free animals cannot develop tolerance unless their colonization occurs in a newborn (25, 22). In a recent article (26), germ-free animals developed natural killer cells (NKC) that influenced intestinal (IBD) and lung (asthma) inflammation, whereas neonatal-colonized infants had decreased number of NKC and developed immune homeostasis (22, 26) again suggesting the importance of early colonization in normal immune function and the absence of disease at a later date.
Under optimal colonizing conditions, a full-term, vaginally delivered newborn ingests a healthy bolus of maternal vaginal/colonic bacteria as it passes through the birth canal. This represents probably the most important phase of initial colonization (phase two). However, additional cause and effect studies need to be carried out to confirm this statement. With the introduction of oral feeding (breast milk vs. formula—to be discussed later), the intestinal microbiota is further stimulated (phase three). Then with weaning to solid foods (4–6 months) (phase four), the gut microbiome has additional stimulus, and by about 18 months–3 years (phase five) the gut is colonized with diverse bacteria (>1,000 species), which represent that child’s signature microbiome for life (27) (Table 1).
Optimal colonization also requires exclusive breastfeeding for the first 4–6 months. Breast milk contains nutrients and other factors that optimize the proliferation of so-called “pioneer” bacteria, which uniquely stimulate the development of immune factors that favor immune homeostasis over inflammation (28, 29). Those bacteria stimulated by breastfeeding (e.g., Bacteroides fragilis, Bifidobacteria infantis, and Lactobacillus acidophilus) uniquely stimulate endogenous production of sIgA (30), activation of T-regulatory cells (23, 31), and anti-inflammation (24, 32), necessary steps to assure homeostasis. Factors in breast milk that stimulate gut development include oligosaccharides (prebiotics), sIgA, lactoferrin, etc., all of which influence the proliferation of healthy indigenous microbiota (33, 34). In addition to “pioneer bacteria” stimulated by exclusive breastfeeding, factors in breast milk can interact directly with the newborn intestine and provide passive protection as well as stimulation to active development of host defense. For example, pIgA and defensins can interfere with pathogen attachment and uptake (33); omega 3 fatty acids (35) and TGF-β (36) can activate enterocytes to cause anti-inflammation; and Lactoferrin can interact with the intestine and promote immune homeostasis (28, 33). In contrast, formula-fed newborns produce a more mature microbiota lacking the “pioneer” bacteria uniquely needed for newborn gut development. A study using conventional culture media techniques has shown that breast-fed infants have higher levels of Bifidobacterium infantis and Lactobacillus alphadophilus, whereas formula-fed infants have increased levels of enterococci and enterobacteria (37). Recently, it has been shown that breast milk has its own microbiome (38) that consists of bacteria from the periauricular alveoli, the infant’s mouth, and the maternal gut (39) and contains about 103 microorganisms per cc of breast milk. How maternal gut microbiota enter breast milk is not known but it is thought to be due to an increase in pericellular intestinal transport of maternal gut bacteria taken up orally during the last stages of pregnancy and transmitted to breast milk through macrophages circulating in the breast itself (19). The breast milk macrophages presumably discharge the engulfed bacteria into milk, which is ingested by the suckling neonate. Although the composition of breast milk microbiome differs between full-term and premature infants (40) and during lactation, its contribution to colonizing bacteria and its effect on neonatal gut defenses at this point are not understood.
Protective functions after initial colonization
The gastrointestinal tract must be colonized before adequate immune function can develop. Adequate immune function occurs with a balanced innate and adaptive immune response (41). With regard to innate immunity, epithelial and immune cells must react to the penetration of pathogens or noxious antigens to prevent expression of disease but at the same time not overreact to innocuous antigens or commensal bacteria to produce a chronic inflammatory state. Protection from penetration of the mucosal barrier within the mucosal surface requires microbial–intestinal crosstalk of an adequately colonized intestine. Numerous immunologic and non-immunologic factors contribute to mucosal barrier function (Figure 1) (42). These factors are all stimulated by colonizing bacteria. They include the production of anti-bacterial substances (defensins, etc.) by paneth epithelial cells (43), a mucus barrier stimulated by activated MUC2 genes in goblet cells (44), pattern recognition receptors (e.g., toll-like receptors) on epithelial and immune cells (45), and a specialized epithelium (so-called microfold or M-cell) (46) to mediate direct antigen/bacteria access from the intestinal lumen to appropriate lymphoid elements (intraepithelial lymphocytes, macrophages, and dendritic cells).
The intestinal epithelial-cell barrier. Simple columnar epithelial cells exhibit physical and biochemical adaptations to microbial colonization to maintain barrier integrity including actin-rich microvillar extensions (a), epithelial-cell tight junctions (b), apically attached and secreted mucins that form a glycocalyx (c), and the production of various antimicrobial peptides (d). Specialized intestinal epithelial cells known as M (microfold) cells overlie Peyer’s patches and lymphoid follicles to facilitate luminal sampling. M cells exhibit reduced mucin secretion and have modified apical and basolateral surfaces (e) to promote uptake and transport of luminal contents to professional antigen-presenting cells that inhabit the subepithelial dome (SED) of the Peyer’s patches and lymphoid follicles (f). Specialized dendritic cell (CD) subsets can also extend dendrites between the tight junctions of intestinal epithelial cells to sample luminal contents (g). (Reproduced with permission from ref. 16).
Protective adaptive immunity requires a balanced response of T-helper cells that mediate humoral, cellular, and tolerogenic immunity. Full-term infants are born with a TH2 bias to protect them from rejection in the womb (47). With initial colonization, the TH2 bias becomes a balanced TH1, TH2, TH17, and T-regulatory helper cell response. Germ-free animals retain the TH2 bias, and colonization is required before a balanced T-helper cell response can develop (25). In fact, colonization must occur in the neonatal period and not later in life for adequate adaptive immunity to develop (22). That is in part why normal initial colonization is essential for immune homeostasis.
Not only do colonizing bacteria per se affect intestinal immune development, but metabolites and secreted products from colonizing bacteria interacting with the intestine can help modulate adaptive function. For example, short-chain fatty acids (SCFA) produced by fermentation of high-fiber diets by “pioneer” bacteria can stimulate a proliferation of FOXP3 T-regulatory cells via a receptor, GFR43, on immune cells (Figure 2). This occurs with increased levels of acetate, butyrate, and proprionic acid production (48). These SCFA alone given to germ-free animals can increase immune tolerance in the absence of active bacteria (49). Similarly, a polysaccharide (PSA) on the surface of Bacteroides fragilis organisms given to uncolonized animals can shift the TH2 to a balanced TH1 and TH2 response and activate a T-regulatory cell response via interaction with TLR2 receptors on dendritic cells (50, 51). Other specific colonizing bacteria (Clostridial species) can interact directly with immune cells to stimulate T-regulatory cells (52). Why is this important? T-regulatory cells and immune tolerance must occur as part of the normal adaptive immune response to prevent increase in autoimmune and allergic diseases that represent the new disease paradigm in developed countries (53). An abnormal colonization process (to be discussed later) can interfere with the development of tolerance and predispose to these conditions (54).
Bacterial metabolites fight intestinal inflammation. Commensal bacteria metabolize fiber and generate short-chain fatty acids. These fatty acids are ligand for GPR43 expressed by Treg cells and stimulate their expansion and immune-suppressive properties such as the production of IL-10, thereby controlling proinflammatory responses in the gut. (Reproduced from ref. 29).
Through the examination of the mucosal barrier, we have begun to understand the importance of the mucus coat overlying epithelial cells as an important component of the mucosal barrier (55). We now know that colonizing bacteria can directly stimulate goblet cells to increase the expression of the MUC2 gene, its translation into glycoproteins, and production of mucus to cover the epithelial cell surface (56). There is a direct inverse association between the thickness of the mucus coat and intestinal inflammation, particularly in inflammatory bowel disease (55). The mucus coat has an outer less-dense component containing protective molecules (sIgA and defensins) and commensal anti-pathogen-producing bacteria to protect against pathogen penetration. The inner component of the mucus coat close to the epithelial surface is very dense, contains no microorganisms or protective molecules, and acts as a physical barrier to pathogen penetration to the epithelium, which can stimulate inflammation (57). This important barrier component is under the control of colonizing bacteria via goblet cell mucus production, and its absence has been implicated in chronic intestinal disease. Thus, normal initial colonization is critical to the development of intestinal function and protection against expression of disease.
Dysbiosis
When there is a disruption in the composition of intestinal microbiota, less diversity and altered ratios of large bacteria taxa occur, a condition called “dysbiosis”. Dysbiosis has been associated with disease states. Its occurrence can be the result of a disruption to the intestinal function (altered diet, antibiotics, intestinal infection, etc.) or can occur as a result of underlying disease (inflammation, malignancy, chemotherapeutic injury, etc.). Regardless, dysbiosis has been associated with numerous disease states, as shown by transplanting the microbiota from either an affected individual or an experimental animal with disease into germ-free animals, which has resulted in the phenotypic expression of that disease (58). Accordingly, dysbiosis has become an area of clinical and research interest.
Dysbiosis can occur frequently during the initial colonization of the newborn intestine (Table 2) (59). As the neonatal phase of colonization is critical to the initial colonization (e.g., the ingested bolus of maternal vaginal/colonic bacteria) process, any disruption in this step can lead to dysbiosis. This occurs with maternal dysbiosis during pregnancy, delivery by cesarean section, premature delivery, and excessive use of antibiotics in the perinatal period. Each circumstance leads to an inadequate phase two of colonization, and despite the stimulus of oral feeding and weaning to solid foods, final colonization is delayed until 4–6 years, during which time the infant is more susceptible to infection and to immune-mediated diseases (60).
Delivery by c-section can be lifesaving for a distressed fetus. However, in many developed countries the procedure has also become increasingly used for the convenience of the mother or obstetrician. The incidence of elective c-section can be as high as 40% in certain developed countries (Denmark (61, 62) and Brazil (60). What happens is the fetus is removed from the womb without ingesting maternal vaginal/colonic microbiota, which occurs during the passage through the birth canal. Instead, the infant is exposed to microbiota from the mother’s skin or the hospital environment, and a less diverse intestinal colonization develops. Many epidemiologic studies have reported a direct association between infants born by c-section, particularly elective c-section, and an increased incidence of autoimmune (IBD, type 1 diabetes) obesity and atopic disease (63, 64, 65, 66). However, a recent study from Houston suggests that c-section for clinical emergencies when the fetus has already entered the birth canal and is in distress results in a colonizing microbiota similar to that in vaginally delivered newborns (67) clarifying discrepancies in c-section studies. A study of allergy-prone infants born initially by c-section showed a strikingly increased incidence of atopy compared with vaginal delivery (68). This dysbiosis, caused by elective c-sections, particularly in specific patent populations, represents a risk factor for disease.
Similarly, when antibiotics are given to infants in the perinatal and newborn environment, the initial colonization process is disrupted, leading to a dysbiotic state (69). Again epidemiologic studies have shown a direct association between the use of antibiotics during the first year of life and the expression of asthma during adolescence (70). The more episodes of antibiotic use in the first year of life and the nature of the antibiotic (broad vs. narrow spectrum), the greater the odds ratio for developing asthma and other diseases (71). Similar observations have been noted for early use of antibiotics and inflammatory bowel disease and type 1 diabetes (72). Recently, an experimental study reported early use of penicillin in an animal model, which initially disrupted colonization but despite later reverting to a normal colonization pattern, reverted in the treated newborns gaining excessive body weight, leading to adult obesity (73). The tendency for obesity worsened when the newborn pups were fed a high-fat diet.
A prematurely born infant rapidly passes through the birth canal, preventing the ingestion of a bolus of maternal/vaginal microbiota. Just as in birth by c-section, the intestinal colonization process is dysbiotic. This dysbiosis has been reported in association with an increased incidence of necrotizing enterocolitis (NEC) (74). We have studied this condition and have hypothesized that a dysbiotic colonization in association with immature intestinal host defense (inflammation vs. homeostasis) is likely to be an important risk factor for NEC (75). We have also reported that the more immature the intestine, e.g., 1,500 vs. 1,000 g infants, the more dysbiotic the colonization process, suggesting that intestinal immaturity contributes to intestinal dysbiosis (74). Recently we reported that when premature infants are fed their mother’s expressed breast milk as against formula, the nature of colonization differs strikingly (76). We speculate that the influence of expressed breast milk, known in part to be the basis for protection against NEC, on intestinal colonization may be in part the basis for prevention of disease.
Finally, the nature of maternal microbiota during pregnancy can influence the initial colonization and may contribute to dysbiosis, leading to disease. This has been shown to be true, with mothers gaining excessive weight during pregnancy, leading to obesity (77). Under these conditions, the mother’s intestinal microbiota becomes dysbiotic and this is passed on to the delivered infant either in utero or at the time of delivery or both (78). As a result, the newborn has dysbiotic initial microbiota, which favors increased absorption of nutrients and excessive weight gain. These infants gain at a rate much faster than infants born to mothers without the dysbiosis of excessive weight gain during pregnancy. Furthermore, the nature of the weaning diet (e.g., high fat vs. high protein) can contribute to the infant’s excessive weight gain (79).
These examples of dysbiosis in initial colonization underscore the importance of normal colonization of the newborn in preventing expression of disease during infancy, childhood, and adulthood. It also strongly supports striving to provide appropriate conditions for normal colonization in infancy—full-term vaginal delivery and exclusive breastfeeding for 4–6 months and a balanced diet of weaning food.
How do we combat dysbiosis
The best way to combat dysbiosis is to have a better understanding of the basis for the condition and to recognize how the western lifestyle, particularly the western diet, has affected the composition of microbiota in the gastrointestinal tract (80, 81, 82). Once the cause of dysbiosis is established, addressing the condition will be easier for the physician. Approaches thus far include dietary changes, prebiotics, probiotics, and fecal microbiota transplants (FMT).
Diet
We know that diet can influence the composition of colonizing bacteria from studies on breastfeeding in the neonatal period and its influence on the developing intestine (28). Dietary influence extends beyond that time period. A recent study (83) comparing the composition of intestinal microbiota in children raised in Florence, Italy, on a westernized high-fat/high-protein dietary intake compared with that in children raised in a remote village in Africa ingesting complex carbohydrates and a high-fiber diet showed that the intestinal microbiota in these two populations was strikingly different, as was the nature of disease in these populations. Children in Florence have increased immune-mediated diseases (atopy and autoimmune diseases), whereas children in Africa have principally infectious disease. At this point, this is only an association, and other factors such as genetics and geographic disease issues may be relevant here. Additional cause and effect studies are needed before specific microbial causes can be ascribed to the differences. A clinical study of healthy adults placed on either a high-protein, high-fat, or high-carbohydrate diet for prolonged periods of time showed a striking difference in intestinal microbiota (84). Composition of the diet has also shown changes in bacterial genetic expression and in secretions of microbial metabolites (85). These studies suggest that diet influences the intestinal microbiota and may be involved in health and disease. The infant when weaned to solid foods at 4–6 months should be given a healthy balanced diet consisting of fiber, vegetables, and fruit, and fewer processed foods. A recent study has shown that emulsifiers in processed foods affect the mucus layer overlying epithelial cells in the intestine and allow microbes to cause inflammation leading to increased incidence of metabolic syndrome (85, 86).
Prebiotics
Prebiotics are complex carbohydrates (fructo-oligosaccharides, galacto-oligosaccharide, etc.) that are ingested into the intestine and enter the colon intact, where they are metabolized by resident microbiota (87, 88, 89, 90, 91). The microenvironment created by their metabolism results in the proliferation of health-promoting indigenous microbiota that positively affects the gut function. A proteotypic prebiotic is breast milk, which contains 8% total milk solids such as oligosaccharides and, as stated above, affects intestinal colonization (28). Studies have been carried out using prebiotics to treat dysbiosis associated with disease (allergy, diarrhea, autoimmune disease) (92, 93, 94, 95, 96, 97) with mixed success suggesting that this approach is only partially successful in combating dysbiosis.
Probiotics
Probiotics are live microorganisms isolated from the human intestine that have a health-promoting effect beyond their nutritive value (98). Probiotics have been used extensively in pediatrics to prevent the anticipated dysbiosis seen in allergic disease (99), inflammatory bowel disease (100), irritable bowel syndrome, (92) NEC (98), and obesity (101) with mixed results. This approach will become more successful when we identify the specific disruption in intestinal microbiota associated with a specific disease condition. When prebiotics and probiotics are used in combination (symbiotics), correction of dysbiosis has been somewhat more successful (101).
Fecal microbial transplant
Fecal microbial transplant uses the intestinal microbiota environment from a healthy individual to treat a patient with a known condition (102). It has been primarily carried out in adult patients, and success has been shown principally in patients with recurrent C. difficile colitis (103). Trials, with limited success, have also been tried in patients with inflammatory bowel disease (102), diabetes (104), and obesity (105). Unfortunately, because large multi-center clinical trials with an established protocol and a long-term follow-up component have not been conducted to determine the potential side effects, the enormous microbial community that is “normal” for one individual may potentially be dangerous for the FMT recipient and the composition of an intestinal microbiota from one donor may differ from another. The approach and methods need to be further standardized before the treatment can be considered for routine therapy. When the entire intestinal microbial contents from one person is given to another, trillions of microorganisms and their metabolites are transferred, some of which could potentially be detrimental to the transplant recipient.
Summary and conclusions
This review has underscored the role of initial colonizing intestinal bacteria as an ancillary body organ in the appropriate development of immune and metabolic function in the newborn intestine and in determining health and disease in the infant, child, and adult. Initial colonization occurs at a time when the newborn infant is adapting to the extrauterine environment, and this component of colonizing bacteria on gut function has a lifelong effect. Evidence is provided that dysbiosis, occurring at the time of birth, can have an effect on the expression of disease later in life. Although we do not completely understand the role of intestinal bacterial colonization in phenotypic expression of disease, several current approaches are suggested to combat dysbiosis to prevent or to treat disease. When we have a better understanding of the disruption in intestinal microbiota associated with specific disease conditions, we should be more effective in preventing/treating the disease by preventing dysbiosis.
References
Rautava S, Luoto R, Salminen S et al, Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012;9:565–576.
Shanahan F . Host-flora interactions in inflammatory bowel disease. Inflamm Bowel Dis 2004;10:S16–S24.
Renz H, Brandtzaeg P, Hornef M . The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol 2012;12:9–23.
Martin CR, Caicedo RA, Walker WA Development of intestinal mucosal barrier. In: Neu J, ed. Gastroenterology and Nutrition: Neonatology Questions and Controversies. St Louis, MO: Elsevier/Saunders, 2012: pp 39–58.
Alenghat T, Osborne KC, Saenz SA et al, Histone deacetylase 3 coordinate commensal-bacteria-dependent intestinal homeostasis. Nature 2013;504:153–7.
Zilbauer M, Zellos A, Jenke A et al, Epigenetics in paediatric gastroenterology, hepatology, and nutrition: present trends and future perspectives. J Pediatr Gastroenterol Nutr 2016;62:521–9.
Choi S, Friso S . Epigenetics: a new bridge between nutrition and health. Adv Nutr 2010;1:8–16.
Barua S, Junaid M . Lifestyle, pregnancy and epigenetic effects. Epigenomics 2015;7:85–102.
Takahashi K, Sugi Y, Hosono A et al, Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol 2009;183:6522–29.
Koenig JE, Spor A, Scalfone N et al, Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci 2011;108:4578–85.
Mackie RI, Sghir A, Gaskins HR . Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035s–45s.
Bach JF . The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–20.
Thorburn AN, Macia L, Mackay CR . Diet, metabolites and “Western-Lifestyle” inflammatory bowel disease. Immunity 2014;40:833–42.
Kaplan J, Shi H, Walker W . The role of microbes in developmental immunologic programming. Pediatr Res 2011;69:465–72.
Guarner F, Bourdet-Sicard R, Rook G et al, Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol 2006;3:275.
Wills-Karp M, Santeliz J, Karp C . The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol 2001;1:69–75.
Shanahan F . The gut microbiota—a clinical perspective on lessons learned. Nat Rev Gastroenterol Hepatol 2012;9:609–14.
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J . The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237.
Donnet-Hughes A, Perez PF, Doré J et al, Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nut Soc 2010;69:407–15.
Perez PF, Dore J, Leclerc M et al, Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 2007;119:e725–32.
Ardissone AN, de la Cruz DM, Davis-Richardson A et al, Meconium microbiome analysis identifies bacteria correlated with preterm birth. PLoS ONE 2014;9:e90784.
Karlsson M, Kahu H, Hanson L et al, Neonatal colonization of rats induces immunological tolerance to bacterial antigens. Eur J Immunol 1999;29:109–118.
Schwartz S, Friedberg I, Ivanov IV et al, A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol 2012;13:r3.
Chichlowski M, De Lartigue G, German JB et al, Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastroenterol Nutr 2012;55:321–7.
Sudo N, Sawamura S, Tanaka K et al, The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 1997;159:1739–45.
Olszak T, An D, Blumberg R et al, Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012;6080:489–93.
Weng M, Walker WA . The role of gut microbiota in programming the immune phenotype. J Dev Origin Health Dis 2013;4:203–14.
Walker WA, Iyengar RS . Breast milk, microbiota and intestinal immune homeostasis. Pediatr Res 2015;77:220–8.
Torrow N, Hornef M . The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol 2017;198:557–63.
Jost T, Lacroix C, Braegger CP et al, New insights in gut microbiota establishment in healthy breast fed neonates. PLoS ONE 2012;7:e44595.
Furuta G, Walker WA . Non-immune defense mechanisms of the gastrointestinal tract. In: Blaser MJ, Smith PD, Ravdin JI et al., eds. Infections of the Gastrointestinal Tract. New York, NY: Raven Press Ltd, 1995:89–98.
Rautava S, Walker WA . Breatfeeding—an extrauterine link between mother and child. Breastfeed Med 2009;4:3–10.
Newburg DS, Walker WA . Protection of the neonate by the immune system of developing gut and of human milk. Pediatr Res 2007;61:2–8.
Gregory KE, Walker WA . Immunologic factors in human milk and disease prevention in the preterm infant. Curr Pediatr Rep 2013;1:222–8.
Wijendran V, Brenna JT, Walker WA et al, Long chain poly-unsaturated fatty acids attenuate the IL-1β-induced pro-inflammatory response in human fetal intestinal epithelial cells. Pediatr Res 2015;78:626–33.
Rautava S, Nanthakumar NN, Walker WA et al, Breast milk transforming growth factor-β2 specifically attenuates IL-1β-induced inflammatory responses in the immature human intestine via a SMAD6 and ERK-dependent mechanism. Neonatology 2010;99:192–201.
Yoshioka H, Iseki K, Fujita K . Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics 1983;72:317–21.
Jeurink P, van Bergenhenegouwen J, Martín R et al, Human milk: a source of more life than we imagine. Benef Microbes 2013;4:17–30.
Martín R, Heilig G, Zoetendal E et al, Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol 2007;103:2638–44.
Cabrera-Rubio R, Collado M, Laitinen K et al, The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012;96:544–51.
Martin CR, Walker WA . Innate and mucosal immunity in the developing GI tract: relationship to early and later disease. In: Gleason CA, Devaskar SU, eds. Avery’s Diseases of the Newborn, 9th edn. St Louis, MO: Elsevier/Saunders, 2011:994–1006.
Artis D . Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008;8:411–420.
Ganz T . Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003;3:7190–720.
Johansson MEV, Larsson JMH, Hansson GC . The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc Natl Acad Sci 2011;108:4659–65.
Sansonetti PJ . War and peace at mucosal surfaces. Nat Rev Immunol 2004;4:953–64.
Neutra MR . M cells in antigen sampling in mucosal tissues. Curr Top Microbial Immunol 1999;236:17–32.
Hansen CHF, Nielsen DS, Kverka M et al, Patterns of early gut colonization shape future immune responses of the host. PLoS ONE 2012;7:e34043.
Bollrath F, Powrie F . Feed your Tregs more fiber. Science 2013;341:463–4.
Smith PM, Howitt MR, Panikov N et al, The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73.
Mazmanian SK, Liu CH, Tzianabos AO et al, An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005;122:107–18.
Mazmanian SK, Kasper DL . The love–hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Imnunol 2006;6:849–58.
Atarashi K, Tanoue T, Shima T et al, Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011;331:337–41.
Kiss EA, Vonarbourg C, Kopfmann S et al, Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011;334:1561–5.
Weiner HL, da Cunha AP, Quintana F, Wu H . Oral tolerance. Immunol Rev 2011;241:241–59.
Petersson J, Schreiber O, Hansson GC et al, Importance and regulation of the colonic mucus barrier in a mouse model of colitis. J Physiol Gastrointest Liver Physiol 2011;300:G327–33.
McAuley JL, Linden SK, Png CW et al, MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest 2007;117:2313–24.
Frey A, Giannasca KT, Weltzin R et al, Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells implications for microbial attachment and oral vaccine targeting. J Exp Med 1996;184:1045–59.
Ahern PP, Faith JJ, Gordon JI . Mining the human gut microbiota for effector strains that shape the immune system. Immunity 2014;40:815–23.
Walker WA . Dysbiosis: In: Floch MH, Ringel Y, Walker WA, eds. The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics and Dysbiosis, Ch 25. Elsevier Inc: New York, USA, 2016:227–231.
Jakobsson HE, Abrahamsson TR, Jenmalm MC et al, Decreased gut microbiota diversity, delayed bacteroides colonization and reduced Th1 responses in infants delivered by caesarean section. Gut 2013;63:559–66.
Chu D, Ma J, Prince A et al, Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017;1:1–15.
Sevelsted A, Stokholm J, Bønnelykke K et al, Cesarean section and chronic immune disorders. Pediatrics 2015;135:e92–8.
Mårild K, Stephansson O, Montgomery S et al, Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology 2012;142:39–45.
Decker E, Engelmann G, Findeisen A et al, Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics 2010;125:e1433–40.
Mesquita DN, Barbieri MA, Goldani HA et al, Cesarean section is associated with increased peripheral and central adiposity in young adulthood: cohort study. PLoS ONE 2013;8:e66827.
Thavagnanam S, Fleming J, Bromley A et al, A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy 2008;38:629–633.
Chu D, Ma J, Prince A et al, Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017;23:314–26.
Penders J, Stobberingh EE, van den Brandt et al, The role of the intestinal microbiota in the development of atopic disorders. Allergy 2007;62:1223–36.
Zeissig S, Blumberg RS . Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nature Immunol 2014;12:307–10.
An D, Oh SF, Olszak T et al, Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 201:123–33.
Marra F, Marra CA, Richardson K et al, Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009;123:1003–1010.
Giongo A, Gano KA, Drabb DB et al, Toward defining the autoimmune microbiome for type 1 diabetes. ISME J 2011;5:82–91.
Trasande L, Blustein J, Liu M et al, Infant antibiotic exposures and early-life body mass. Int J Obes 2013;37:16–23.
Zhou Y, Shan G, Sodergren E et al, Longitudinal analysis of preterm intestinal microbiome prior to necrotizing enterocolitis: a case–control study. PLoS ONE 2015;10:e0118632.
Nanthakumar N, Meng D, Goldstein AM et al, The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE 2011;6:e17776.
Gregory KE, Samuel BS, Houghteling P et al, Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 2016;4:68.
Million M, Lagier J-C, Yahav D, Paul M . Gut bacterial microbiota and obesity. Clin Microbiol Infect 2013;19:305–13.
Galley JD, Bailey M, Dush CK, Schoppe-Sullivan S, Christian LM . Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS ONE 2014;9:e-112026.
Baker JL, Michalesen KF, Rasmussen KM, Sorensen TIA . Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr 2004;80:1579–88.
McFarland L . Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open 2014;4:1.
Belizário J, Napolitano M . Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol 2015;6:1050.
Petersen C, Round J . Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 2014;16:1024–33.
De Filippo C, Cavalieri D, Di Paola M et al, Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci 2010;107:14691–6.
Wu GD, Chen J, Hoffmann C et al, Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8.
Faith JJ, Guruge JL, Charbonneau M et al, The long-term stability of the human gut microbiota. Science 2013;341:1237439.
Chassaing B, Koren O, Godrich JK et al, Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92–6.
Zenhom M, Hyder A, de Vrese M et al, Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and preptidoglycan recognition protein 3. J Nutr 2011;141:971–7.
Bakker-Zieikzee AM, van Tol EAF, Kroes H, Alles MS, Kok FJ, Bindels JD . Fecal SIgA secretion in infants fed on pre- or probiotic infant formula. Pediatr Allergy Immunol 2006;17:134–40.
Chichlowski M, DeLartigue G, German JB, Raybould HE, Mills DA . Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastro Nutr 2012;55:321–7.
Newburg DS, Ko JS, Leone S, Nanthakumar NN . Human milk oligosaccharides and synthetic galactosyloligosaccharides contain 3-, 4-4, and 6-galactosyllactose and attenuate inflammation in human T84, NCM-460, and H4 cells and intestinal tissue ex vivo. J Nutr 2016;146:358–67.
Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Letink-Wissink MLG, Kata MB, ven der Merr R . Dietary fructooligosaccarides affect intestinal barrier function in healthy men. J Nutr 2006;136:70–4.
Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G . A mixture of prebiotic oligosaccharides reduces the incident of atopic dermatitis during the first six months of age. Arch Dis Child 2006;91:814–19.
Lindsay JO, Whelan K, Stagg AJ et al, The clinical, microbiological and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006;55:348–55.
Gruber C, van Stujvengerg M, Mosca F et al, Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol 2010;126:791–797.
Boehm G, Jelinek J, Stahl B et al, Prebiotics in infant formulas. J Clin Gastroenterol 2004;38:S76–9.
Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR . Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 2009;29:508–18.
Xia Q, Williams T, Hustead D, Price P, Morrison M, Yu Z . Quantitative analysis of intestinal bacterial populations from term infants fed formula supplemented with fructo-oligosaccharides. J Pediatr Gastroenterol Nutr 2013;55:314–20.
Gareau MG, Sherman PM, Walker WA . Probiotics and the gut microbiota in intestinal health and disease. Nature Rev: Gastroenterol Hepatol 2010;7:503–14.
Kamada N, Chen G, Nunez G . Harnessing pathogen–commensal relations. Nature Med 2012;18:1190–1191.
Mack DR, Michael S, Wei S, McDougall L, Hollingsworth MA . Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol Gastrointest Liver Physiol 1999;39:G941–50.
Thomas DW, Greer FR et al, Clinical report—probiotics and prebiotics in pediatrics. Pediatrics 2010;126:1217–31.
Smits LP, Bouter KEC, de Vos WM, Borody T, Nieuwdorp M . Therapeutic potential of fecal microbiota transplantation. Gastroenterology 2013;145:946–53.
Dutta SK, Girota M, Garg S et al, Efficacy of combined jejuna and colonic fecal microbiota transplantation for recurrent clostridium difficile infection. Clin Gastroenterol Hepatol 2014;12:1572–6.
Hourigan SK, Oliva-Hemker M . Fecal microbiota transplantation in children: a brief review. Pediatr Res 2016;80:2–6.
Knaapen M, Kootte RS, Zoetendal EG et al, Obesity, non-alcoholic fatty liver disease and atherothrombosis: a role for the intestinal microbiota? Clin Microbiol Infect 2013;19:331–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Additional information
STATEMENT OF FINANCIAL SUPPORT
Research Grants have been obtained from the National Institutes of Health, Bethesda, MD, USA (P30 DK040561 and P01 DK033506).
Rights and permissions
About this article
Cite this article
Walker, W. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr Res 82, 387–395 (2017). https://doi.org/10.1038/pr.2017.111
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.111
This article is cited by
-
Metabolic and fecal microbial changes in adult fetal growth restricted mice
Pediatric Research (2024)
-
Is there a placental microbiota? A critical review and re-analysis of published placental microbiota datasets
BMC Microbiology (2023)
-
Mechanisms and regulation of defensins in host defense
Signal Transduction and Targeted Therapy (2023)
-
Edge and modular significance assessment in individual-specific networks
Scientific Reports (2023)
-
Gut microbiota transfer evidence from mother to newborn
European Journal of Pediatrics (2023)