Abstract
Background:
Cannabidiol (CBD), a nonpsychoactive cannabinoid, has shown neuroprotective actions after neonatal hypoxia-ischemia (HI) in animals. We wanted to further explore the effects of CBD, alone and in conjunction with hypothermia, in a piglet model of global HI.
Methods:
Fifty-five anesthetized newborn piglets were randomized to either controls (n = 7) or HI (n = 48) by ventilation with 8% O2 until mean arterial blood pressure reached 20 mmHg and/or base excess reached −20 mmol/l. After resuscitation piglets were randomized to either: vehicle (VEH), CBD 1mg/kg, VEH+hypothermia (H) or CBD 1mg/kg+H (each n = 12). Piglets were euthanized 9.5 h after HI and plasma, urine, cerebrospinal fluid, and brain tissue were sampled for analysis.
Results:
HI induced global damage with significantly increased neuropathology score, S100B in cerebrospinal fluid, hippocampal proton magnetic resonance spectroscopy biomarkers, plasma troponin-T, and urinary neutrophil gelatinase-associated lipocalin. CBD alone did not have any significant effects on these parameters while CBD+H reduced urinary neutrophil gelatinase-associated lipocalin compared with VEH+H (P < 0.05). Both hypothermic groups had significantly lower glutamate/N-acetylaspartate ratios (P < 0.01) and plasma troponin-T (P<0.05) levels compared with normothermic groups.
Conclusion:
In contrast to previous studies, we do not find significant protective effects of CBD after HI in piglets. Evaluation of CBD in higher doses might be warranted.
Similar content being viewed by others
Main
Perinatal hypoxia-ischemia (HI) is a major cause of death and neurodisability in children worldwide (1,2). Disordered brain function as a consequence of HI is referred to as hypoxic ischemic encephalopathy (HIE) and occurs at a rate of 1:2 per 1,000 live births in high-income countries and up to 26 per 1,000 live births in low and middle-income countries (3). In preventing HIE the initial handling in the delivery room is important (4). Further, the only available intervention is therapeutic hypothermia which improves survival and reduces neurodisability (5). Still only one of eight infants who meet current eligibility criteria benefit from the treatment (5,6) and in low and middle-income countries, where the burden of HIE is greatest, therapeutic hypothermia has not yet been shown to reduce mortality or morbidity (7,8). Finding new and adjuvant neuroprotective strategies are therefore important (8).
Cannabidiol (CBD), a nonpsychoactive cannabinoid, has over the last decade emerged as a promising neuroprotectant in animal models of stroke, neurodegenerative disease and neurotoxicity (9,10,11). Meta-analysis of preclinical studies on stroke found that CBD reduced infarct volume and improved functional outcome (12).
CBD has also shown neuroprotective abilities in animal models of neonatal HIE (13,14,15,16,17) and recently received orphan drug designation status for this condition. It is believed to exert neuroprotection by modulating several of the key pathogenic processes involved in HIE such as excitotoxicity, inflammation, oxidative stress, blood-brain barrier integrity, and apoptosis. The exact molecular targets of CBD are not yet established, but neuroprotective effects are probably not mediated through the endocannabinoid system and classical cannabinoid receptors, but rather through other molecular pathways (18).
Three studies have previously examined the effects of CBD in piglet models of neonatal HI. CBD 0.1 mg/kg i.v. improved short-term cerebral hemodynamics and brain metabolic activity as well as reduced severity of brain edema and seizure burden after HI (15). CBD reduced levels of S100B and neuron specific enolase in cerebrospinal fluid (CSF) 6 h after HI as well as improved neurobehavioral score at 72 h (16). CBD 1mg/kg mitigated the increase in the proton magnetic resonance spectroscopy (H+MRS) biomarkers lactate/n-acetylaspartate (Lac/NAA) and glutamate/n-acetylaspartate ratios (Glu/NAA) and reduced protein carbonylation as a sign of antioxidative effects (17). All three studies found reduced neuronal damage on histopathological examination in piglets that received CBD (15,16,17).
CBD is believed to be safe and well tolerated in adults, however safety studies in children are still lacking (19). In the above mentioned piglet studies no adverse effects were found after doses up to 1 mg/kg intravenously (i.v.).
Until now CBD has only been studied in one large animal model of neonatal HI. Before considering clinical trials it is essential to further evaluate the safety and efficacy of CBD in different preclinical models. Also in combination with therapeutic hypothermia since this has not been done before. The piglet is well suited for these kind of studies due to its similarity to the human in brain anatomy, growth, and development and piglet models have previously contributed significantly to our understanding of the pathophysiology of HIE and possible new interventions (23, 24). Our goal was to further investigate possible short-term protective effects of CBD, alone and in combination with therapeutic hypothermia, in a well established piglet model of neonatal HI (20,21,22). Compared with previous studies we induce a more severe and global hypoxic-ischemic insult.
Results
There was no difference in postnatal age, body weight or baseline physiological, and biochemical variables between study groups. The severity of the HI insult, as measured by base excess, drop in mean arterial blood pressure (MABP) and lactate, was similar among groups ( Table 1 ). Two piglets in the CBD group had a rapid fall in MABP which explains the nonsignificant difference in BE and mean duration of HI in this group. From the initiation of treatment until end of study the heart rate was higher in the normothermic piglets compared with hypothermic as expected. Apart from this there were no significant differences between the groups during study period ( Table 1 ). A total number of four piglets suffered fatal cardiac arrest during the study (7.5% mortality), all in the period immediately after HI and before treatment was initiated. These piglets have been excluded from analysis.
Plasma Concentrations CBD
CBD was detectable in plasma 9.5 h after i.v. administration in all CBD treated animals. Levels were similar in normothermic (CBD 15.5 (± 10.9) µg/l) and hypothermic animals (CBD + H 16.1(± 8.1) µg/l).
Neuropathological Examination
HI induced significant damage as measured by the percentage of piglets with evidence of neuronal damage (VEH 83% vs. controls 14%, P = 0.0015) ( Figure 1a ) and mean neuropathology score (VEH 1.0 ± 0.6 vs. controls 0.1 ± 0.2, P = 0.002) ( Figure 1b ). There were no significant differences between CBD and VEH groups and the lower percentage of damaged piglets in hypothermic groups was not significant (VEH 83% vs. VEH+H 50%, P = 0.08) ( Figure 1a ). The degree of damage ranged from mild to total autolysis of the cerebrum ( Figure 1b ). All piglets noted to have macroscopic autolysis at autopsy also received the highest neuropathology scores and the score correlated well with S100B in cerebrospinal fluid and hippocampal Lac/NAA ratio (P < 0.0001, r2 = 0.83) ( Figure 1c ). Analysis was also performed after excluding piglets with autolysis of the brain and this did not produce significant changes in the results.
Neuropathological analysis of brain from newborn piglets 9.5 h after hypoxia-ischemia treated with vehicle (VEH, white bar), cannabidiol (CBD, gray bar), vehicle + hypothermia (VEH+H, white bar) or cannabidiol + hypothermia (CBD+H, gray bar). (a) Percentage of animals per group with evidence of neuronal damage. (b) Degree of damage (neuropathological score), data presented as individual scores (dots), grey lines representing group mean. Dotted line representing mean value in controls. (c) Scatter plot of neuropathological score (x-axis) vs log S100B (black dots) and Lac/NAA ratio (grey dots) (y-axis). Multiple regression: P < 0.0001, r2 = 0.83.
S100B in Cerebrospinal Fluid
Levels of S100B in CSF were significantly increased 9.5 h after HI (VEH 6.9 ± 6.2 ng/ml vs. control 2.3 ± 0.6 ng/ml, P = 0.004), but there were no significant effects of CBD or hypothermia on these levels ( Figure 2a ).
Biomarkers in different body fluids 9.5 h after hypoxia-ischemia in newborn piglets treated with vehicle (VEH, white bar), cannabidiol (CBD, gray bar), vehicle + hypothermia (VEH+H, white bar) or cannabidiol + hypothermia (CBD+H, gray bar). S100B in CSF (a), plasma Troponin-T (b) and urinary Neutrophil Gelatinase Associated Lipocalin (uNGAL) (c). Results expressed as mean with SD. Dotted line representing mean value in controls. *P < 0.05.
Biomarkers of Myocardial and Renal Injury
Plasma troponin-T was significantly elevated 9.5 h after HI (VEH 195 ± 150 ng/l vs. controls 19 ± 5 ng/l, P < 0.0001). There were no differences between CBD and VEH groups, but significantly lower levels in hypothermic groups compared with normothermia (VEH 195 ± 150 ng/l vs. VEH+H ng/l 80 ± 42, P = 0.03 and CBD 274 ± 167 vs. CBD+H 146 ± 74, P = 0.02) ( Figure 2b ).
HI significantly increased levels of urinary neutrophil gelatinase-associated lipocalin (uNGAL) (VEH 909 ± 698 ng/ml vs. control 139 ± 124 ng/ml, P = 0.001). Levels in normothermic groups were similar, but in contrast piglets receiving CBD+H had significantly lower levels than VEH + H (392 ± 491 ng/ml vs. 945 ± 648 ng/ml respectively, P = 0.04) ( Figure 2c ).
Proton Magnetic Resonance Spectroscopy
HI led to significantly increased Lac/NAA ratio (VEH 2.5 ± 0.5 vs. control 1.9 ± 0.4, P = 0.02) ( Figure 3a ) and Glu/NAA ratio (VEH 1.2 ± 0.2 vs. control 0.9 ± 0.1, P = 0.04) ( Figure 3b ) as well as reduced NAA (VEH 3.5 ± 1.9 vs. control 5.9 ± 2.7, P = 0.04) ( Figure 3c ). There were no differences between CBD and VEH groups. Lower ratios were found in both hypothermic groups, however only significant for the Glu/NAA ratio (VEH 1.2 ± 0.2 vs. VEH + H 0.7 ± 0.3, P = 0.004 and CBD 1.2 ± 0.4 vs. CBD + H 0.8 ± 0.2, P = 0.02) ( Figure 3b ).
Proton magnetic resonance spectroscopy (H+MRS) biomarkers in hippocampal tissue collected 9.5 h after HI from newborn piglets treated with vehicle (VEH, white bar), cannabidiol (CBD, gray bar), vehicle + hypothermia (VEH+H, white bar) or cannabidiol + hypothermia (CBD+H, gray bar). Lactate/NAA ratio (a), glutamate/NAA ratio (b) and NAA (c). Data presented as median with IQR a or mean with SD b,c. Dotted line representing mean/median value in controls. *P < 0.05, **P < 0.005.
Gene and Protein Expression in Cortex and Hippocampus
mRNA expression of IL-6, IL1-b, TNFα, Bax, Bcl-2, and Caspase-3 in cortex at end study was not significantly altered by HI ( Figure 4 ). Neither was protein expression of IL-6, IL1-b, and TNFα in cortex or hippocampus ( Table 2 ).
mRNA expression in brain samples from prefrontal cortex collected 9.5 h after hypoxia-ischemia in newborn piglets treated with vehicle (VEH, white bar), cannabidiol (CBD, gray bar), vehicle + hypothermia (VEH+H, white bar) or cannabidiol + hypothermia (CBD+H, gray bar). Data expressed as median fold change relative to median in controls (dotted line).
Neuronal Oxidative Stress
We found no significant differences between HI and controls in urinary levels of neuroprostanes or neurofuranes at 210 min post-HI or at end of study ( Table 3 ).
Discussion
In contrast to previous animal studies on CBD in the setting of neonatal HI we do not find evidence of short-term protective effects. Compared with VEH, CBD did neither prevent neuronal and astrocyte damage, nor improve cerebral energy metabolism or mitigate myocardial injury. Combining CBD and therapeutic hypothermia did not produce unwanted effects, but neither offered significant synergy.
The degree and frequency of early neuronal damage on neuropathological analysis was similar among CBD and VEH groups ( Figure 1a , b ). We are aware of the limitations of assessing histological damage at such an early time-point, however early signs of brain damage has previously been demonstrated in similar models (17,22,23) and we found a good correlation between neuropathological score and both S100B in cerebrospinal fluid and hippocampal Lac/NAA ratio ( Figure 1c ) that in clinical studies are linked to long-term outcome (24,25).
S100B reflects astrocytic injury and blood-brain barrier integrity and correlate with outcome in asphyxiated infants at 15 mo (24). In contrast to the study of Lafuente et al. (16), CBD did not affect levels of S100B in our piglets ( Figure 3 ).
The Lac/NAA ratio measured in deep gray matter by H+MRS is considered an accurate quantitative MR biomarker within the neonatal period for prediction of neurodevelopmental outcome after HIE (25). In contrast to the study of Pazos et al. (17), CBD did not mitigate the short-term rise in hippocampal Lac/NAA or Glu/NAA ratios compared with vehicle ( Figure 3 ). Therapeutic hypothermia mitigated the increase in both ratios, although only significant for the Glu/NAA ratio ( Figure 3b ). This might be interpreted as reduced exitotoxicity in hypothermic animals. In addition to the above mentioned ratios, we present data of NAA since this metabolite is more robustly detected by H+MRS compared with e.g., lactate (26). The finding of significantly decreased concentration of NAA in our hypoxic-animals could therefore strengthen the validity of the results ( Figure 3c ).
The degree of myocardial and renal injury after neonatal HI can be reliably determined by biomarkers (27). Plasma levels of cardiac troponin-T have been shown to be significantly higher in nonsurvivors and neonates with abnormal MRI findings following neonatal HI (28). Previous studies in our animal model have shown that increasing levels of troponin-T correlate with myocardial and cerebral damage on histopathology after 27 h observation time (21). In contrast to the study of Alvarez et al (15), we found no effects of CBD on Troponin T levels while both groups receiving therapeutic hypothermia had significantly lower levels ( Figure 2b ). CBD alone did not have any effect on levels of uNGAL, a marker of hypoxic-ischemic renal injury and a predictor of HIE severity (29). In contrast CBD + H significantly mitigated the increase in uNGAL compared with VEH + H. This might be interpreted as a synergistic effect of CBD and hypothermia and is the only finding of synergy between CBD and therapeutic hypothermia in our study.
To elucidate possible effects of CBD on inflammation, apoptosis, and oxidative stress we analyzed the expression of a selected number of mRNAs ( Figure 4 ), protein expression of inflammatory cytokines in brain tissue ( Table 2 ) and urinary markers of neuronal oxidative stress ( Table 3 ). Contrary to what we expected HI did not significantly alter these parameters and neither were there any significant differences between treatment groups. As seen in the control group the piglets are exposed to considerable inflammation and oxidative stress alone by surgery, anesthesia, and mechanical ventilation. Markers of inflammation and oxidative stress might be particularly vulnerable to these baseline stress factors and this illustrates the strength of including a nonhypoxic control group. Another important consideration is the large temporal variation in gene and protein expression after neonatal hypoxia (30,31,32) which mean that a significant difference could be present at other time points and that our results from a single time-point must be interpreted with caution.
In summary, we were not able to reproduce the previous findings with protective effects of CBD after neonatal HI. An explanation for this can be the differences in the character and severity of the hypoxic-ischemic insult given. We induce ischemia by hypoxic ventilation alone until cardiovascular compensatory mechanisms fail rather than by external means such as carotid clamping. This results in a more global insult with severe multiorgan involvement as reflected in the degree of hypotension, metabolic acidosis, and also increase in troponin-T and uNGAL. Multiorgan involvement is common in newborns with HIE (27) and our model thus allow us to study effects that might not be fully accounted for in models relying on more localized brain damage with milder hypoxia accompanied by acute reversible bilateral common artery occlusion. In fact acute reversible bilateral common artery occlusion has not been shown to significantly reduce cerebro-cortical blood flow in piglets and thus might not significantly exacerbate the effects of hypoxic ventilation alone (33).
We do acknowledge the fact that there are limitations to our study. The early end-point only allows us to study short-term effects. We have tried to compensate for this by analyzing biomarkers that correlate both with injury after longer follow up in the same piglet model (21) as well as long term outcome in clinical studies (24,25). The concurrent findings of limited hypothermic neuroprotection pose another challenge when interpreting the missing CBD effects. One might argue that the hypoxic-ischemic insult is too severe for neuroprotection to be found or that the end-point used for analysis is premature. The previous studies on hypothermic neuroprotection in piglets have primarily found neuroprotective effects after more moderate insults and using later end-points for evaluation (>24 h) (34,35,36). In contrast the previous studies on CBD (15,16,17) have found protective effects at even earlier time points indicating that the effects of CBD on these parameters in fact can be found early after HI. Therefore, we argue that our findings are relevant despite the limited effects of therapeutic hypothermia. Another challenge when interpreting our results is the large variation among piglets in tolerance and response to HI which is reflected in our data with large variation. This is mainly related to genetic diversity, but we could have reduced intragroup variability by attempting to further individualize the hypoxic-ischemic insult using for example, amplitude-integrated electroencephalography (aEEG) or other methods (37,38).
Although our study failed to show protective effects of CBD, an orphan designated drug for treatment of newborns with HIE, the majority of existing evidence point in the other direction. Our animal model accounts for parts of HIE pathophysiology not accounted for in previous studies, and thus provide valuable new knowledge about the limitations of CBD in this setting. We do however speculate that the severe multiorgan involvement might have “overwhelmed” the protective effects of CBD 1 mg/kg, something that is supported by the findings of limited hypothermic neuroprotection in our study. Thus higher doses of CBD should be evaluated in our animal model and we will aim to do this in future studies.
Methods
Approval
The Norwegian Council for Animal Research approved the experimental protocol. The animals were cared for and handled in accordance with the European Guidelines for Use of Experimental Animals, by certified FELASA (Federation of European Laboratory Animals Science Associations) Category C researchers.
Surgical Preparation and Anesthesia
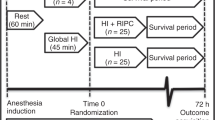
A total of 55 newborn Noroc (LyxLD) piglets were included in the study, with inclusion criteria of age 12–36 h, Hb > 5 g/dl and good general condition. Number of animals needed was roughly estimated by the resource equation method. 48 piglets were exposed to global HI while seven piglets went through the same experimental set-up apart from HI, and served as controls.
Anesthesia was induced by cannulating an ear vein and giving midazolam at 1 mg/kg, fentanyl at 50 µg/kg , and pentobarbital sodium at 15 mg/kg i.v. as a bolus injection. The piglets were then orally intubated and ventilated with a pressure-controlled ventilator (Babylog 8000+; Drägerwerk, Lübeck, Germany). Then piglets were placed in the supine position and washed for sterile procedures. The left jugular vein was cannulated with an arterial cannula with FloSwitch (20 G/1,10 mm 645 mm, Swindon, UK), and the right carotid artery was cannulated using a venflon (BD Venflon Pro, 22GA, 0.9 mm, 625 mm, Becton Dickinson Infusion Therapy AB, Helsingborg, Sweden). Anesthesia was maintained by continuous infusion of fentanyl (50 µg/kg/h) and midazolam (0.25 mg/kg/h) in mixtures, giving 1 ml/kg/h for each drug applied by IVAC P2000 infusion pump. When necessary, a bolus of fentanyl (10 µg/kg) or midazolam (1 mg/kg) was added. If not effective a bolus of pentobarbital (2.5 mg/kg) was added. The need for medication being defined as signs of insufficient depth of anesthesia such as triggering on the respirator, increased muscle tone assessed by passive movements of the limbs, increase in blood pressure and/or pulse and excessive shivering. In severe refractory cases of shivering leading to increased body temperature a bolus of pancuronium (0.1 mg/kg) was added. A continuous i.v. infusion of Salidex (saline 0.3% and glucose 3.5%) 10 ml/kg/h was given until 15 min into HI, then stopped until 15 min after resuscitation, restarted at 5 ml/kg/h−1 and given throughout the rest of the experiment. Normoventilation (arterial carbon dioxide tension (PaCO2) 4.5–5.5 kPa), and a tidal volume of 6–8 ml/kg were achieved by adjusting the peak inspiratory pressure or ventilatory rate. The ventilatory rate was 15–40 respirations/min. The inspiratory time of 0.45 s and the positive end-expiratory pressure of 4.5 cm H2O were kept constant throughout the experiment. The MABP was measured continuously in the left carotid artery using BioPac systems MP150-CE. The O2 saturation was continuously monitored (Masimo RAD-5 pulsoxymeter, Neuchatel, Switzerland). Rectal temperature was maintained between 38.5 and 40.0°C with a heating blanket. 1 h of stabilization was allowed after surgery. At the end of the experiment, the piglets were given an overdose of 150 mg/kg pentobarbital i.v.
Randomization
We performed two randomizations, by sealed envelopes, in blocks of 12. The first block-randomization between HI and controls took place after surgery and stabilization. The second block-randomization took place after end of HI between the different treatment groups.
Experimental Protocol
HI was achieved by ventilation with a gas mixture of 8% O2 in N2 until either the MABP decreased to 20 mmHg or the base excess reached −20 mmol/l. CO2 was added during hypoxemia, aiming at a PaCO2 of 8.0–9.5 kPa to imitate hypercapnia during perinatal HI. Immediately after resuscitation the hypoxic piglets were block-randomized to receive either vehicle (VEH) (n:12), VEH + hypothermia (n:12), CBD (n:12) or CBD + hypothermia (n:12). VEH and CBD were given i.v. in a central vein 30 min after end hypoxia at a dose of 1 mg/kg. The CBD (GW Pharmaceuticals, Cambridge, UK) was prepared in a 5 mg/ml formulation of ethanol:solutol:saline at a ratio of 2:1:17 by a collaborating unit (Experimental Unit, Pediatric Department, University Hospital Puerto de Hierro Majadahona, Madrid, Spain) and shipped to us.
Piglets randomized to therapeutic hypothermia was cooled to 35°C (± 0.5°C) by a cooling mattress (Tecotherms TSmed 200; TecCo, Halle, Germany) perfused with circulating liquid set at desired temperature while all other groups were kept normothermic (39°C) (34,35,39). Cooling was initiated 30 min post-HI. Throughout the whole experiment, there was a continuous surveillance of blood pressure, saturation, pulse, temperature, and blood gas measurements. Deep rectal temperature was recorded by digital rectal thermometer. Temperature-corrected arterial acid/base status, glucose, and hemoglobin were regularly measured throughout the experiment on a Blood Gas Analyzer 860 (Ciba Corning Diagnostics, Midfield, MA). Apart from during the hypoxic insult mechanical ventilation settings were adjusted to maintain PaO2 and PaCO2 at 8–12 kPa and 4.0–6.5 kPa respectively, allowing for temperature correction of the arterial blood sample.
Blood samples of 2 ml were drawn from the carotid artery before initiating the hypoxia, at the end of hypoxia, 30 min after end hypoxia, 210 min after end hypoxia and at end study (570 min after end hypoxia) and were handled according to standard operating procedures and then stored at −80°C until subsequent analysis. All blood samples obtained were replaced by normal saline 1.5× the volume drawn. Urine was collected at 210 min after end hypoxia and at end study by suprapubic thin needle aspiration. CSF was collected at end study by lumbar puncture. All samples collected were immediately snap-frozen in liquid nitrogen and then stored at −80°C. The animals were killed with an overdose of pentobarbital (150 mg/kg i.v.) 9.5 h (570 min) after the end of HI. The brain and cerebellum were immediately removed and sagitally divided. From the right half, specimens from the fronto-parietal cortex, the hippocampus, corpus striatum, white matter, and the cerebellum were immediately snap-frozen in liquid nitrogen and stored at −80°C until subsequent analysis. The left hemisphere was placed in cold 4% buffered formalin for subsequent histopathological analysis.
Dose and Plasma Concentrations CBD
An i.v. dose of 1 mg/kg was chosen based on previous experiments in newborn piglets (16,17). The concentration of CBD was determined in plasma samples at the end of study, 9 h after i.v. administration, by gas chromatography–mass spectrometry.
Neuropathological Analysis
At autopsy the left hemisphere of the brain was immediately fixed in cold buffered formalin. By help of a stereotaxic atlas of the pig brain, coronal blocks (~0.4 cm thick) from areas sensitive to hypoxic-ischemic damage, namely prefrontal cortex, parietal cortex/striatum, hippocampus, and cerebellum were prepared. Tissue blocks were processed in a Leica TP1050 tissue processor (GMI, Ramsey, MN), embedded in paraffin, sliced in 4-μm-thick sections, and stained with hematoxylin and eosin (H&E).
Neuropathological analysis of H&E sections was performed by an experienced pathologist blinded to the randomization and clinical details. Brain regions analyzed were: prefrontal, parietal and dorsal cortex, hippocampus, white matter, and cerebellum. In the cerebrum areas with vacuolated neuropil, shrunken neurons with pyknotic nuclei and scattered eosinophilic neurons were defined as early neuronal damage while for the cerebellum eosinophilic Purkinje cells were the indicators of hypoxic/ischemic damage. A modified version of a validated scoring system (35) was used. Based on all regions assessed a global score, ranging from 0 to 4 was given to each piglet. Zero representing no damage, 1: mild/moderate, 2: moderate/severe, 3: severe, and 4: massive damage with total autolysis of the cerebrum.
Biomarkers
S100B in CSF and plasma Troponin-T were measured using an electrochemiluminescent immunometric assay on the Cobas e601 immunoassay platform (Roche Diagnostics, Mannheim, Germany). uNGAL was measured by using a porcine-specific ELISA (Kit-044; Bioporte) according to the manufacturer’s instructions.
Proton-magnetic-resonance-spectroscopy (H+MRS)
H+MRS was performed in the MRI Unit of the Instituto Pluridisciplinar (Universidad Complutense, Madrid, Spain) at 500.13 MHz using a Bruker AMX500 spectrometer 11.7 T operating at 4°C on frozen brain samples from hippocampus (5–10 mg weight) placed within a 50 μl zirconium oxide rotor with cylindrical insert and spun at 4,000 Hz spinning rate. Standard solvent suppressed spectra were acquired into 16 k data points, averaged over 256 acquisitions, total acquisition 14 min using a sequence based on the first increment of the NOESY pulse sequence to effect suppression of the water resonance and limit the effect of B0 and B1 inhomogeneities in the spectra (relaxation delay-90°-t1-90°-tm-90°-acquire free induction decay) in which a secondary radio frequency irradiation field is applied at the water resonance frequency during the relaxation delay of 2 s and during the mixing period (tm = 150 ms), with t1 fixed at 3 μs. A spectral width of 8,333.33 Hz was used. All spectra were processed using TOPSPIN software, version 1.3 (Bruker, Rheinstetten, Germany). Prior to Fourier transformation, the free induction decays were multiplied by an exponential weight function corresponding to a line broadening of 0.3 Hz. Spectra were phased, baseline-corrected and referenced to the sodium (3-trimethylsilyl)-2,2,3,3-tetradeuteriopropionate singlet at δ 0 ppm. By using the 3.1.7.0 version of the SpinWorks software (University of Manitoba, Winnipeg, Canada) curve fitting was performed, and concentration and ratios were calculated, including: lactate/N-acetylaspartate (Lac/NAA) and glutamate/N-acetylaspartate (Glu/NAA) ratios.
mRNA Expression
Total RNA was extracted from tissue samples using EZNA Total RNA Kit II (Omega Bio-Tek, Norcross, GA) according to manufacturers’ instructions. Purified RNA was quantified using NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE) and 2 µg were reverse transcribed into cDNA with the High capacity cDNA Reverse Transcription kit (Applied Biosystems, Life Tech, Warrington, UK) Primers were designed using Primer Express 3.0 Software (Applied Biosystems, Life Tech).
Primer Sequences (forward, reverse)
IL 6-CACAAGCGCCTTCAGTCCA, TGTCCGGAGAGGTGAAGAGC; IL1b-GTGATGCCAACGTGCAGTCT, GTGGGCCAGCCAGCACTA; TNFα-CAAGGACTCAGATCATCGTCTCA, CATACCCACTCTGCCATTGGA; Bax-AGCGAGTGTCTCAAGCGCAT, ACACCTCTGCAGCTCCATGTTAC; Bcl 2-TGGTGAGTCGGATCGCAACT, AGAGTTCCACAAAAGTGTCCCAG; Caspase3 GACGGACAGTGGGACTGAAGA, GCCAGGAATAGTAACCAGGTGC; PO (housekeeping gene) ACAATGTGGGCTCCAAGCA, CATCAGCACCACGGCTTTC.
A 10-fold dilution of each primer showed efficiency of between 90 and 110%. Amplification was performed for both target genes and housekeeping gene PO in a ViiA 7 Real Time PCR System, universal settings (Applied Biosystems, Life Tech). 50 ng of each sample were run in duplicate with 400 nmol/l primers and Power SYBR Green Master Mix (Applied Biosystems). Data were analyzed by the comparative Ct method (ΔΔCt method) and expressed as fold change relative to median among controls.
Preparation of Brain Tissue Extracts for ELISA
Cerebral tissue from prefrontal cortex and hippocampus, 100 mg from each, was homogenized in ice-cold lysis buffer (TrisHCl (pH 7.5) with 1% NP-40 and a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and OmniTip Tissue Homogenizing Kits (Omni International, Kennesaw, Georgia, USA) and centrifuged a 12,000 × rcf for 15 min at 4ºC. Supernatants were spun for 5 min, and the protein concentration was measured by the BCA method (Pierce, Cheshire, UK).
ELISA
Cytokines: IL-1b, IL-6 and TNF-α were measured using a commercial available enzyme immunoassay, performed according to the manufacturer´s instructions (R&D Systems, Oxford, UK).
Determination of Neuroprostanes and Neurofuranes in Urine
Urinary neuroprostanes (NeuPs) and neurofurans (NeuFs) were determined by ultracromatography coupled to tandem mass spectrometry (UPLC-MS/MS) as described in detail by Máximo Vento’s group (Kuligowski et al. ARS 2014 (40). After thawing on ice, urine samples were centrifuged at 7,500×g and at 4ºC for 10 min. 297 µl of H2O (pH 3, adjusted with formic acid):CH3OH (85:15, v/v) were added to 600 µl of supernatant and spiked with 3 µl of internal standard solution PGF2α -D4 (20 µmol/l). Discovery DSC-18 SPE 96-well plates (Sigma-Aldrich, St. Louis, MO)) were conditioned with 1 ml CH3OH and 1ml H2O before loading diluted samples. Each well was washed with 500 µl H2O and 500 µl heptane, and samples were eluted using four times 100 µl ethyl acetate. Recovered extracts were evaporated to dryness under a stream of N2 and dissolved in 60 µl of H2O (pH 3):CH3OH (85:15 v/v). Ultra Performance Liquid Chromatography (UPLC)-MS/MS analysis was carried out by employing an Acquity–Xevo TQ system (Waters, Milford, MA) in the negative electrospray ionization (ESI-) mode using the following conditions: capillary 3.5 kV, source temperature 120ºC, desolvation temperature 300ºC, dwell time 5 ms; nitrogen cone and desolvation gas flows were 25 and 680 l/h respectively. Separation were carried out using a Kinetex UPLC C18 reversed-phase column (2.1 × 100 mm, 1.7 µm) and precolumn (2.1 × 2 mm) from Phenomenex (Torrance, CA), and a CH3OH (0.1% v/v HCOOH):H2O (0.1% v/v HCOOH) binary gradient. Flow rate, column temperature, and injection volume were set at 400 µl/min, 37ºC, and 5 µl respectively. The following gradient was employed: From 0 to 1 min, 30% v/v CH3OH (0.05% v/v HCOOH) (i.e., channel B) were used and from 1 to 4.0 min, %B increased till 90%. Return to initial conditions was achieved at 4.1 min, and conditions were maintained for 3.9 min. Acquisition parameters of MS detection carried out by multiple reaction monitoring were selected from Kuligowski et al. (40). Chromatographic area values were normalized using PGF2α-D4 as Internal Standard.
Statistical Analysis
Statistical analyses were performed by SPSS version 21 (SPSS, Chicago, IL) and GraphPad Prism 6 (GraphPad Prism Software, La Jolla, CA). When the distribution of the data deviated markedly from the normal distribution, a suitable transformation was applied. If transformation did not produce a normal distribution nonparametric methods were used. mRNA expression data was normalized to baseline (control group median) and expressed as fold change. All other analyses were performed as follows: firstly a significant effect of HI on the parameter of interest, defined as significant difference between control group and VEH group, was established. Then comparison of treatment effects was performed by one-way ANOVA (normally distributed data) or Kruskall–Wallis test (non-normally distributed data) with relevant post-hoc tests. A 5% significance level was used.
Statement of Financial Support
We thank the Lebara Foundation (London EC2R 7BP, UK), Laerdal Foundation (4003, Stavanger, Norway) and Renée og Bredo Grimsgaard’s Foundation (0133, Oslo, Norway) for generous grants.
Disclosure
We have nothing to disclose and no conflicts of interest.
References
Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40.
Lawn JE, Blencowe H, Oza S, et al.; Lancet Every Newborn Study Group. Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014;384:189–205.
Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:329–38.
Saugstad OD. Delivery room management of term and preterm newly born infants. Neonatology 2015;107:365–71.
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;1:Cd003311.
Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363.
Pauliah SS, Shankaran S, Wade A, Cady EB, Thayyil S. Therapeutic hypothermia for neonatal encephalopathy in low- and middle-income countries: a systematic review and meta-analysis. PLoS One 2013;8:e58834.
Higgins RD, Raju T, Edwards AD, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Hypothermia Workshop Speakers and Moderators. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 2011;159:851–858.e1.
Martín-Moreno AM, Reigada D, Ramírez BG, et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol 2011;79:964–73.
Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med 2011;51:1054–61.
da Silva VK, de Freitas BS, da Silva Dornelles A, et al. Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 2014;49:222–33.
England TJ, Hind WH, Rasid NA, O’Sullivan SE. Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2015;35:348–58.
Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis 2010;37:434–40.
Pazos MR, Cinquina V, Gómez A, et al. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacol 2012;63:776–83.
Alvarez FJ, Lafuente H, Rey-Santano MC, et al. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res 2008;64:653–8.
Lafuente H, Alvarez FJ, Pazos MR, et al. Cannabidiol reduces brain damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr Res 2011;70:272–7.
Pazos MR, Mohammed N, Lafuente H, et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacol 2013;71:282–91.
Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015;12:699–730.
Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 2011;6:237–49.
Solberg R, Longini M, Proietti F, Vezzosi P, Saugstad OD, Buonocore G. Resuscitation with supplementary oxygen induces oxidative injury in the cerebral cortex. Free Radic Biol Med 2012;53:1061–7.
Andresen JH, Carlsen B, Solberg R, et al. Newborn piglets exposed to hypoxia after nicotine or saline pretreatment: long-term effects on brain and heart. J Matern Fetal Neonatal Med 2009;22:161–8.
Munkeby BH, De Lange C, Emblem KE, et al. A piglet model for detection of hypoxic-ischemic brain injury with magnetic resonance imaging. Acta Radiol 2008;49:1049–57.
Martin LJ, Brambrink AM, Price AC, et al. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol Dis 2000;7:169–91.
Massaro AN, Chang T, Baumgart S, McCarter R, Nelson KB, Glass P. Biomarkers S100B and neuron-specific enolase predict outcome in hypothermia-treated encephalopathic newborns*. Pediatr Crit Care Med 2014;15:615–22.
Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatr 2010;125:e382–95.
Robertson NJ, Thayyil S, Cady EB, Raivich G. Magnetic resonance spectroscopy biomarkers in term perinatal asphyxial encephalopathy: from neuropathological correlates to future clinical applications. Curr Pediatr Rev 2014;10:37–47.
Aslam S, Molloy EJ. Biomarkers of multiorgan injury in neonatal encephalopathy. Biomark Med 2015;9:267–75.
Armstrong K, Franklin O, Sweetman D, Molloy EJ. Cardiovascular dysfunction in infants with neonatal encephalopathy. Arch Dis Child 2012;97:372–5.
Sweetman DU, Molloy EJ. Biomarkers of acute kidney injury in neonatal encephalopathy. Eur J Pediatr 2013;172:305–16.
Shrivastava K, Llovera G, Recasens M, et al. Temporal expression of cytokines and signal transducer and activator of transcription factor 3 activation after neonatal hypoxia/ischemia in mice. Dev Neurosci 2013;35:212–25.
Rognlien AG, Wollen EJ, Atneosen-Åsegg M, Saugstad OD. Increased expression of inflammatory genes in the neonatal mouse brain after hyperoxic reoxygenation. Pediatr Res 2015;77:326–33.
Wollen EJ, Sejersted Y, Wright MS, et al. Transcriptome profiling of the newborn mouse brain after hypoxia-reoxygenation: hyperoxic reoxygenation induces inflammatory and energy failure responsive genes. Pediatr Res 2014;75:517–26.
Domoki F, Zolei-Szenasi D, Olah O, et al. Comparison of cerebrocortical microvascular effects of different hypoxic-ischemic insults in piglets: a laser-speckle imaging study. J Physiol Pharmacol 2014;65:551–8.
Gressens P, Dingley J, Plaisant F, et al. Analysis of neuronal, glial, endothelial, axonal and apoptotic markers following moderate therapeutic hypothermia and anesthesia in the developing piglet brain. Brain Pathol 2008;18:10–20.
Haaland K, Løberg EM, Steen PA, Thoresen M. Posthypoxic hypothermia in newborn piglets. Pediatr Res 1997;41(4 Pt 1):505–12.
Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol 2003;53:65–72.
Kyng KJ, Skajaa T, Kerrn-Jespersen S, Andreassen CS, Bennedsgaard K, Henriksen TB. A piglet model of neonatal hypoxic-ischemic encephalopathy. J Vis Exp 2015:e52454.
Björkman ST, Foster KA, O’driscoll SM, et al. Hypoxic/Ischemic models in newborn piglet: comparison of constant FiO2 versus variable FiO2 delivery. Brain Res 2006;1100:110–7.
W.O R. Dukes’ Physiology of Domestic Animals. 13th edn. NJ: Wiley Blackwell: Hoboken, NJ; 2012:151.
Kuligowski J, Aguar M, Rook D, et al. Urinary lipid peroxidation byproducts: Are they relevant for predicting neonatal morbidity in preterm infants? Antioxid Redox Signal 2015;23:178–84.
Acknowledgements
We thank Professor in biostatistics Leiv Sandvik at the University of Oslo for statistical advice; MSc Dag Helge Strand at the National Health Institute for analysis of plasma CBD concentrations; Ingeborg Løstegaard Goverud for assistance with histopathology; MSc Monica Åsegg-Atneosen and Grethe Dyrhaug for help with analysis of mRNA and protein expression; and Department of Medical Biochemistry, Oslo University Hospital, for analysis of S100B in CSF and Troponin T in plasma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garberg, H., Huun, M., Escobar, J. et al. Short-term effects of cannabidiol after global hypoxia-ischemia in newborn piglets. Pediatr Res 80, 710–718 (2016). https://doi.org/10.1038/pr.2016.149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.149
This article is cited by
-
Gene expression in the intestine of newborn piglets after hypoxia-reoxygenation
Pediatric Research (2023)
-
Neuroprotective therapies in the NICU in term infants: present and future
Pediatric Research (2023)
-
Splanchnic oxygen saturation during reoxygenation with 21% or 100% O2 in newborn piglets
Pediatric Research (2022)
-
Cannabinoid Analogue WIN 55212–2 Protects Paraquat-Induced Lung Injury and Enhances Macrophage M2 Polarization
Inflammation (2022)
-
New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy
European Journal of Pediatrics (2022)