Abstract
Background:
Premature infants are at risk of vascular neurologic insults. Hypotension and hypertension are considered injurious, but neither condition is defined with consensus. Cerebrovascular critical closing pressure (CrCP) is the arterial blood pressure (ABP) at which cerebral blood flow (CBF) ceases. CrCP may serve to define subject-specific low or high ABP. Our objective was to determine the ontogeny of CrCP.
Methods:
Premature infants (n = 179) with gestational age (GA) from 23–31 wk had recordings of ABP and middle cerebral artery flow velocity twice daily for 3 d and then daily for the duration of the first week of life. All infants received mechanical ventilation. CrCP was calculated using an impedance-model derivation with Doppler-based estimations of cerebrovascular resistance and compliance. The association between GA and CrCP was determined in a multivariate analysis.
Results:
The median (interquartile range) CrCP for the cohort was 22 mm Hg (19–25 mm Hg). CrCP increased significantly with GA (r = 0.6; slope = 1.4 mm Hg/wk gestation), an association that persisted with multivariate analysis (P < 0.0001).
Conclusion:
CrCP increased significantly from 23 to 31 wk gestation. The low CrCP observed in very premature infants may explain their ability to tolerate low ABP without global cerebral infarct or hemorrhage.
Similar content being viewed by others
Main
Cerebrovascular critical closing pressure (CrCP) is the arterial blood pressure (ABP) at which blood flow to the brain ceases due to vascular collapse. Also referred to as collapsing pressure, CrCP can be easily and noninvasively monitored at the bedside using Doppler ultrasound and ABP measurements (1). CrCP is posited to be the sum of vascular wall tension and intracranial pressure (2). CrCP can be conceptualized as a factor for the normalization of ABP to an “effective cerebral perfusion pressure” or “closing margin” (3,4). The “effective cerebral perfusion” or “closing margin” (ABP–CrCP) can be determined for any phase of the cardiac cycle by subtracting the CrCP from systolic, mean, or diastolic ABP.
Limited data sets report CrCPs in term and preterm newborns ranging from 24 to 33 mm Hg, which is similar to reported values in mature subjects (5,6). Low ABP in premature infants results in a closing margin that is strikingly low ( Figure 1 ). Premature infants are commonly treated for low values of blood pressure based on mean ABP thresholds related to their gestational age (GA) (7,8,9), and these thresholds are within the range of published CrCP values (5,6). Moreover, during postnatal transition, a period of time often characterized by shock, premature infants can have CBF limited to the systolic phase of the cardiac cycle (10).
Critical closing pressure (CrCP) is illustrated for a single premature infant born at 28 wk gestation. CrCP is shown during hypertension caused by (a,b) tracheal suctioning and (c,d) at rest. (a) Arterial blood pressure (ABP) and cerebral blood flow velocity (CBFV) are both elevated during tracheal suctioning. (b) The data from a is shown as a pressure flow loop, with the calculated CrCP at 30 mm Hg shown by the vertical dashed lines. Cerebral blood flow is present throughout the cardiac cycle because ABP is greater than CrCP. (c) At rest, the ABP returns to baseline, and CBFV is zero during diastole. (d) Pressure flow loops constructed with the data from c show that during diastole the ABP is less than the CrCP, which remains at 30 mm Hg. While the ABPs were different during suctioning and at rest, the CrCP was the same.
Disparity in the definition of hypotension and treatment thresholds for low ABP are likely attributable to a lack of outcome data in premature infants demonstrating a benefit for treating low ABP (11). We propose that an important confounding factor may be the low closing margin relative to the range of CrCP in this population. For example, a difference of 10 mm Hg CrCP (from 20 to 30 mm Hg) could profoundly increase or decrease CBF velocity (CBFV) in a premature infant with a mean ABP of 30 mm Hg. In contrast, such changes in CrCP would have trivial consequences to the CBF of mature subjects with a mean ABP of 70 mm Hg. Simply stated, the “effective cerebral perfusion pressure” or “closing margin” may be more relevant to define brain perfusion than ABP in premature newborns.
Our objective then was to measure and characterize the ontogeny of CrCP in premature infants and to evaluate potential determinants of CrCP in this cohort. We hypothesized that CrCP would increase with GA.
Results
Infant Characteristics
Premature newborns (n = 179) with GA 26.2 ± 2 wk (mean ± SD; range: 23–31 wk) and birth weight 824 ± 237 g had 1-h recordings of ABP and middle cerebral artery CBFV twice daily for the first 3 d and then daily for the next 4 d, during the first week of life (median 6 recording sessions per subject). Of this cohort, 96 (54%) were female, 127 (70%) were born by cesarean section, 163 (91%) were exposed to antenatal steroids, 47 (26%) were from multiple gestation pregnancies, 63 (34%) received vasopressor support, and all were mechanically ventilated during the monitoring sessions. The Apgar scores (median, interquartile range) at 1 and 5 min were 4 (2–6) and 6 (5–7), respectively. A total of 172 (92%) of infants survived to hospital discharge, with three deaths occurring outside of the first week of life.
CrCP (Univariate Analysis)
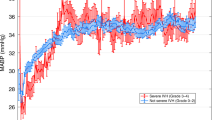
With the method used to calculate CrCP, 752 subject sessions had sufficient data, and 630 subject sessions had sufficient data to render all variables included in the final multivariate model, which included at least one session for all 179 subjects. The median (interquartile range) CrCP was 22 mm Hg (19–25). CrCP increased significantly with GA from 19 mm Hg (15–22 mm Hg) at 23 wk to 31 mm Hg (30,31 mm Hg) at 31 wk gestation. The full spectrum of CrCP is summarized across GA ( Table 1 ). GA was strongly related to CrCP, which increased 1.4 mm Hg per week of gestation (P < 0.0001; Figure 2 ). Five-min Apgar scores were positively correlated to CrCP (r = 0.26; P = 0.0005), escalation of vasopressor therapy was associated with lower CrCP (r = −0.25; P = 0.0007), and PaCO2 measures were not associated with CrCP ( Table 2 ).
Critical closing pressure (CrCP) increases with gestational age (GA) at a rate of 1.4 mm Hg per week of gestation (P < 0.0001)
Multivariate Analysis
The multivariate regression model showed that higher GA was both strongly and independently associated with a higher CrCP (P < 0.0001). The trend for CrCP to increase with hour of life was also significant in this cohort (P < 0.0001; Table 3 ). Five-min Apgar score, vasopressor use, and PaCO2 fluctuation were not associated with CrCP.
Discussion
This is the first study using a sufficiently large cohort of premature infants spanning a range of GA needed to demonstrate developmental changes of CrCP. This is also the first study of CrCP in any pediatric population using the nonlinear, impedance-modeled method. Prior to this study, CrCP in human premature infants was estimated from small cohorts of infants using linear assumptions that could render nonphysiologic negative values (6,12). The main finding of this study is that CrCP increases significantly with GA from a median of 19 mm Hg (15–22 mm Hg) at 23 wk to 31 mm Hg (30–31 mm Hg) at 31 wk gestation, at a rate of 1.4 mm Hg per week. While the relatively low CrCP in most premature infants permits effective cerebral perfusion during states of hypotension, a subset of premature infants will suffer cerebral underperfusion with profound hypotension.
In theory, the increase in CrCP with development is caused by either increased vascular tone or increased intracranial pressure. Because we did not invasively measure intracranial pressure, it is unknown which effect predominates. In fetal sheep, the cerebral vasculature develops a muscularis layer at ~0.67 gestation, which corresponds to ~26–27 wk in humans, and is the developmental stage studied in this cohort (13,14). One reasonable hypothesis is that vascular tone imparted by the newly developed muscularis is a contributor to the increase in CrCP at this stage of development.
Numerous methods for calculating CrCP have been published, with different assumptions and limitations. CrCP is a measurement that assumes static cerebral vasomotor tone, an assumption that is only valid when the measurement of CrCP is performed using high frequency changes of ABP and CBFV (15). The cardiac cycle in premature newborns is considered adequately rapid that changes in vascular wall tension are negligible at normal pulse frequencies. Previous methods examining CrCP were performed using small populations, and CrCP was determined by the x-intercept of the line described by a Cartesian plot of paired ABP/middle cerebral artery CBFV values (5,6). The method used in this study was developed from a model of impedance in the cerebral vasculature with resistance and compliance in parallel. This method renders a CrCP that is bounded to be nonnegative. The impedance method has excellent agreement with the traditional method, except for instances where the traditional method renders clearly nonsensical low and negative values (16,17). In these cases, the new method gave results that made sense (4,18).
In the multivariate analysis, in addition to GA, hour of life was found to be significantly associated with increased CrCP. This finding may suggest that time after birth may result in further development of the vascular muscularis layer; however, this remains only speculative. Vasopressor use was significantly associated with lower CrCP in univariate analysis and therefore was included in the final multivariate analysis. We defer speculative interpretation of the effect of vasopressor use because our vasopressor scale has not been validated elsewhere, and in this study, it served only as a marker of provider perception of hemodynamic compromise. The 5-min Apgar score was not predictive of CrCP; this may be due to the relative stability of the infants in this cohort given their median score 6 at 5 min of life.
Additionally, in a rabbit study of changes in PCO2, CrCP was not altered during hypercapnia (4). This lack of response is possibly explained by simultaneous changes of vasodilation from decreasing wall tension and elevated intracranial pressure and increased ABP inducing autoregulatory changes that counteract the vasodilation during hypercapnia. In our cohort, PaCO2 measures were also not predictive of CrCP, although the mechanism behind this could not be elucidated by this study.
This study has a few possible limitations. The need for high-quality resolution of systolic and diastolic ABP resulted in some data dropout and may have added unexpected bias. Additionally, despite concerns that CBFV measurements may not be reliable proxies for CBF due to possible vessel diameter changes, good correlations have been observed between relative changes of CBFV and near-infrared spectroscopy measures of cerebral hemodynamics as well as by magnetic resonance angiography (19,20,21,22). Further, without using Doppler CBFV, we would not have been able to calculate the CrCP. Selection bias for more critically ill infants may have been introduced, as part of the inclusion criteria was the need for mechanical ventilation. Another limitation of the study is that we used averaged PaCO2 and CrCP, and this could have blunted the intersubject contribution of PaCO2 on CrCP. Routine echocardiography was also not performed at the time of this study. Measurements of cardiac function and the presence of the patent ductus arteriosus may have led to a better understanding of the ontogeny of CrCP and will be added in our future investigations. Finally, ABP is a component of the CrCP equation and is collinear with GA. It is conceivable that this collinearity contributed to the finding that CrCP increased with GA. However, CrCP is not linearly related to ABP, rather it is determined in the method used here by the interaction between ABP and CBFV, expressed as the time constant τ. This explains why the subject shown in Figure 1 rendered the same CrCP at two different states of ABP.
Despite these limitations, this is the first study using a large population of premature infants examining the ontogeny of CrCP beginning at 23–24 wk gestation. CrCP is a significant determinant of CBF at this developmental stage. The prominent role of CrCP in determining the CBF is unique to premature newborns. CrCP and the “effective cerebral perfusion pressure” derived from CrCP warrant further study in premature infants. This study suggests that ABP alone is an insufficient measurement to determine cerebral hemodynamics and that the assessment of CrCP to “normalize” ABP to CBF may be a more effective tool to determine who may require treatment for hemodynamic compromise.
While current guidelines suggest treating infants with low mean ABP, this study highlights that treatment may not be necessary and could in fact be injurious for this very fragile population. Since ABP is a poor predictor of intraventricular hemorrhage or periventricular leukomalacia (11,23,24,25,26), we propose that the closing margin may identify individualized ABP targets in premature infants better than standard gestational, postnatal, and normative values of ABP presently used by many neonatologists.
In conclusion, CrCP increased significantly from 23 to 31 wk gestation at a rate of 1.4 mm Hg per week of gestation. The low CrCP observed in very premature infants may explain their ability to tolerate low ABP without global cerebral infarct or hemorrhage. Results from this study should be used to inform future prospective studies and not to change current clinical management.
Methods
This study is a re-analysis of previously published data prospectively collected from infants born from July 2002 to April 2008 (ref. 27,28,29,30,31). Approval was obtained by the University of Arkansas for Medical Sciences Institutional Review Board, and informed parental consent was obtained before enrollment. Very-low-birth-weight infants (<1,500 g) who were mechanically ventilated and had an umbilical artery catheter in place were potential subjects. Those with major congenital anomalies and chromosomal abnormalities and those in extremis were excluded.
Monitoring Equipment, Data Acquisition, and Data Handling
All infants had measurement of ABP with an umbilical artery catheter placed for clinical monitoring. Middle cerebral artery CBFV was recorded with transcranial Doppler (Nicolet Vascular/Natus Medical Incorporated, San Carlos, CA). PaCO2 was continuously recorded with a Neotrend-L fiber-optic sensor (Diametrics Medical, St Paul, MN). Analog data was collected simultaneously using a PowerLab 8 Channel data acquisition system (AD Instruments, Mountain View, CA). Digitized files were subsequently analyzed using ICM+ software (ICM+, Cambridge Enterprise, UK). Artifact was removed using valid values range filters and by visual inspection of each individual recording.
Calculating CrCP
We employed a recently proposed new method to calculate CrCP using ABP and CBFV tracings (4). In this method, CBFV is described by an alternating flow velocity at the frequency of the cardiac cycle, and CrCP is derived from an equation of impedance to flow velocity. The full derivation of this method is briefly summarized below.
Digitized ABP and middle cerebral artery CBFV recordings at 200 Hz were analyzed using ICM+. CrCP was given by the equation:

where mABP is mean arterial blood pressure; τ is the time constant, a product of arterial resistance and arterial compliance; and HR is heart rate. τ is estimated by the equation:

where CBV1 is the amplitude of the fundamental harmonic of cerebral arterial blood volume approximated from a time-integral of the difference between instantaneous and averaged (over a few heart cycles) middle cerebral artery CBFV; mCBFV is mean middle cerebral artery CBFV (32); and A1 is the amplitude of the fundamental harmonic of ABP.
It is important to remember that “effective cerebral perfusion” or “closing margin” = ABP–CrCP and can be determined during systole and diastole separately.
An example of ABP, CBFV, and CrCP for a single premature infant, before and after endotracheal suctioning, is shown in Figure 1 . This figure demonstrates that although ABP is a component in the derivation of CrCP, it is not deterministic of CrCP, as CrCP was the same for two different ABP in the same infant. Rather, it is the interaction between ABP and CBFV, rendering the time constant τ, which is most deterministic of CrCP. CrCP was calculated from consecutive 10-s sampling epochs and updated at 10-s intervals. A single mean CrCP was reported for each infant recording session for subsequent analysis.
Statistics
Variables and characteristics potentially related to CrCP were tested in univariate analyses with an a priori threshold of P < 0.1 for inclusion in the multivariate model using SigmaPlot (Systat Software, San Jose, CA). For the univariate analysis, repetitive measures were collapsed to single data points per subject by averaging variables from each recording session with equal weight. GA at birth, 5-min Apgar scores, vasopressor score, and PaCO2 variables were tested as potential predictors of CrCP. The use of vasopressors was scored from 0 to 6 according to the progressive vasopressor support strategy used clinically. (0 = no vasopressors; 1 = dopamine; 2 = dobutamine; 3 = hydrocortisone; 4 = dopamine + dobutamine; 5 = dopamine + hydrocortisone; and 6 = dopamine + dobutamine + hydrocortisone). This vasopressor score is conceptualized as a marker of provider perception of hemodynamic compromise. Minimum, maximum, and fluctuation (maximum–minimum) PaCO2 were averaged to a single value per subject.
Tests of normality (Shapiro-Wilk) and constant variance were performed for values averaged from each recording session. Since CrCP is not normally distributed given its mathematical bound from 0 to infinity, the logarithm of CrCP was used and verified with the Lilliefors and Jarque–Bera tests. Linear regression with generalized estimation of equations, accounting for the different number of recording sessions per subject, using a robust covariance matrix, was performed in the final multivariate model with the MATLAB toolkit of Ratcliffe and Shults, which is appropriate for inconsistent repetitive measures (33,34).
Statement of Financial Support
J.R.K. was supported by the National Institutes of Health (1K23NS43185, RR20146, and 1R01NS060674; Bethesda, MD) and the University of Arkansas for Medical Sciences Translational Research Institute (1UL1RR029884). C.J.R. is supported by the Chao Physician Scientist Award (Baylor College of Medicine; Houston, TX).
Disclosures
The authors do not have any potential conflicts of interest, real or perceived, to disclose.
References
Varsos GV, Budohoski KP, Kolias AG, et al. Relationship of vascular wall tension and autoregulation following traumatic brain injury. Neurocrit Care 2014;21:266–74.
Nichol J, Girling F, Jerrard W, Claxton EB, Burton AC. Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am J Physiol 1951;164:330–44.
Jägersberg M, Schaller C, Boström J, Schatlo B, Kotowski M, Thees C. Simultaneous bedside assessment of global cerebral blood flow and effective cerebral perfusion pressure in patients with intracranial hypertension. Neurocrit Care 2010;12:225–33.
Varsos GV, Richards H, Kasprowicz M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab 2013;33:235–43.
Panerai RB, Coughtrey H, Rennie JM, Evans DH. A model of the instantaneous pressure-velocity relationships of the neonatal cerebral circulation. Physiol Meas 1993;14:411–8.
Panerai RB, Kelsall AW, Rennie JM, Evans DH. Estimation of critical closing pressure in the cerebral circulation of newborns. Neuropediatrics 1995;26:168–73.
Report of a Joint Working Group of the British Association of Perinatal Medicine and the Research Unit of the Royal College of Physicians. Development of audit measures and guidelines for good practice in the management of neonatal respiratory distress syndrome. Arch Dis Child 1992;67:1221–7.
Dempsey EM, Barrington KJ. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol 2007;27:469–78.
Batton B, Li L, Newman NS, et al.; Eunice Kennedy Shriver National Institute of Child Health & Human Development Neonatal Research Network. Use of antihypotensive therapies in extremely preterm infants. Pediatrics 2013;131:e1865–73.
Rhee CJ, Fraser CD 3rd, Kibler K, et al. The ontogeny of cerebrovascular pressure autoregulation in premature infants. J Perinatol 2014;34:926–31.
Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed 2009;94:F241–4.
Michel E, Zernikow B, von Twickel J, Hillebrand S, Jorch G. Critical closing pressure in preterm neonates: towards a comprehensive model of cerebral autoregulation. Neurol Res 1995;17:149–55.
Helou S, Koehler RC, Gleason CA, Jones MD Jr, Traystman RJ. Cerebrovascular autoregulation during fetal development in sheep. Am J Physiol 1994;266(3 Pt 2):H1069–74.
Szymonowicz W, Walker AM, Yu VY, Stewart ML, Cannata J, Cussen L. Regional cerebral blood flow after hemorrhagic hypotension in the preterm, near-term, and newborn lamb. Pediatr Res 1990;28:361–6.
Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodynamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg 1974;41:597–606.
Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys 2003;25:621–32.
Aaslid R, Lash SR, Bardy GH, Gild WH, Newell DW. Dynamic pressure–flow velocity relationships in the human cerebral circulation. Stroke 2003;34:1645–9.
Soehle M, Czosnyka M, Pickard JD, Kirkpatrick PJ. Critical closing pressure in subarachnoid hemorrhage: effect of cerebral vasospasm and limitations of a transcranial Doppler-derived estimation. Stroke 2004;35:1393–8.
Bassan H, Gauvreau K, Newburger JW, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res 2005;57:35–41.
Pellicer A, Valverde E, Gayá F, Quero J, Cabañas F. Postnatal adaptation of brain circulation in preterm infants. Pediatr Neurol 2001;24:103–9.
Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr 1997;131:549–54.
Benders MJ, Hendrikse J, de Vries L, Groenendaal F, van Bel F. Doppler-assessed cerebral blood flow velocity in the neonate as estimator of global cerebral blood volume flow measured using phase-contrast magnetic resonance angiography. Neonatology 2013;103:21–6.
Batton B, Batton D, Riggs T. Blood pressure during the first 7 days in premature infants born at postmenstrual age 23 to 25 weeks. Am J Perinatol 2007;24:107–15.
Logan JW, Westra SJ, Allred EN, et al.; ELGAN Study investigators. Antecedents of perinatal cerebral white matter damage with and without intraventricular hemorrhage in very preterm newborns. Pediatr Neurol 2013;49:88–96.
Perlman JM, Risser R, Broyles RS. Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics 1996;97(6 Pt 1):822–7.
Limperopoulos C, Bassan H, Kalish LA, et al. Current definitions of hypotension do not predict abnormal cranial ultrasound findings in preterm infants. Pediatrics 2007;120:966–77.
Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr 2004;144:809–14.
Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res 2005;58:931–5.
Kaiser JR, Gauss CH, Williams DK. Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J Perinatol 2008;28:34–41.
Kaiser JR, Gauss CH, Williams DK. The effects of closed tracheal suctioning plus volume guarantee on cerebral hemodynamics. J Perinatol 2011;31:671–6.
Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr 2009;154:824–8.
Kasprowicz M, Czosnyka M, Soehle M, et al. Vasospasm shortens cerebral arterial time constant. Neurocrit Care 2012;16:213–8.
Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30.
Ratcliffe S, Shults J. GEEQBOX: A Matlab toolbox for generalized estimating equations and quasi-least squares. J Stat Softw 2008;25:1–14.
Acknowledgements
The technical assistance of Natalie C. Sikes and Melanie J. Mason and the support of the University of Arkansas for Medical Sciences neonatologists, NICU nurses, respiratory therapists, and ultrasound technicians are gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Rhee, C., Fraser, C., Kibler, K. et al. Ontogeny of cerebrovascular critical closing pressure. Pediatr Res 78, 71–75 (2015). https://doi.org/10.1038/pr.2015.67
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.67
This article is cited by
-
Observed and calculated cerebral critical closing pressure are highly correlated in preterm infants
Pediatric Research (2019)
-
Neonatal cerebrovascular autoregulation
Pediatric Research (2018)