Abstract
Background:
To assess the ability of urinary neutrophil gelatinase-associated lipocalin (UNGAL) to discriminate between culture-positive vs. culture-negative late-onset sepsis evaluations.
Methods:
This is a prospective observational study of 136 neonates who underwent ≥1 sepsis evaluation at >72 h of age. Urine was obtained at the time of sepsis evaluation to measure UNGAL concentration. Using generalized estimating equations controlling for gender, gestational and postnatal age, acute kidney injury, and within-patient correlations, pair-wise contrasts between mean log UNGAL concentrations of infants with negative sepsis evaluations vs. culture-positive sepsis and presumed sepsis were assessed. Discrimination characteristics at several UNGAL cutoff concentrations were assessed using receiver-operating characteristic curves.
Results:
The predicted mean log UNGAL values of culture-positive sepsis and presumed sepsis vs. negative sepsis evaluations differed significantly (P < 0.001 and P = 0.02, respectively). At a cutoff ≥ 50 ng/ml, UNGAL discriminated between culture-positive sepsis and culture-negative sepsis evaluations with sensitivity = 86%, specificity = 56%, positive predictive value = 41%, negative predictive value = 92%, and number needed to treat = 3.
Conclusion:
UNGAL is a noninvasive biomarker with high negative predictive value at the time of late-onset sepsis evaluation in neonates and could be a useful adjunct to traditional components of sepsis evaluations.
Similar content being viewed by others
Main
Late-onset sepsis, by definition, occurs at >72 h of life and can affect as many as 20–30% of infants hospitalized in the neonatal intensive care unit (NICU), with varying mortality rates depending on gestational age (GA) (1,2). Accurate diagnosis of late-onset sepsis in this vulnerable population can be difficult due to nonspecific signs and symptoms, as well as due to difficulties in obtaining sufficient diagnostic specimens, such as an adequate volume of blood samples. In order to determine which infants are most likely to be infected, biomarkers have been utilized with varying success. The ideal biomarker should have rapid onset of expression, reversibility of expression when the disease abates, ease of collection and measurement, high sensitivity, and high negative predictive value (NPV). Currently, C-reactive protein (CRP) is the most frequently used serum biomarker in the NICU population. As described by Benitz et al. (3), a single CRP at the time of a sepsis evaluation has a modest sensitivity (65%) and NPV (79%). In septic infants, CRP peaks 24–48 h after the onset of symptoms; a second sample obtained during this time period increases the sensitivity to 97%. Although CRP can help determine which neonates may not be septic, it is a relatively invasive, late biomarker and the results of the second sample may have limited additional diagnostic utility compared with blood culture results, which are often available about the same time. A noninvasive biomarker with high sensitivity, high NPV, and results available at the time of sepsis evaluation might be more useful than currently available biomarkers.
Urinary neutrophil gelatinase-associated lipocalin (UNGAL) is a 25-kDa protein of the lipocalin family produced by the kidney (4). UNGAL is an indicator of acute kidney injury (AKI) due to sepsis, ischemia, and nephrotoxins (5,6); concentrations have been shown to rise after cardiopulmonary bypass and renal transplantation in adults and children (7,8,9,10), with contrast administration (11), with hemolytic-uremic syndrome (12), and with lupus nephritis (13). We have previously demonstrated, in a pilot study, that concentrations of UNGAL in urine specimens obtained at the time of sepsis evaluations in very-low-birth-weight infants correlated with the presence of sepsis (14).
The objective of this study was to prospectively measure UNGAL at the time of initial evaluation for late-onset sepsis among infants in a quaternary care NICU to determine a cutoff concentration of UNGAL for culture-positive late-onset sepsis with optimal sensitivity and NPV compared with negative-sepsis evaluations. We hypothesized that (i) UNGAL concentrations would be increased in neonates of all GAs and birth weights (BWs) with culture-positive late-onset sepsis and (ii) UNGAL would have a sensitivity and NPV comparable to those of CRP at the time of the sepsis workup.
Results
Study Subjects
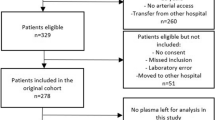
During the study period, 368 late-onset sepsis evaluations were performed in 179 patients. Of these, urine for UNGAL analysis was obtained in 58% (214/368) of evaluations from 76% (136/179) of subjects. Urine was not obtained for 154 evaluations in 43 patients for the following reasons: missed collection (n = 119), oliguria/anuria (n = 23), and parents declined participation (n = 12). Subjects with UNGAL measurements were of lower GA and BW than those infants for whom urine was not collected for UNGAL measurement (P < 0.05) ( Table 1 ).
Sepsis Evaluations
Organisms identified in the culture-positive sepsis group included: Klebsiella pneumoniae (n = 10), methicillin-susceptible Staphylococcus aureus (n = 8), coagulase-negative Staphylococcus (n = 8), Escherichia coli (n = 4), Candida spp. (n = 3), group B Streptococcus (n = 1), Enterobacter spp. (n = 1), methicillin-resistant S. aureus (n = 1), and Actinomyces (n = 1). Three necrotizing enterocolitis (NEC) evaluations yielded a concomitant K. pneumoniae bacteremia and one NEC evaluation yielded a concomitant E. coli urinary tract infection (UTI); these were categorized in the culture-positive sepsis and UTI groups, respectively. When an antimicrobial treatment was given to subjects for wound infections, pneumonia, or cellulitis with negative blood cultures, these episodes were categorized in the “other” group.
Urinary Neutrophil Gelatinase-Associated Lipocalin Concentrations
Figure 1 shows the geometric mean UNGAL concentrations and 95% confidence interval (CI) for each group. Log UNGAL concentrations were highest for the culture-positive and presumed sepsis groups. Pair-wise contrast estimates demonstrated significant differences between the culture-positive sepsis group and the culture-negative sepsis evaluation group and between the presumed sepsis group and the culture-negative sepsis evaluation group (P < 0.001 and P = 0.02, respectively). The UNGAL concentration was similar among other pair-wise comparisons ( Table 2 ). The generalized estimating equation used in this study included initial and subsequent sepsis evaluations. However, when only the first episode was analyzed, the results were similar but effects were not as pronounced.
Geometric mean urinary neutrophil gelatinase-associated lipocalin by culture result. Error bars: 95% CI.
A receiver-operator curve (ROC) was constructed to evaluate the ability of log UNGAL to discriminate between the culture-positive sepsis group and the culture-negative sepsis evaluation group ( Figure 2 ). The area under the curve (AUC) was 0.81 (95% CI (0.72, 0.90)). At a cutoff concentration ≥ 50 ng/ml, UNGAL discriminated between culture-positive sepsis and culture-negative sepsis evaluation groups with sensitivity = 86%, specificity = 56%, positive predictive value (PPV) = 41%, NPV = 92%, positive likelihood ratio (posLR) = 1.95, negative likelihood ratio (negLR) = 0.25, and the number needed to treat (NNT) = 3 ( Table 3 ). The estimated values for screening test parameters for the composite variable, “sepsis-treated” group vs. culture-negative sepsis evaluation group, were similar ( Table 3 ). When comparing those with confirmed sepsis with those with negative blood cultures (excluding NEC, UTI, and other categories), a similar ROC was constructed with an AUC of 0.79 (95% CI (0.70, 0.87)). Negative predictive values (NPVs) increased since the prevalence of culture-positive sepsis decreased by increasing the number of negative cultures, but the negLR ratio was nearly identical and the NNT increased to 5. We compared the performance of UNGAL ≥ 50 ng/ml with that of CRP ≥ 10 mg/l collected at the time of sepsis evaluation. The screening test parameter estimates for the two biomarkers were similar ( Table 3 ).
Receiver-operator curves for culture-positive sepsis vs. culture-negative sepsis evaluation.
Discussion
Few investigations have examined the utility of UNGAL as a potential biomarker for neonatal late-onset sepsis. This study is the largest prospective study demonstrating the use of UNGAL as a noninvasive biomarker for late-onset sepsis in the neonatal population. The inclusion of patients across a range of GA and BW improves the generalizability of these findings. The design of our study offered the opportunity to directly compare the performance of UNGAL with that of another biomarker in common use, CRP. In order to compare our results with those in the study by Benitz et al. (3), the comparison group (culture-negative sepsis evaluation group) was used. This group is most similar to that used by Benitz, in which the negative culture group was defined as follows: “no sepsis if there were no clinical, radiographic, or laboratory findings attributable to sepsis (3).” As noted in Table 3 , the screening test parameter estimates for UNGAL (cutoff ≥ 50 ng/ml) and CRP (cutoff > 10 mg/l) were similar. Moreover, the parameter estimates for both biomarkers were similar to those for the initial CRP published by Benitz et al. (3).
Our study is conditioned on infants experiencing deterioration in status sufficient to warrant a sepsis evaluation. The screening test characteristics derived from this design are likely to resemble those observed in clinical practice. Recently Ertuğrul et al. (15) compared the screening test performance of three biomarkers: CRP, procalcitonin, and UNGAL taken from 24 preterm infants at the onset of culture-proven sepsis. Of the three tests, UNGAL performed best, with sensitivity, specificity, PPV, and NPV values of 91.7, 100, 100, and 90.9%, respectively. However, the design of this study excluded infants with presumed sepsis as well as those with other infectious and noninfectious conditions likely to raise the concentration of a biomarker; moreover, UNGAL from well infants was used as the comparison group rather than UNGAL from infants with culture-negative sepsis evaluations, as in our study. Thus, the results of Ertuğrul et al. (15) cannot be compared with those of this study.
We explored the utility of a single UNGAL determination collected at the time of sepsis evaluation; thus, we have no data on the characteristics of UNGAL as a screening test when additional serially obtained values are combined with the initial result. However, we have previously described the longitudinal behavior of UNGAL in very-low-birth-weight infants with sepsis (14): UNGAL increases from baseline 1 d prior to blood culture sampling and peaks at a mean level six times higher than baseline 2 d after the initiation of the antibiotic treatment, which then begins a slow descent back toward baseline that encompasses the duration of treatment. This pattern suggests that additional UNGAL determinations obtained serially, early in the course of a sepsis evaluation, might enhance the sensitivity and PPV of the biomarker.
In adult humans and in the adult mouse model, neutrophil gelatinase-associated lipocalin (NGAL) exists in two separate body pools: systemic and renal. In the systemic pool, NGAL is normally expressed at very low concentrations by many organs, as well as in neutrophils and macrophages (16,17). NGAL expression is up-regulated by infection, inflammation, ischemia, and neoplastic transformation (18,19,20,21,22,23). Filtered NGAL is captured by megalin in the proximal tubule and degraded; thus, very little (0.1–0.2%) is found in urine, suggesting that systemic (filtered) NGAL contributes little if any NGAL to the urinary pool. In the postischemic mouse model, NGAL production is up-regulated within the kidney in the thick ascending limb of Henle and the collecting ducts (17). Studies looking at the biomarker characteristics of NGAL using the NGAL-reporter mouse model have also demonstrated the α-intercalated cells of the collecting system and the thick ascending limb of Henle as the sources of urinary NGAL (24).
The mechanism by which UNGAL increases in neonatal sepsis is unknown. One possibility is that systemic NGAL elaborated by neutrophils or other tissues in response to infection is less effectively reabsorbed during sepsis by the immature neonatal kidney. Nonetheless, based on studies using mouse models, a toll-like receptor expression is essential to NGAL production (18) and UNGAL excretion in response to infection (25). Filterable bacterial products may reach the kidney tubule to activate these toll-like receptors in the renal tubules.
Limitations
Our study provides support for the use of UNGAL as a biomarker; however, several limitations must be acknowledged. The first is using blood culture result as the “gold standard” for sepsis. Blood cultures have their own inherent false positive and false negative rates (26,27,28). Given this uncertainty, we used the sepsis-treated outcome to evaluate the performance of UNGAL as a screening test to distinguish those infants in whom a decision was made to treat with a full course of antibiotics. Second, in a prior study in which bagged urine specimens were collected daily, the mean UNGAL values from female infants exceeded those of males (29). The authors speculated that the cause of this difference was the contamination of female urine by stool or secretions during collection. In this study, urine was obtained by urethral catheterization or bag specimen for culture at the time of sepsis workup and no male–female difference in UNGAL values was observed. We speculate that in this study greater care may have been taken in collecting specimens to be used for urine culture than in the prior study, in which urine was not usually cultured. Third, GA and AKI (30,31,32) have been associated with changes in UNGAL concentrations in infants, independent of infection. Regression models controlling for the effects of GA, sex, and AKI found no significant interactions between these covariates and culture results; however, our study was neither designed nor powered to detect such effects. Fourth, the results of our study are primarily based on very-low-birth-weight infants, which limit the generalizability of our study. We were unable to further delineate any additional clinical differences among the patients with and without UNGAL levels, which may have contributed to the statistically significant difference between GA and BW. When controlled for GA and BW among patients with UNGAL values, no differences were detected.
Urinary neutrophil gelatinase-associated lipocalin (UNGAL) levels can be elevated due to both ischemic and septic kidney damage; thus, UNGAL levels must be interpreted in the clinical context. The results of our multivariate analysis, which demonstrated significant associations between elevated UNGAL and culture-positive sepsis and presumed sepsis, controlled for the presence of AKI. In practical terms, the presence of AKI reduces the specificity and PPV of UNGAL as a screening test for sepsis.
Conclusion
This study found that UNGAL is a potential biomarker for late-onset neonatal sepsis. When obtained at the time of sepsis evaluation, UNGAL has discriminant characteristics equal to the most widely used biomarker, CRP. Further studies should explore the ability of serial UNGAL measures to improve the sensitivity and NPV of this biomarker.
Methods
Study Design, Site, and Subjects
This is a prospective observational study of hospitalized infants undergoing an evaluation for late-onset sepsis at >72 h of age performed from 1 February 2010 to 30 April 2011 in the quaternary care NICU at the NewYork-Presbyterian Morgan Stanley Children’s Hospital at Columbia University Medical Center. The eligible infants were of any GA and BW, inborn or outborn, who underwent one or more evaluations for late-onset sepsis. Thus, multiple evaluations performed in the same infant were eligible for inclusion. The infants on antibiotics at the time of sepsis evaluation were excluded. The Institutional Review Board of Columbia University Medical Center approved the study with verbal consent from parents prior to the measurement of UNGAL in an aliquot of urine obtained at the time of sepsis evaluation.
Specimen Collection and UNGAL Measurement
At the time of evaluation for late-onset sepsis, the following tests were obtained as per usual practice: complete blood counts, at least one—preferably two—blood culture(s), a CRP at the time of the initial evaluation and 24 h later, urine culture, and (if indicated) cerebrospinal fluid. To measure UNGAL, 500 μl of urine was collected from the bagged urine or the urethral catheter specimen obtained for urine culture. No subjects were catheterized for the sole purpose of obtaining urine for UNGAL. The urine specimens for UNGAL concentrations were refrigerated and centrifuged (5,000 rpm for 5 min) within 12 h of collection and the supernatant was stored at –80 °C for a subsequent batch analysis. As described in a previous study (29), UNGAL was measured by a western immunoblot by a single technician blinded to the results of the late-onset sepsis evaluations. The UNGAL concentrations were not shared with the practitioners in the NICU.
Case Definitions
The study investigators defined seven mutually exclusive outcomes of the late-onset sepsis evaluations ( Table 4 ). These included: (i) culture-positive sepsis; (ii) single positive culture for coagulase-negative Staphylococcus; (iii) presumed (culture-negative) sepsis treated with intravenous antibiotics for ≥ 5 d; (iv) UTI with a urine culture positive for >10,000 colonies of a single pathogen from a catheterized specimen; (v) NEC defined as Bell stage ≥2, with either pneumatosis intestinalis or portal venous gas on abdominal radiograph or consistent surgical pathology; (vi) other infections, e.g., wound infections, pneumonia, and cellulitis not associated with a positive blood culture; and (vii) negative sepsis evaluations that did not fulfill any of the other outcome definitions. A study investigator blinded to UNGAL results then categorized each eligible late-onset sepsis evaluation into one of these seven outcomes. A composite endpoint, “sepsis treated,” included both culture-positive and presumed sepsis outcomes. The episodes of NEC evaluations yielding positive blood cultures were categorized as culture-positive sepsis. Creatinine concentration was measured on the day of sepsis evaluation and/or within 72 h of the evaluation for all but eight of the sepsis evaluations. AKI was defined as an increase in serum creatinine, 26.5 µmol/l (≥0.3 mg/dl) above baseline for ≥48 h within 72 h of the sepsis evaluation (33).
Statistical Analysis
To assess whether infants enrolled in the study were representative of our NICU population undergoing sepsis evaluations, we compared the GA, BW, and gender of study subjects with the GA, BW, and gender of infants undergoing sepsis evaluations without an available UNGAL concentration. These demographic data were collected from the electronic medical record.
Urinary neutrophil gelatinase-associated lipocalin (UNGAL) has a log-normal distribution (29). Exploratory bivariate comparisons were done using standard parametric and nonparametric techniques, as appropriate. The log UNGAL concentrations for each category were graphically displayed as geometric means with 95% CIs. We used generalized estimating equations (SPSS, version 20, IBM Corporation, Armonk, NY) controlling for sex, GA, postnatal age, AKI, and within-patient correlations resulting from one or more evaluations per subject to assess the pair-wise contrasts between the mean log UNGAL concentrations of the reference group (culture-negative sepsis evaluations) and other outcomes. The initial and all subsequent sepsis evaluations were included in the model.
We used ROCs to assess discrimination characteristics at several UNGAL cutoff concentrations. Based on these cutoffs, we estimated the screening test parameters including sensitivity, specificity, PPV, NPV, posLRs and negLRs, and NNT for the culture-positive sepsis vs. culture-negative sepsis evaluations and for the “sepsis-treated” outcome vs. culture-negative sepsis evaluations. For comparison, the screening test parameter estimates for CRP collected at the same time as UNGAL were computed using a cutoff of ≥10 mg/l.
Statement of Financial Support
All phases of this study were supported by the Family HSU Donation (New York, NY) and by the National Institute for Nursing Research—National Institutes of Health (R01 NR010821).
Diclosure
Columbia University (New York, NY) has licensed neutrophil gelatinase-associated lipocalin (NGAL) to Abbott Labs (North Chicago, IL) and Alere (San Diego, CA) for the detection of AKI. The authors have no other financial relationship relevant to this article to disclose. The authors have no conflicts of interest to disclose.
References
Brodie SB, Sands KE, Gray JE, et al. Occurrence of nosocomial bloodstream infections in six neonatal intensive care units. Pediatr Infect Dis J 2000;19:56–65.
Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285–91.
Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998;102:E41.
Yang J, Mori K, Li JY, Barasch J. Iron, lipocalin, and kidney epithelia. Am J Physiol Renal Physiol 2003;285:F9–18.
Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 2007;11:R84.
Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 2012;59:246–55.
Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008;3:665–73.
Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care 2007;11:R127.
Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231–8.
Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 2006;6:1639–45.
Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 2007;22:2089–95.
Trachtman H, Christen E, Cnaan A, et al.; Investigators of the HUS-SYNSORB Pk Multicenter Clinical Trial. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr Nephrol 2006;21:989–94.
Brunner HI, Mueller M, Rutherford C, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum 2006;54:2577–84.
Parravicini E, Nemerofsky SL, Michelson KA, et al. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res 2010;67:636–40.
Ertuğrul S, Annagur A, Kurban S, Altunhan H, Ors R. Comparison of urinary neutrophil gelatinase-associated lipocalin, C-reactive protein and procalcitonin in the diagnosis of late onset sepsis in preterm newborns. J Matern Fetal Neonatal Med 2013;26:430–3.
Gwira JA, Wei F, Ishibe S, Ueland JM, Barasch J, Cantley LG. Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J Biol Chem 2005;280:7875–82.
Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 2007;18:407–13.
Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917–21.
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 2002;10:1033–43.
Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 2009;75:285–94.
Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14:2534–43.
Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005;115:610–21.
Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int 2007;71:967–70.
Paragas N, Qiu A, Zhang Q, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 2011;17:216–22.
Paragas N, Kulkarni R, Werth M, et al. The α-intercalated cell defend the urinary system from bacterial infection. J Clin Invest 2014; 124:2963–76.
Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J 1997;16:381–5.
Snyder SR, Favoretto AM, Baetz RA, et al. Effectiveness of practices to reduce blood culture contamination: a Laboratory Medicine Best Practices systematic review and meta-analysis. Clin Biochem 2012;45:999–1011.
Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics 2007;119:891–6.
Huynh TK, Bateman DA, Parravicini E, et al. Reference values of urinary neutrophil gelatinase-associated lipocalin in very low birth weight infants. Pediatr Res 2009;66:528–32.
Lavery AP, Meinzen-Derr JK, Anderson E, et al. Urinary NGAL in premature infants. Pediatr Res 2008;64:423–8.
Askenazi DJ, Koralkar R, Levitan EB, et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res 2011;70:302–6.
Tabel Y, Elmas A, Ipek S, Karadag A, Elmas O, Ozyalin F. Urinary neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury in preterm infants. Am J Perinatol 2014;31:167–74.
Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr 2012;24:191–6.
Acknowledgements
We thank the neonatal intensive care unit (NICU) staff for their assistance in obtaining urine specimens and the members of the Barasch Lab (New York, NY, USA) for performing the neutrophil gelatinase-associated lipocalin (NGAL) assays.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Pynn, J., Parravicini, E., Saiman, L. et al. Urinary neutrophil gelatinase-associated lipocalin: potential biomarker for late-onset sepsis. Pediatr Res 78, 76–81 (2015). https://doi.org/10.1038/pr.2015.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.62
This article is cited by
-
Urinary Biomarkers of Aminoglycoside-Induced Nephrotoxicity in Cystic Fibrosis: Kidney Injury Molecule-1 and Neutrophil Gelatinase-Associated Lipocalin
Scientific Reports (2018)
-
Is urinary neutrophil gelatinase-associated lipocalin able to predict acute kidney injury episodes in very low birth weight infants in clinical settings?
Pediatric Research (2016)