Abstract
Viral infections in the fetus or newborn often involve the central nervous system (CNS) and can lead to significant morbidity and mortality. Substantial progress has been made in identifying interventions decreasing adverse neurodevelopmental outcomes in this population. This review highlights progress in treatment of important viruses affecting the CNS in these susceptible hosts, focusing on herpes simplex virus (HSV), cytomegalovirus (CMV), human immunodeficiency virus (HIV), and enteroviruses. The observation that high-dose acyclovir improves mortality in neonatal HSV disease culminated decades of antiviral research for this disease. More recently, prolonged oral acyclovir was found to improve neurologic morbidity after neonatal HSV encephalitis. Ganciclovir, and more recently its oral prodrug valganciclovir, is effective in improving hearing and neurodevelopment after congenital CMV infection. Increasing evidence suggests early control of perinatal HIV infection has implications for neurocognitive functioning into school age. Lastly, the antiviral pleconaril has been studied for nearly two decades for treating severe enteroviral infections, with newer data supporting a role for this drug in neonates. Identifying common mechanisms for pathogenesis of viral CNS disease during this critical period of brain development is an important research goal, highlighted by the recent emergence of Zika virus as a potential cause of fetal neurodevelopmental abnormalities.
Similar content being viewed by others
Main
Improvement in neonatal mortality has been among the more noteworthy advances in child health over the last 50 y, yet for developmental reasons newborns remain more susceptible to severe disease from infection than older children and adults (1,2,3). Neurologic involvement is also more frequent, and improvements in survival of both premature and term newborns suggest that interventions which address morbidity of these diseases may take on increasing importance. Persisting neurodevelopmental deficits are associated with infection in the early newborn period, particularly in preterm infants (4). High rates of neurologic deficits are described in patients with perinatal insults, with more than 40% of survivors of herpes, encephalitis, or sepsis in the neonatal period having subsequent neurologic impairment (5). In developed countries, neurologic impairment in children is associated with a substantial and increasing demand for inpatient hospital resources (6).
Vaccines have greatly reduced the prevalence of certain viral pathogens in many populations, including measles, mumps, polio, rubella, and varicella (7,8,9,10), all of which can affect the central nervous system (CNS). However, for some medically important viruses for which vaccines are not yet available, antiviral treatment has been found to reduce both mortality and neurologic morbidity. Among these are herpes simplex virus (HSV), cytomegalovirus (CMV), human immunodeficiency virus (HIV), and potentially enteroviruses. This review will focus on studies from the past several decades which have defined the impact of antiviral interventions on neurologic outcomes from these perinatal viral infections involving the CNS ( Table 1 ), and discuss additional research needs in this area.
Acyclovir for Neonatal HSV Infection
Disease consistent with HSV infection has been described as long ago as Hippocrates (11), and the first reported case of presumed neonatal HSV disease appeared in 1935 (12). Antiviral treatment with the thymidine analogue 5-iodo-2′-deoxyuridine was identified as effective for keratitis in the 1960’s (13), and although systemic treatment was attempted in some newborns with apparent success (14), myelosuppressive toxicity and subsequent development of alternative agents ultimately limited its use (15). Through studies driven by Richard J. Whitley and the National Institute of Allergy and Infectious Diseases-sponsored Collaborative Antiviral Study Group (CASG) (16,17,18), the thymidine analogue acyclovir eventually emerged as the treatment of choice for neonatal HSV disease.
A seminal report from the CASG by Kimberlin et al. established the current standard practice of treating neonatal HSV disease involving the CNS or disseminated disease with 20 mg/kg/dose given every 8 h for 21 d (19). This study, conducted between 1989 and 1997, compared data from a trial using a “standard dose” of acyclovir (10 mg/kg/dose) (18) with “intermediate” and “high” doses (15 and 20 mg/kg/dose, respectively). Although designed as a phase II study to evaluate the safety of higher doses of the antiviral, the results demonstrated a significant survival advantage at 24 mo of age in newborns with disseminated disease treated with high-dose acyclovir when compared with the data from the prior study using a standard dose, though survival was not statistically different based on treatment dose for newborns classified as having CNS disease. Importantly, a greater percentage of patients who received the high-dose regimen were assessed to be developmentally normal at 12 mo of age than in the prior group treated with the standard dose, though again this result did not attain statistical significance. Neutropenia was identified as a fairly common adverse effect of acyclovir (21% of patients with localized disease), though it was transient and did not have apparent sequelae.
Despite the observed improvement in neurodevelopment with high-dose vs. standard dose acyclovir, more than two-thirds of patients with CNS disease had at least mild neurologic morbidity at 12 mo of age, with more than one-third of such patients classified as having severe morbidity (19). Although it was not uncommon for physicians to use oral suppressive acyclovir in newborn survivors of neonatal HSV disease, recurrent encephalitis while on suppressive treatment was reported (20), leading to uncertainty about whether this practice provided benefit on a population level (21,22). This question was ultimately answered in 2011, again by a CASG study, in which improved neurologic outcomes were demonstrated in patients with neonatal HSV and a history of CNS involvement, which were treated with oral acyclovir for 6 mo after their initial i.v. course (23). In a decade-long randomized trial, 45 infants from 19 institutions were enrolled to receive either suppressive oral acyclovir or placebo three times daily for 6 mo, with the primary neurodevelopmental outcome an assessment of Bayley Scales of Infant Development score at 12 mo of age. Of the 28 infants in whom this assessment was made, those patients randomized to acyclovir had statistically significantly higher mean scores (88.2 vs. 68.1, P = 0.046), and a higher percentage were reported to have normal neurologic outcomes (69% vs. 33%). This study provided the first controlled data showing that newborns with CNS disease due to HSV have ongoing neurologic injury even after treatment with i.v. acyclovir for 21 d, and that suppressive acyclovir treatment can decrease this injury. However, it is important to recognize that even with suppressive treatment, a significant proportion (~30%) of survivors of neonatal HSV involving the CNS have abnormal neurologic outcomes, suggesting that an improved understanding of the pathogenesis of neurologic injury in this population, and additional treatment interventions, remain important medical goals. Application of molecular diagnostic techniques as a means of providing prognostic information has not proved useful for identifying newborns at risk of abnormal neurologic outcomes as yet; Melvin et al. recently reported for HSV encephalitis in newborns that DNA copy numbers in cerebrospinal fluid or plasma did not correlate with neurodevelopmental outcomes (24). There are still not sufficient data to be certain how to use quantitative PCR to best guide prognosis or treatment in these infections.
Ganciclovir for Congenital CMV Infection
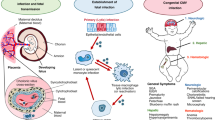
CMV is the most common identified congenital infection in the developed world (25), and is the leading viral cause of sensorineural hearing loss and adverse neurodevelopmental outcomes (26). Either symptomatic or asymptomatic congenital infection may lead to morbidity (27,28,29,30), leading the Institute of Medicine to classify development of vaccines against CMV infection as a level I (most favorable) priority (31). Primary maternal CMV infection carries a 30–40% risk of vertical transmission, with 0.2–2% of secondary infections (reactivated latent infection or reinfection with a new strain in seropositive women) leading to fetal infection (32,33,34,35).
As with acyclovir, the CASG spearheaded the clinical evaluations of antiviral interventions to improve outcomes from symptomatic congenital CMV infection. In the early 1980’s, ganciclovir was identified as an inhibitor of CMV replication in vitro and in animal models (36), ultimately leading to phase I/II and phase II trials to establish safe dosing for newborns with symptomatic congenital infection (37,38,39). Importantly, the phase II trial also demonstrated virologic response and stabilization or improvement in hearing for a subset of infected babies (39), paving the way for a larger, randomized clinical trial to establish efficacy.
The challenging but pivotal CASG trial evaluating efficacy of i.v. ganciclovir for symptomatic congenital CMV infection enrolled 100 patients at 18 centers, and took 8 y to complete (40). In this trial, infants under 1 mo of age with confirmed CMV infection and CNS involvement (microcephaly, intracranial calcifications, abnormal cerebrospinal fluid for age, chorioretinitis, and/or hearing deficits) were randomized to 6 wk of i.v. ganciclovir or no treatment, with the primary outcome measure a comparison of hearing as assessed by brainstem auditory evoked response between baseline and 6 mo follow-up. The difficulty in conducting this study is underscored by the high loss to follow-up: only 42 of the 100 enrolled subjects completed the primary endpoint, with 43 patients completing the secondary endpoint comparing brainstem auditory evoked response at baseline to ≥12 mo follow-up. Despite the limitations of the poor follow-up, this study demonstrated improvement or stabilization in hearing in the “best ear” for 84% of subjects receiving ganciclovir as compared with 59% of controls receiving no treatment (P = 0.06), and no hearing deterioration in subjects receiving ganciclovir as compared with deterioration in 41% of controls (P < 0.01). There was also less deterioration of hearing in the treated group as compared with controls at 12-month follow-up. Neutropenia as a complication of treatment was common, with about half of participants requiring dose adjustments and one needing to be treated for gram negative septicemia.
This cohort of patients was also studied for developmental outcomes after randomization to i.v. ganciclovir vs. no treatment (41). Participants in the study were evaluated using the Denver II developmental test for attainment of developmental milestones at 6 wk, 6 mo, and 12 mo of age. Developmental testing was completed for most of the patients than in the prior study, with more than 70 patients evaluated at any individual time point and 60 patients completing testing at all three time points. Patients in the “no treatment” group were found to have delays in attaining significantly more milestones than those in the ganciclovir group at both 6 mo and 12 mo, an observation that also held for the individual components of the developmental assessment (personal/social, fine motor, gross motor, and language). To avoid the confounding contribution of hearing loss, the investigators also determined that the difference held when the language component was removed from the analyses. These observations support the conclusion that antiviral treatment of symptomatic congenital CMV infection results in a neurodevelopmental benefit. Importantly, however, the investigators noted that even the treatment group had delays at all three time points relative to population norms, so that ganciclovir treatment did not prevent delays in development for most patients.
While these data supported a role for ganciclovir in treatment of symptomatic congenital CMV infection, the risks of prolonged i.v. treatment and drug toxicity (neutropenia) made thorough discussion of risk and benefit critical in evaluating whether any individual patient should receive ganciclovir for cCMV (40,41). The development of the valyl ester of ganciclovir, valganciclovir, allowed for an oral alternative to be considered for this indication, eliminating the need for long-term i.v. access. After a favorable pharmacokinetic profile of valganciclovir oral solution was established in this age group (42), the CASG conducted a placebo-controlled study comparing a 6 wk course of valganciclovir to a 6 mo course of that medication in 96 newborns with symptomatic congenital CMV infection, with longitudinal follow-up out to 24 mo of age (43). Hearing at 6 mo did not differ between the two treatment groups, but hearing at 12 mo was more likely to be normal or improved in the patients who received the longer course of treatment, a benefit which was maintained at 24 mo. Neurodevelopmental outcomes, assessed in this study using the Bayley Scales of Infant and Toddler Development, were modestly improved in the longer duration treatment group as well. Neutropenia (occurring in 19% of participants) was a common adverse effect associated with the initial 6 wk of treatment when both groups were receiving drug, though this was a lower percentage than in the prior study with i.v. ganciclovir (63%) (40). Interestingly, during the subsequent 4.5 mo when one group continued to receive drug while the other received placebo, comparable percentages of patients in the two groups had grade 3 or 4 neutropenia, suggesting that congenital CMV may itself compromise neutrophil production. Together, these studies support the use of 6 mo of valganciclovir for treatment of symptomatic congenital CMV infection. However, as was observed in the studies of prolonged acyclovir in neonatal HSV, a significant proportion of patients had developmental scores below the normative means, supporting the need for ongoing research to understand the interactions between the viral pathogen and the developing nervous system to improve outcomes.
Influence of Controlling Perinatal HIV Infection on Cognition
Peripartum antiretroviral (ARV) prophylaxis to decrease mother-to-child transmission of HIV represents one of the major public health successes of recent times; in the United States, vertical transmission has been reduced from ~25% with no treatment to <1% when maternal viral load is reduced below the limit of detection of sensitive PCR assays (44). However, when vertical transmission of HIV occurs, neurocognitive deficits in affected children are well-described even in the setting of suppressive ARV treatment (45).
Studies of neurocognitive outcomes after perinatal HIV infection which used HIV-exposed, uninfected subjects as controls, did not identify cognitive differences between children with HIV and HIV-exposed uninfected children, unless there was a history of an early AIDS-defining illness (46,47). It was not known from these reports whether early initiation of ARV treatment might provide a neurocognitive benefit: the developing brain may be more susceptible to injury after HIV infection (48), suggesting that minimizing the effects of HIV replication during a developmental window might provide benefit for later cognitive measures.
A prospective cohort study in the Democratic Republic of Congo associated early access to care for HIV-infected children with longitudinal gains in cognitive development; the increase in the mean cognitive score 12 mo after entry into care of infected children between 18 and 71 mo of age was higher among those children presenting “early” for care (defined as prior to eligibility for highly-active ARV treatment, or HAART, using established clinical criteria to determine eligibility for HAART) when compared with patients eligible for HAART at the time of presentation (49). To test whether earlier initiation of ARV treatment would improve neurodevelopmental outcomes of HIV-infected infants, Laughton et al. studied South African infants involved in a study randomizing HIV-infected newborns to early ARV treatment (before 12 wk of age) vs. those in whom ARV treatment was delayed until clinical or immunological progression of HIV (50). The mean age of starting ARV treatment in the delayed group was 31.4 wk, and the mean time on ARV treatment before neurodevelopmental assessments were conducted was 40.9 wk in the early ARV group vs. 18.7 wk in the delayed group (P < 0.01). Neurodevelopmental scores were significantly higher at 11 mo of age for the 64 HIV-infected infants randomized to early ARV treatment when compared with 26 infants for whom ARV was delayed, supporting the ability of early ARV treatment to improve short-term neurodevelopmental outcomes in HIV-infected infants.
A recent study involving two US-based prospective cohorts of patients with either perinatal HIV infection or exposure reported by participants in the Pediatric HIV/AIDS Cohort Study (PHACS) showed that the benefits to neurodevelopment associated with early ARV treatment may persist into school age (51). Crowell et al. showed for 396 perinatally HIV-infected children enrolled in these cohorts that improved neurologic outcomes at school age (mean age of evaluation 9.6 y) were associated with HIV suppression during infancy or early childhood. Again, however, as seen in studies of neonatal HSV infection or congenital CMV infection, even in patients with improved outcomes associated with antiviral treatment, cognitive assessments were on average below population norms.
Severe Enteroviral Infection and Pleconaril
Enteroviruses are well known causes of CNS illness, with poliovirus perhaps the best known historical example (52). As in the case of decreasing perinatal transmission of HIV through ARV treatment, the near eradication of polio by vaccination efforts represents another significant achievement of modern global public health (53). Despite this success, recent years have seen outbreaks of enterovirus infections causing CNS disease, including enterovirus 71 (54,55) and enterovirus D68 (56,57). Although neurologic disease and neurodevelopmental sequelae in these recent outbreaks are more commonly reported in young children than newborns (55,56,57,58,59), newborns and infants are at higher risk of poor outcomes after enteroviral infection than older children or adults (60,61). There are no licensed antiviral agents for treatment of severe enteroviral disease.
Pleconaril was initially developed as an inhibitor of uncoating and attachment of picornaviruses, including rhinovirus and enterovirus, to host cell receptors (62,63). Administration of pleconaril to 38 patients of a range of ages with severe enterovirus infections under a compassionate use protocol led to clinical, virologic, and radiologic responses in the majority of individuals, including five of six neonates treated for enteroviral sepsis (64). A prior phase I study in newborns with presumed viral sepsis showed no apparent adverse effects, and established a dose for further clinical studies in this population (65).
The pharmacokinetics and efficacy of pleconaril in infants <1 y of age with meningitis due to enterovirus infection was evaluated in a double blind and placebo-controlled trial conducted through the CASG (66). This trial was unable to demonstrate virologic or clinical efficacy, which was attributed to the generally self-limited and clinically benign course of enteroviral meningitis in the population studied. However, in another CASG-organized study, a double blind and randomized controlled trial of pleconaril in newborns with enteroviral sepsis occurring prior to 15 d of life enrolled 61 subjects at 19 centers over more than 10 y period (67). The primary study endpoint was the percentage of patients with a positive viral culture from the oropharynx 5 d after initiating study drug, with secondary and tertiary endpoints including virologic, laboratory, and clinical measures. Patients treated with pleconaril had had negative viral cultures and PCR at multiple anatomic sites more quickly than the placebo group, and data suggested improved survival among those who received pleconaril. The potential for efficacy of pleconaril in this population supports further development of this medication for this indication. Although the study did not evaluate neurologic outcomes of patients, the high concentrations achieved by pleconaril in brain tissue (66) suggest that evaluation of this endpoint could be embedded into future studies with this drug or others with potential efficacy against enterovirus, if their development continues (68).
Emerging Viral Infections with Potential to Affect Neurodevelopment in Newborns
The recent Zika virus epidemic in Brazil, and the association between infection during pregnancy and brain anomalies including microcephaly (69), has attracted widespread attention in the United States and other countries which harbor the Aedes mosquito vector. Vector-borne arboviruses are important causes of viral CNS infection in developing countries (70,71), with newborn disease generally rarely reported. In the United States, congenital West Nile virus infection leading to significant neurologic damage was described soon after the epidemic started in the late 1990’s (72), and CNS involvement in newborns has been described for chikungunya (73) and Japanese encephalitis virus (74,75). Perinatal infection with California group virus (LaCrosse subtype) has been associated with subsequent adverse neurocognitive outcomes (76). Perinatal transmission of dengue appears to be uncommon, and the degree to which it is associated with severe outcomes in newborns is unclear (77,78,79,80). The global spread of vector-borne viruses, and their potential for CNS involvement, highlights the importance of improving our understanding of underlying pathophysiologic mechanisms which may compromise brain development when viral CNS infection may occur during particularly critical developmental periods.
Common Themes in Pathogenesis of Viral CNS Disease in the Newborn
Differences in immunity have been identified between newborns and adults that may contribute to the enhanced susceptibility of newborns to infection (1,2,3). Identifying shared mechanisms that may be used by diverse viral pathogens to mediate CNS disease during critical periods of brain development could lead to interventions specifically modulating disease in this age group. For example, using models of HSV encephalitis, we have shown that there may be differences between newborn and adult animals in cellular tropism of virus within the CNS (81,82), in production of inflammatory mediators in brain tissue (82,83), and in intrinsic cellular antiviral responses in the CNS (84). This latter study in particular provides an important example of the need to distinguish newborns from older animals in pathogenesis studies: we found that autophagy during HSV infection the newborn brain was significantly less important than in adults for protection from mortality, suggesting that proposed pharmacologic strategies for HSV encephalitis to augment autophagy (85) might be protective in older but not younger brains.
Additional important common mechanistic themes emerge from considering perinatal CNS infection with these different viruses. Direct infection of neurons does not appear to be required for adverse cognitive outcomes, as neither CMV nor HIV primarily target neurons, and there may also be a contribution to disease in newborns from glial cell infection with HSV (81) and enterovirus (86). Results from murine models of neonatal HSV (83) and congenital CMV (87,88) infection suggest that host inflammation may induce at least some of the observed CNS pathology, as opposed to direct effects of virus infection and replication. However, although inflammatory responses may contribute to CNS disease in the young host, the clinical studies discussed in this review are evidence that persisting viral replication also leads to ongoing damage, as for at least the herpesviruses, prolonged treatment with antivirals improves neurodevelopmental outcomes (23,43). Infection of neural progenitors and immature neurons may be important to outcomes in the developing host, particularly cells originating from periventricular areas near the choroid plexus, with evidence of infection in these cells observed in model systems for HSV (82), CMV (89,90,91), HIV (92,93), and enteroviruses (94,95,96). There is also evidence that Zika virus can target human neural stem and progenitor cells in the fetal cortex, potentially explaining the defects in brain development associated with infection in utero (97). Further understanding of age-dependent differences in host-pathogen interactions may lead to interventions that target specific, developmentally-regulated pathways of host responses or viral replication.
The clinical trials of antiviral treatment for viral CNS infection in newborns highlighted in this review share an important further similarity: in general, studies of these relatively uncommon diseases in newborns are very difficult to conduct. The long duration required to enroll sufficient numbers to provide statistical power, and the long follow-up periods needed to assess certain developmental outcomes, frequently led to limitations in assessing outcome measures due to loss to follow-up (19,23,40), and one study even needed to be terminated prior to completing accrual (67). Longitudinal follow-up in these populations may be challenged by demographics, as risk factors for neonatal infection with many of the pathogens discussed include socioeconomic factors associated with difficulty in accessing health care. Multicenter studies are required to accrue sufficient numbers of patients, and large organizational structures such as the CASG, Comprehensive International Program for Research in AIDS (involved in the infant HIV neurodevelopmental study (50)), and PHACS network are critical to successful completion.
Global Research Needs for Perinatal CNS Infections
Despite the advances in the treatment of perinatal CNS infection discussed in this review, the persisting neurologic deficits associated with such insults result in a significant and rising burden of disease globally (98), putting strain on health care resources of countries at all economic levels (6,99). All tiers of research—basic, translational, clinical, and population-level – need continued financial investment to facilitate development and implementation of effective interventions (100). Continued support for organizations capable of supporting multisite trials, such as the CASG, the Comprehensive International Program for Research in AIDS, and PHACS, is critical to ensuring the clinical study infrastructure needed to study new interventions to improve newborn outcomes. Bench studies of common and emerging viruses that can affect the developing CNS may promote a better understanding of shared mechanisms of pathogenesis and could identify additional interventions for treatment or prevention.
Statement of Financial Support
No financial assistance was received to support this study.
Disclosure
None.
References
Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr Workshop Ser Pediatr Program 2008;61:183–95.
Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7:379–90.
Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Pediatr 2012;101:120–7.
Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014;14:751–62.
Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 2012;379:445–52.
Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med 2012;9:e1001158.
Papania MJ, Wallace GS, Rota PA, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatr 2014;168:148–55.
McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS ; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013;62(RR-04):1–34.
Wassilak SG, Oberste MS, Tangermann RH, Diop OM, Jafari HS, Armstrong GL. Progress toward global interruption of wild poliovirus transmission, 2010–2013, and tackling the challenges to complete eradication. J Infect Dis 2014;210 Suppl 1:S5–S15.
Shah SS, Wood SM, Luan X, Ratner AJ. Decline in varicella-related ambulatory visits and hospitalizations in the United States since routine immunization against varicella. Pediatr Infect Dis J 2010;29:199–204.
Scott TF, Tokumaru T. Herpesvirus hominis (virus of herpes simplex). Bacteriol Rev 1964;28:458–71.
Hass GM. Hepato-adrenal necrosis with intranuclear inclusion bodies: report of a case. Am J Pathol 1935;11:127–142.5.
Kaufman H, Martola EL, Dohlman C. Use of 5-iodo-2′-deoxyuridine (IDU) in treatment of herpes simplex keratitis. Arch Ophthalmol 1962;68:235–9.
Overall JC Jr, Glasgow LA. Virus infections of the fetus and newborn infant. J Pediatr 1970;77:315–33.
Whitley RJ. Treatment of human herpesvirus infections with special reference to encephalitis. J Antimicrob Chemother 1984;14 Suppl A:57–74.
Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics 1980;66:495–501.
Whitley RJ, Yeager A, Kartus P, et al. Neonatal herpes simplex virus infection: follow-up evaluation of vidarabine therapy. Pediatrics 1983;72:778–85.
Whitley R, Arvin A, Prober C, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious diseases collaborative antiviral study group. N Engl J Med 1991;324:444–9.
Kimberlin DW, Lin CY, Jacobs RF, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 2001;108:230–8.
Fonseca-Aten M, Messina AF, Jafri HS, Sánchez PJ. Herpes simplex virus encephalitis during suppressive therapy with acyclovir in a premature infant. Pediatrics 2005;115:804–9.
Gutierrez K, Arvin AM. Long term antiviral suppression after treatment for neonatal herpes infection. Pediatr Infect Dis J 2003;22:371–2.
Frenkel LM. Challenges in the diagnosis and management of neonatal herpes simplex virus encephalitis. Pediatrics 2005;115:795–7.
Kimberlin DW, Whitley RJ, Wan W, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med 2011;365:1284–92.
Melvin AJ, Mohan KM, Schiffer JT, et al. Plasma and cerebrospinal fluid herpes simplex virus levels at diagnosis and outcome of neonatal infection. J Pediatr 2015;166:827–33.
Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother 2009;63:862–7.
Griffiths P, Plotkin S, Mocarski E, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine 2013;31 Suppl 2:B197–203.
Pass RF, Stagno S, Myers GJ, Alford CA. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 1980;66:758–62.
Williamson WD, Percy AK, Yow MD, et al. Asymptomatic congenital cytomegalovirus infection. Audiologic, neuroradiologic, and neurodevelopmental abnormalities during the first year. Am J Dis Child 1990;144:1365–8.
Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 1992;90:862–6.
Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 1992;11:93–9.
Institute of Medicine. Vaccines for the 21st Century. Washington, DC: National Academy Press; 2000.
Carlson A, Norwitz ER, Stiller RJ. Cytomegalovirus infection in pregnancy: should all women be screened? Rev Obstet Gynecol 2010;3:172–9.
Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am 2013;60:335–49.
Mendelson E, Aboudy Y, Smetana Z, Tepperberg M, Grossman Z. Laboratory assessment and diagnosis of congenital viral infections: Rubella, cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), parvovirus B19 and human immunodeficiency virus (HIV). Reprod Toxicol 2006;21:350–82.
Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001;344:1366–71.
Matthews T, Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis 1988;10 Suppl 3:S490–4.
Trang JM, Kidd L, Gruber W, et al. Linear single-dose pharmacokinetics of ganciclovir in newborns with congenital cytomegalovirus infections. NIAID Collaborative Antiviral Study Group. Clin Pharmacol Ther 1993;53:15–21.
Zhou XJ, Gruber W, Demmler G, et al. Population pharmacokinetics of ganciclovir in newborns with congenital cytomegalovirus infections. NIAID Collaborative Antiviral Study Group. Antimicrob Agents Chemother 1996;40:2202–5.
Whitley RJ, Cloud G, Gruber W, et al. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: results of a phase II study. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 1997;175:1080–6.
Kimberlin DW, Lin CY, Sánchez PJ, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003;143:16–25.
Oliver SE, Cloud GA, Sánchez PJ, et al.; National Institute of Allergy, Infectious Diseases Collaborative Antiviral Study Group. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 2009;46 Suppl 4:S22–6.
Kimberlin DW, Acosta EP, Sánchez PJ, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis 2008;197:836–45.
Kimberlin DW, Jester PM, Sánchez PJ, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015;372:933–43.
American Academy of Pediatrics Committee on Pediatric AIDS. HIV testing and prophylaxis to prevent mother-to-child transmission in the United States. Pediatrics 2008;122:1127–34.
Paramesparan Y, Garvey LJ, Ashby J, Foster CJ, Fidler S, Winston A. High rates of asymptomatic neurocognitive impairment in vertically acquired HIV-1-infected adolescents surviving to adulthood. J Acquir Immune Defic Syndr 2010;55:134–6.
Smith R, Malee K, Leighty R, et al.; Women and Infants Transmission Study Group. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics 2006;117:851–62.
Smith R, Chernoff M, Williams PL, et al.; Pediatric HIV/AIDS Cohort Study (PHACS) Team. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J 2012;31:592–8.
Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol 2014;24:316–31.
Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr 2009;52:636–42.
Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 2012;26:1685–90.
Crowell CS, Huo Y, Tassiopoulos K, et al.; PACTG 219C Study Team and the Pediatric HIVAIDS Cohort Study (PHACS). Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS 2015;29:295–304.
Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 2010;172:1213–29.
Garon JR, Cochi SL, Orenstein WA. The challenge of global poliomyelitis eradication. Infect Dis Clin North Am 2015;29:651–65.
Lee TC, Guo HR, Su HJ, Yang YC, Chang HL, Chen KT. Diseases caused by enterovirus 71 infection. Pediatr Infect Dis J 2009;28:904–10.
Chang LY, Huang LM, Gau SS, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med 2007;356:1226–34.
Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015;385:1662–71.
Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): a retrospective cohort study. Lancet Infect Dis 2015;15:671–82.
Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med 1999;341:936–42.
Pfeiffer HC, Bragstad K, Skram MK, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill 2015;20:21062.
Abedi GR, Watson JT, Pham H, Nix WA, Oberste MS, Gerber SI. Enterovirus and human parechovirus surveillance–United States, 2009–2013. MMWR Morb Mortal Wkly Rep 2015;64:940–3.
Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J 1993;12:820–4.
Pevear DC, Fancher MJ, Felock PJ, et al. Conformational change in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol 1989;63:2002–7.
Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother 1999;43:2109–15.
Rotbart HA, Webster AD ; Pleconaril Treatment Registry Group. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis 2001;32:228–35.
Kearns GL, Bradley JS, Jacobs RF, et al. Single dose pharmacokinetics of pleconaril in neonates. Pediatric pharmacology research unit network. Pediatr Infect Dis J 2000;19:833–9.
Abzug MJ, Cloud G, Bradley J, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr Infect Dis J 2003;22:335–41.
Abzug MJ, Michaels MG, Wald E, et al.; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. A randomized, double-blind, placebo-controlled trial of pleconaril for the treatment of neonates with enterovirus sepsis. J Pediatric Infect Dis Soc 2016;5:53–62.
Modlin JF. Treatment of neonatal enterovirus infections. J Pediatric Infect Dis Soc 2016;5:63–4.
Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med 2016;374:1981–7.
Depoortere E, Kavle J, Keus K, Zeller H, Murri S, Legros D. Outbreak of West Nile virus causing severe neurological involvement in children, Nuba Mountains, Sudan, 2002. Trop Med Int Health 2004;9:730–6.
Venturi G, El-Sawaf G, Arpino C, et al. Arboviral infections in Egyptian and Sardinian children and adults with aseptic meningitis and meningo-encephalitis. Scand J Infect Dis 2009;41:898–9.
Alpert SG, Fergerson J, Noël LP. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol 2003;136:733–5.
Robin S, Ramful D, Le Seach’ F, Jaffar-Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol 2008;23:1028–35.
Mathur A, Tandon HO, Mathur KR, Sarkari NB, Singh UK, Chaturvedi UC. Japanese encephalitis virus infection during pregnancy. Indian J Med Res 1985;81:9–12.
Chaturvedi UC, Mathur A, Chandra A, Das SK, Tandon HO, Singh UK. Transplacental infection with Japanese encephalitis virus. J Infect Dis 1980;141:712–5.
Gauld LW, McMillan BC, Sinha SK. Relationship of California group virus infection and mental retardation: seroepidemiological observations. J Ment Defic Res 1979;23:63–8.
Poli L, Chungue E, Soulignac O, Gestas P, Kuo P, Papouin-Rauzy M. [Materno-fetal dengue. Apropos of 5 cases observed during the epidemic in Tahiti (1989)]. Bull Soc Pathol Exot 1991;84(5 Pt 5):513–21.
Kerdpanich A, Watanaveeradej V, Samakoses R, et al. Perinatal dengue infection. Southeast Asian J Trop Med Public Health 2001;32:488–93.
Phongsamart W, Yoksan S, Vanaprapa N, Chokephaibulkit K. Dengue virus infection in late pregnancy and transmission to the infants. Pediatr Infect Dis J 2008;27:500–4.
Chye JK, Lim CT, Ng KB, Lim JM, George R, Lam SK. Vertical transmission of dengue. Clin Infect Dis 1997;25:1374–7.
Kopp SJ, Karaba AH, Cohen LK, Banisadr G, Miller RJ, Muller WJ. Pathogenesis of neonatal herpes simplex 2 disease in a mouse model is dependent on entry receptor expression and route of inoculation. J Virol 2013;87:474–81.
Wilcox DR, Folmsbee SS, Muller WJ, Longnecker R. The type I interferon response determines differences in choroid plexus susceptibility between newborns and adults in herpes simplex virus encephalitis. MBio 2016;7:e00437–16.
Kopp SJ, Ranaivo HR, Wilcox DR, Karaba AH, Wainwright MS, Muller WJ. Herpes simplex virus serotype and entry receptor availability alter CNS disease in a mouse model of neonatal HSV. Pediatr Res 2014;76:528–34.
Wilcox DR, Wadhwani NR, Longnecker R, Muller WJ. Differential reliance on autophagy for protection from HSV encephalitis between newborns and adults. PLoS Pathog 2015;11:e1004580.
Shoji-Kawata S, Sumpter R, Leveno M, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 2013;494:201–6.
Zhang X, Zheng Z, Shu B, et al. Human astrocytic cells support persistent coxsackievirus B3 infection. J Virol 2013;87:12407–21.
Bradford RD, Yoo YG, Golemac M, Pugel EP, Jonjic S, Britt WJ. Murine CMV-induced hearing loss is associated with inner ear inflammation and loss of spiral ganglia neurons. PLoS Pathog 2015;11:e1004774.
Cekinović D, Golemac M, Pugel EP, et al. Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. J Virol 2008;82:12172–80.
Kawasaki H, Kosugi I, Arai Y, Tsutsui Y. The amount of immature glial cells in organotypic brain slices determines the susceptibility to murine cytomegalovirus infection. Lab Invest 2002;82:1347–58.
Poland SD, Bambrick LL, Dekaban GA, Rice GP. The extent of human cytomegalovirus replication in primary neurons is dependent on host cell differentiation. J Infect Dis 1994;170:1267–71.
van den Pol AN, Reuter JD, Santarelli JG. Enhanced cytomegalovirus infection of developing brain independent of the adaptive immune system. J Virol 2002;76:8842–54.
Petito CK, Chen H, Mastri AR, Torres-Munoz J, Roberts B, Wood C. HIV infection of choroid plexus in AIDS and asymptomatic HIV-infected patients suggests that the choroid plexus may be a reservoir of productive infection. J Neurovirol 1999;5:670–7.
Burkala EJ, He J, West JT, Wood C, Petito CK. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS 2005;19:675–84.
Feuer R, Ruller CM, An N, et al. Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period. J Virol 2009;83:9356–69.
Puccini JM, Ruller CM, Robinson SM, et al. Distinct neural stem cell tropism, early immune activation, and choroid plexus pathology following coxsackievirus infection in the neonatal central nervous system. Lab Invest 2014;94:161–81.
Feuer R, Mena I, Pagarigan RR, Harkins S, Hassett DE, Whitton JL. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. Am J Pathol 2003;163:1379–93.
Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 2016;18:591–6.
Silberberg D, Anand NP, Michels K, Kalaria RN. Brain and other nervous system disorders across the lifespan—global challenges and opportunities. Nature 2015;527:S151–4.
Birbeck GL, Meyer AC, Ogunniyi A. Nervous system disorders across the life course in resource-limited settings. Nature 2015;527:S167–71.
John CC, Carabin H, Montano SM, Bangirana P, Zunt JR, Peterson PK. Global research priorities for infections that affect the nervous system. Nature 2015;527:S178–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muller, W. Treatment of perinatal viral infections to improve neurologic outcomes. Pediatr Res 81, 162–169 (2017). https://doi.org/10.1038/pr.2016.191
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.191
This article is cited by
-
The University of Zimbabwe College of Health Sciences (UZ-CHS) BIRTH COHORT study: rationale, design and methods
BMC Infectious Diseases (2020)