Abstract
Background:
Placental lesions are associated with neurological morbidity but the mechanism leading to morbidity is unclear. To provide insight into such a possible mechanism, we determined whether placental lesions were associated with regional cerebral tissue oxygen saturation (rcSO2) and fractional tissue oxygen extraction (FTOE) in preterm infants during their first 5 d after birth. We hypothesized that as a result of cerebral hypoperfusion, rcSO2 would be lower and FTOE would be higher.
Method:
In a prospective, observational study of 42 preterm infants (gestational age <32 wk), the infants’ placentas were examined for histopathology. We measured rcSO2 and transcutaneous arterial oxygen saturation (SpO2) on days 1–5. FTOE was calculated as FTOE = (transcutaneous SpO2 − rcSO2)/transcutaneous SpO2.
Results:
Only three placentas showed no pathology. Ascending intrauterine infection (AIUI) (n = 16) was associated with lower rcSO2 and higher FTOE values on days 2, 3, and 4 (P ≤ 0.05). Other placental lesions were not associated with rcSO2 and FTOE.
Conclusion:
AIUI is associated with lower rcSO2, and higher FTOE shortly after birth. The effect it has on cerebral oxygenation might be the mechanism leading to neurodevelopmental problems.
Similar content being viewed by others
Main
The placenta is the link between mother and fetus during pregnancy and as such it is an essential organ for the development of the fetus. It is the only organ that enables the exchange of nutrients and oxygen from mother to fetus and removes fetal waste products (1). Placental lesions carry the risk of fetal hypoxia, neonatal morbidity, and even perinatal death (2,3,4,5,6). Moreover, such lesions are associated with several neurological problems including intraventricular hemorrhage, white matter injury, cerebral palsy, and long-term neurodevelopmental problems (7,8,9,10,11,12,13).
To date, the mechanism whereby placental lesions lead to cerebral damage is unclear. One study hypothesized that chronic placental insufficiency could induce fetal hypoxia that in turn could result in cerebral hypoperfusion, which leads to cerebral damage (14). Long-standing placental hypoperfusion can result in a non-optimal intrauterine environment. The placental underperfusion can lead to a reduction of perfusion surface and, as a consequence, nonoptimal oxygen delivery to the fetal circulation. This might result in some degree of intrauterine cerebral underperfusion, and as a consequence to a (transitional) effect on postnatal cerebral blood flow. On the other hand, cerebral hyperperfusion could also lead to cerebral damage (15). Understanding the mechanism of placental lesions leading to neurodevelopmental problems is necessary to provide possible clues for early interventions aiming to improve neurological outcome. To determine whether the disturbances in hemodynamics shortly after birth could be a possible mechanism underlying cerebral damage caused by placental lesions, it would be useful if we could measure cerebral tissue oxygen saturation and extraction. A noninvasive method of doing so is near-infrared spectroscopy (NIRS). It measures regional cerebral tissue oxygen saturation (rcSO2). From this value, fractional cerebral tissue oxygen extraction (FTOE) can be calculated, which reflects the balance between cerebral oxygen supply and cerebral oxygen consumption (16).
Our objective was to determine whether placental lesions were associated with cerebral tissue oxygen saturation and extraction shortly after birth. We hypothesized that in the presence of placental lesions cerebral tissue oxygen saturation would be lower due to cerebral hypoperfusion.
Results
The patient characteristics are presented in Table 1 . No infants died during the first 5 d after birth. Four infants died between 6 and 19 d after birth; three infants died of respiratory and circulatory insufficiency due to sepsis and one infant died of gastrointestinal perforation.
Placental Lesions
Of the 42 placentas we examined, only three showed no lesions ( Table 1 ). The largest group of placental lesions consisted of maternal vascular underperfusion (MVU) (25 placentas). Sixteen placentas, representing the second largest group, showed signs of ascending intrauterine infection (AIUI). A maternal response was present in 15 placentas, a fetal response was present 13 placentas, and a maternal and fetal response was present in 12 placentas. In 25 placentas, we found more than one placental lesion.
Relationship between Placental Lesions and rcSO2 and FTOE
On the first day after birth, none of the placental lesions were associated with rcSO2 or FTOE.
On the second day, the presence of AIUI was associated with lower rcSO2 and higher FTOE (P = 0.05 and P = 0.04, respectively). AIUI with a fetal response was associated with lower rcSO2 (P = 0.05) and higher FTOE (P = 0.06), although neither were statistically significant. AIUI with a maternal response was also associated with lower rcSO2 (P = 0.11) and higher FTOE (P = 0.07), but not significant. There was a trend toward an association between MVU and higher rcSO2 (P = 0.08) and lower FTOE (P = 0.06).
On the third and fourth days, AIUI was still associated with lower rcSO2 (day 3 P = 0.008, day 4 P = 0.007) and higher FTOE (day 3 P = 0.01, day 4 P = 0.01). AIUI with a fetal response was also associated with lower rcSO2 (day 3 P = 0.009, day 4 P = 0.002) and higher FTOE (day 3 P = 0.02 day 4 P = 0.002). AIUI with a maternal response was associated with lower rcSO2 values (day 3 P = 0.05, day 4 P = 0.06) and higher FTOE values (day 3 P = 0.05 day 4 P = 0.09), although not statistically significant. On the fourth day, a tendency toward an association between elevated nucleated red blood cells (NRBCs) and lower rcSO2 (P = 0.09) and higher FTOE (P = 0.06) was seen.
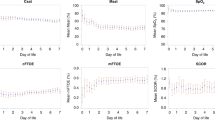
On the fifth day after birth, we found no association between AIUI and cerebral oxygenation. Figure 1 shows the relation between AIUI and rcSO2 (a) and FTOE (b) during the first 5 d after birth. Arterial oxygen saturation (SpO2) did not differ in the presence or absence of AIUI. No other placental lesions were associated with rcSO2 or FTOE ( Table 2 ) during the first 5 d after birth.
Relation between ascending intrauterine infection and rcSO2 and FTOE. The course of rcSO2 (a) and FTOE (b) during the first 5 d after birth in the presence and absence of ascending intrauterine infection. Data are shown in box and whisker plots. Dots represent outliers. FTOE, fractional tissue oxygen extraction; rcSO2, regional cerebral tissue oxygen saturation. Ascending intrauterine infection: white bars, absent; hatched bars, present. Significant differences between the two groups are marked with asterisks (*P < 0.05, **P < 0.01).
Clinical Variables
To test whether the clinical variables we collected had confounded our findings regarding AIUI and rcSO2 and FTOE, we performed multiple linear regressions. We first tested whether these variables differed between the groups of infants with and without AIUI (P < 0.1). Gestational age (GA), birth weight, Apgar scores, umbilical cord pH, birth asphyxia, early-onset sepsis (EOS) and late-onset sepsis, C-reactive protein (CRP), intraventricular hemorrhage, signs of circulatory failure, ventilatory status, patency of the ductus arteriosus, medication (including antibiotic therapy for at least 48 h after birth), and maternal antihypertensive medication were not significantly associated with AIUI in our group. The variables that differed were small for gestational age (SGA), whether the mother had preeclampsia, whether the mother had preterm prelabor rupture of the membranes (PPROM), and whether the child was delivered by cesarean section. On days 3 and 4 after birth, in the presence of AIUI, the infants’ heart rates were higher: mean 155 vs. 145.7 and 155.5 vs. 148, i.e., P = 0.007 and P = 0.08, respectively. We entered these variables into the regression model, separately for each day.
On day 2, the parameters applied in our final model for rcSO2 and FTOE consisted of AIUI, whether the mother had preeclampsia, whether the infant was SGA, whether the mother had PPROM, and whether the child was delivered by cesarean section. The final models for days 3 and 4 consisted of AIUI, whether the mother had preeclampsia, whether the infant was SGA, whether the mother had PPROM, whether the child was delivered by cesarean section, and the infant’s heart rate.
On day 2, only preeclampsia remained significant in the regression model with rcSO2 and FTOE. On day 3, AIUI remained significant in the model for rcSO2. For FTOE only SGA remained significant in the model. On day 4, only AIUI remained significant in the regression model for rcSO2 ( Table 3 ). AIUI, PPROM, and the infant’s heart rate remained significant in the model for FTOE ( Table 4 ).
The presence of a clinical infection was also associated with placentas, showing signs of AIUI. We did not adjust for this because the relation between AIUI and clinical infection is interdependent and can therefore falsely affect our results. In a univariate analysis, clinical infection was not associated with the NIRS values during the first 5 d after birth (independent t-test, P > 0.1)
Subanalysis of the AIUI Group
Of the 16 infants with AIUI, one infant had a culture-proven EOS and three infants had signs of a clinical infection. In the group of 16 infants with AIUI, no difference was found in cerebral tissue oxygen saturation and oxygen extraction in the presence or absence of a clinical/culture-proven infection (P > 0.10).
Discussion
Our study indicated that AIUI was associated with lower rcSO2 and higher FTOE on the second, third, and fourth days after birth. SpO2 did not differ in the presence or absence of AIUI. During the first 5 d after birth, we found no other placental lesions that associated with rcSO2 and FTOE. Therefore, in the case of AIUI, our hypothesis that cerebral oxygen saturation is lower in the presence of placental lesions was confirmed, albeit not for other placental lesions.
The lower rcSO2 and higher FTOE in the presence of AIUI may be either due to reduced cerebral oxygen supply or due to higher cerebral oxygen consumption (17). Firstly, the reduced cerebral oxygen supply might be the result of lower cerebral blood flow. Due to the presence of AIUI, several immune-derived cytokines may be induced: interleukin (IL)-1, IL -6, and tumor necrosis factor (TNF)-α (18). These cytokines are major initiators of acute-phase liver response that leads to an increase in chemokines, cytokines, and prostaglandins. In turn, prostaglandin activation leads to vasodilatation (19). Such systemic vasodilatation may lead to lower cerebral blood flow. We would expect to find lower blood pressures in the presence of vasodilatation. Systemic blood pressure, however, did not differ between the group with and without AIUI. We did find higher heart rates in the presence of AIUI. It is possible that blood pressure is maintained through a higher heart rate. Conversely, it is known that blood pressure in preterm infants is not a reliable measure of cardiac output and, therefore, low cerebral blood flow (20). This means that, in the presence of adequate blood pressure, microcirculation might be disturbed and end-organ perfusion is reduced. As a consequence, it might be that in the presence of AIUI blood pressure is adequate, but that the microcirculation is reduced. This could result in lower end-organ perfusion and, therefore, lower cerebral oxygen supply, which results in higher FTOE. This is, however, highly speculative. It is, for example, not supported by clinical findings as clear signs of circulatory failure were absent in the infants with AIUI.
A second possible explanation for lower rcSO2 and higher FTOE was higher cerebral oxygen consumption. Higher cerebral oxygen consumption may reflect increased cerebral metabolic activity (17). We surmise that increased metabolic activity might be due to cytokine activation in the presence of AIUI.
Our results could also be explained by higher rcSO2 and lower FTOE in the group without AIUI compared with the group with AIUI. Even though our cohort consisted of preterm infants, the etiology of their preterm births differed. Some infants were born preterm after PPROM, while others were born following maternal or fetal indications such as preeclampsia or fetal growth restriction. Indeed, we found a higher rate of preeclampsia and SGA in the group without AIUI compared with the group with AIUI. It was suggested that maternal antihypertensive drugs, often prescribed in cases of preeclampsia, are associated with a decrease in cerebral oxygen consumption (↓FTOE) (21). Nevertheless, we did not find a difference in antihypertensive drug use between the group with and the group without AIUI. Other factors that are presumed to be associated with a decrease in cerebral oxygen consumption, e.g., medication for the infant such as morphine and midazolam, did not differ on the research days between the group with and without AIUI (21,22). Although we were unable to find a difference in clinical variables that could affect cerebral oxygenation between the group with and without AIUI, we could not completely exclude this option.
Another possibility is that the effect of AIUI on rcSO2 and FTOE is secondary to a systemic inflammatory response in EOS. We did not find a relation between the presence of AIUI and culture-proven EOS. This can be due to the small number of infants with EOS (n = 2). We did find a relation between AIUI and clinical infection. However, clinical infection was not associated with rcSO2 and FTOE.
Ascending intrauterine infection (AIUI) is known to be associated with neonatal morbidity, like low Apgar scores shortly after birth, a higher incidence of neonatal infections, necrotizing enterocolitis, and bronchopulmonary dysplasia (23,24,25,26). In addition, AIUI is also associated with neurological problems such as intraventricular hemorrhages, periventricular leukomalacia, cerebral palsy, and poorer neurodevelopmental outcomes at toddler and school ages (7,13,26,27). We now add the association between lower cerebral oxygen saturation, higher cerebral oxygen extraction, and the presence of AIUI. To the best of our knowledge, only one other study investigated the relation between AIUI with a fetal response (fetal vasculitis) and cerebral tissue oxygen saturation and extraction shortly after birth (28). These authors found no difference in cerebral oxygenation in the presence or absence of AIUI with a fetal response. However, in their study, cerebral oxygenation was only measured during the first 24 h after birth. Likewise, in our study we also found no relation between AIUI and cerebral oxygenation on the first day. During this transitional day, other factors might exert more influence on cerebral oxygenation than AIUI. However, we did find an association on days 2, 3, and 4.
It was suggested that during the first 2 wk after birth, cerebral oxygenation is associated with neurodevelopmental outcome. Lower rcSO2 and higher FTOE are associated with poorer neurodevelopmental outcome at 2–3 y of age (29). AIUI is also known to be associated with poorer neurodevelopmental outcome (27). The status of cerebral oxygenation shortly after birth might be the mediating factor for AIUI to lead to neurodevelopmental problems.
The strength of this study was that we investigated the relation between a broad spectrum of placental lesions and cerebral oxygenation. This might contribute toward gaining insight into the pathogenesis of placental lesions leading to neurological problems. Nevertheless, we need to point out several limitations of our study. Firstly, only three children in our group had no placental lesions. All the others had one or more placental lesions. When determining the associations between placental lesions and cerebral oxygenation, the control group consisted partly of infants with other placental lesions than the one studied. Because of the high incidence of placental lesions in a premature group, it was difficult to include a large control group with no placental lesions. Secondly, we performed multiple testing in the univariate analyses. We chose not to adjust our significance level, as this was an explorative study. Thirdly, we only included singletons so as to be certain that each infant was linked to its own placenta. Placental lesions might also differ between twins, e.g., twin-to-twin transfusion. Finally, we studied rcSO2 and FTOE values during a 2-h stable period each day. However, these values might be different during other moments of the day. Mean arterial blood pressures were also studied during a 2-h stable period, and might therefore not be sufficient for the interpretation of hemodynamics.
Conclusion
Our study indicated that AIUI was associated with lower rcSO2 and higher cerebral oxygen extraction on the second, third, and fourth days after birth. Both AIUI and lower cerebral oxygen saturation and a higher oxygen extraction shortly after birth are associated with neurodevelopmental problems. The effect AIUI has on cerebral oxygenation might be the mechanism that causes it to lead to neurodevelopmental problems.
Methods
Patient Population
Our cohort consisted of 42 preterm, singleton infants who had been admitted to the neonatal intensive care unit of Beatrix Children’s Hospital in Groningen, the Netherlands, between May 2006 and February 2008. The inclusion criteria were singleton birth and a GA of less than 32 wk. Infants with major chromosomal and congenital abnormalities were not included. The review board of University Medical Center Groningen approved the study. Written, informed parental consent was obtained in all cases.
The majority of the infants included in this study were also part of another study concerning placental lesions and outcome (30). In that study, we determined the relation between placental lesions and early neurological outcome based on the quality of general movements.
Placental Lesions
A perinatal pathologist examined the placentas in accordance with the guidelines of the Royal College of Obstetricians and Gynaecologists and the Royal College of Pathologists in Britain, and of the College of American Pathologists (31,32). Apart from knowing the infants’ GAs, the pathologist was blinded as to their clinical outcomes. We examined all placentas for lesions suspected of having an association with neurological impairment (13,33). Such lesions are: placental pathology consistent with MVU (34), AIUI (35), chronic villitis of unknown origin (36), chronic deciduitis (37), perivillous fibrinoid (38), fetal thrombotic vasculopathy (39), meconium-associated vascular necrosis (40), chorioamniotic hemosiderosis (41), elevated NRBCs (42), chorangiosis (43), and umbilical cord abnormalities (44). A single placenta can have more than one lesion. All lesions presented in a single placenta were scored separately. In Table 5 , we present the diagnostic terminologies and definitions of these placental lesions. In addition to the placental lesions, we also collected data on placental weights and umbilical cord lengths.
Near-Infrared Spectroscopy (NIRS)
We used an INVOS 4100 near-infrared spectrometer (Somanetics Corporation, Troy, MI) in combination with the pediatric Somasensor to obtain rcSO2 values. We placed the SomaSensor on the left frontoparietal side of the infant’s head and it was held in place by elastic bandaging. A more detailed description of the method was published previously (45).
We measured rcSO2 within the first 24 h after birth and subsequently on the second, third, fourth, and fifth days. On these days, rcSO2 was measured over a clinically stable two-h period. Fifteen minutes were allowed for stabilization of the measurement. Simultaneously, we measured SpO2 by pulse oximetry. We calculated FTOE using the equation FTOE = (SpO2 – rcSO2)/SpO2 (16).
Clinical Variables
Prospectively, we collected details on perinatal and neonatal characteristics that might influence hemodynamics. These included GA, birth weight, SGA, Apgar score, umbilical cord pH, birth asphyxia, EOS and late-onset sepsis (culture proven), clinical infection, CRP, intraventricular hemorrhage, signs of circulatory failure, ventilatory status, patency of the ductus arteriosus, and medication. Clinical infection was defined as maternal fever during labor and/or fetal tachycardia and/or a CRP value ≥10 during the first 72 h after birth, and/or positive blood cultures during the first 48 h after birth. Maternal and pregnancy variables included antihypertensive medication (labetalol, MgSO4, nifedipine), fetal growth restriction, PPROM, preeclampsia, and mode of delivery.
The infants’ heart rates, respiratory rates, and mean arterial blood pressures were recorded simultaneously with the rcSO2 and SpO2 measurements. Blood gas values, blood glucose, and hemoglobin concentrations were recorded on the day of NIRS measurements.
Statistical Analysis
We used SPSS 20.0 software for Windows (SPSS, Chicago, IL) for the statistical analyses. The rcSO2 and SpO2 values were collected every 5 s. The mean values for rcSO2, SpO2, and FTOE were calculated for the 2-h recording period. We used the Kolmogorov–Smirnov test to determine the normality of the rcSO2 and FTOE values. Both showed a normal distribution. For the analyses of the relationships between placental lesions and NIRS parameters, we used a univariate linear regression. We included those placental lesions in our analyses, which were five or more times present in our study group.
When determining the associations between a specific placental lesion and cerebral oxygenation, the control group consisted of infants with no placental lesions and infants with other placental lesions than the one under study. We used backward multiple linear regression analyses to determine which variables were independently associated with rcSO2 and FTOE throughout the analyses. For placental lesions, we chose a univariate level of significance of P ≤ 0.05 to be included into the multivariate analyses. The variables that were potential confounders and differed between the group with and without a placental lesion were included in the regression analyses at P < 0.1. A predetermined P value of <0.05 was considered statistically significant.
Author Contributions
A.M.R. was involved in the design of the study, data acquisition, analyzed and interpreted the data, drafted the initial manuscript, and approved the final manuscript as submitted. A.T. was involved in the design of the study, data acquisition, interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted. M.E.v.d.L. contributed to data acquisition, interpretation of data, reviewed and revised the manuscript, and approved the final manuscript as submitted. J.J.H.M.E. contributed to the design of the study, interpretation of data, reviewed and revised the manuscript, and approved the final manuscript as submitted. A.F.B. contributed to the conception and design of the study, interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted. E.M.W.K. contributed to the design of the study, interpretation of data, reviewed and revised the manuscript, and approved the final manuscript as submitted. E.A.V. contributed to the conception and design of the study, data acquisition and supervised data collection, data analysis and interpretation of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Statement of Financial Support
This study was part of the research program of the Postgraduate School for Behavioural and Cognitive Neurosciences (BCN), University of Groningen, Groningen, the Netherlands. A.M.R. was financially supported by a Junior Scientific Master Class grant of the University of Groningen.
Disclosure
All the authors declare that they have nothing to disclose.
References
Larsen W. Human Embryology. 3rd edn. Philadelphia, PA: Churchill Livingstone, 2001.
Wintermark P, Boyd T, Gregas MC, Labrecque M, Hansen A. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am J Obstet Gynecol 2010;203:579.e1–9.
de Laat MW, Franx A, Bots ML, Visser GH, Nikkels PG. Umbilical coiling index in normal and complicated pregnancies. Obstet Gynecol 2006;107:1049–55.
Moscuzza F, Belcari F, Nardini V, et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol 2011;27:319–23.
Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med 2003;13:102–9.
Korteweg FJ, Erwich JJ, Holm JP, et al. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol 2009;114:809–17.
Wu YW, Colford JM Jr . Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000;284:1417–24.
Maleki Z, Bailis AJ, Argani CH, Askin FB, Graham EM. Periventricular leukomalacia and placental histopathologic abnormalities. Obstet Gynecol 2009;114:1115–20.
Salafia CM, Minior VK, Rosenkrantz TS, et al. Maternal, placental, and neonatal associations with early germinal matrix/intraventricular hemorrhage in infants born before 32 weeks’ gestation. Am J Perinatol 1995;12:429–36.
Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch Pediatr Adolesc Med 2005;159:1032–5.
Leviton A, Allred EN, Kuban KC, et al. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res 2010;67:95–101.
Redline RW, Minich N, Taylor HG, Hack M. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr Dev Pathol 2007;10:282–92.
Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med 2000;124:1785–91.
Blair E, de Groot J, Nelson KB. Placental infarction identified by macroscopic examination and risk of cerebral palsy in infants at 35 weeks of gestational age and over. Am J Obstet Gynecol 2011;205:124.e1–7.
Alderliesten T, Lemmers PM, Smarius JJ, van de Vosse RE, Baerts W, van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr 2013;162:698–704.e2.
Naulaers G, Meyns B, Miserez M, et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 2007;92:120–6.
Kissack CM, Garr R, Wardle SP, Weindling AM. Cerebral fractional oxygen extraction is inversely correlated with oxygen delivery in the sick, newborn, preterm infant. J Cereb Blood Flow Metab 2005;25:545–53.
Elgan Study Investigators; Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A. Relationship between neonatal blood protein concentrations and placenta histologic characteristics in extremely low GA newborns. Pediatr Res 2011;69:68–73.
Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol 2005;18:117–23.
Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J Pediatr 1996;129:506–12.
Verhagen EA, Kooi EM, van den Berg PP, Bos AF. Maternal antihypertensive drugs may influence cerebral oxygen extraction in preterm infants during the first days after birth. J Matern Fetal Neonatal Med 2013;26:871–6.
van Alfen-van der Velden AA, Hopman JC, Klaessens JH, Feuth T, Sengers RC, Liem KD. Effects of midazolam and morphine on cerebral oxygenation and hemodynamics in ventilated premature infants. Biol Neonate 2006;90:197–202.
Beebe LA, Cowan LD, Altshuler G. The epidemiology of placental features: associations with gestational age and neonatal outcome. Obstet Gynecol 1996;87(5 Pt 1):771–8.
Been JV, Zimmermann LJ. Histological chorioamnionitis and respiratory outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed 2009;94:F218–25.
Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med 2009;14:2–7.
Beaudet L, Karuri S, Lau J, Magee F, Lee SK, von Dadelszen P. Placental pathology and clinical outcomes in a cohort of infants admitted to a neonatal intensive care unit. J Obstet Gynaecol Can 2007;29:315–23.
Rovira N, Alarcon A, Iriondo M, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Hum Dev 2011;87:253–7.
Sorensen LC, Maroun LL, Borch K, Lou HC, Greisen G. Neonatal cerebral oxygenation is not linked to foetal vasculitis and predicts intraventricular haemorrhage in preterm infants. Acta Paediatr 2008;97:1529–34.
Verhagen EA, Van Braeckel KNJA, van der Veere CN, et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev Med Child Neurol 2014; epub ahead of print, 2014 Nov 8. doi: 10.1111/dmcn.12622.
Roescher AM, Timmer A, Hitzert MM, et al. Placental pathology and neurological morbidity in preterm infants during the first two weeks after birth. Early Hum Dev 2014;90:21–5.
Royal College of Obstetricians and Gynaecologists. Fetal and perinatal pathology. Report of a joint working party. London: RCOG Press, 2001.
Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med 1997;121:449–76.
Redline RW. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am J Obstet Gynecol 2005;192:452–7.
Society for Pediatric Pathology, Perinatal Section, Maternal Vascular Perfusion Nosology Committee; Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2004;7:237–49.
Society for Pediatric Pathology, Perinatal Section, Amniotic Fluid Infection Nosology Committee; Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6:435–48.
Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol 2007;38:1439–46.
Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol 2000;31:292–5.
Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol 2002;5:159–64.
Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2004;7:443–52.
Altshuler G, Arizawa M, Molnar-Nadasdy G. Meconium-induced umbilical cord vascular necrosis and ulceration: a potential link between the placenta and poor pregnancy outcome. Obstet Gynecol 1992;79(5 Pt 1)):760–6.
Ohyama M, Itani Y, Yamanaka M, et al. Maternal, neonatal, and placental features associated with diffuse chorioamniotic hemosiderosis, with special reference to neonatal morbidity and mortality. Pediatrics 2004;113:800–5.
Lewis S, Perrin E. Pathology of the Placenta. 2nd edn. New York: Churchill Livingstone, 1999.
Ogino S, Redline RW. Villous capillary lesions of the placenta: distinctions between chorangioma, chorangiomatosis, and chorangiosis. Hum Pathol 2000;31:945–54.
Baergen RN. Cord abnormalities, structural lesions, and cord “accidents”. Semin Diagn Pathol 2007;24:23–32.
Verhagen EA, Keating P, ter Horst HJ, Martijn A, Bos AF. Cerebral oxygen saturation and extraction in preterm infants with transient periventricular echodensities. Pediatrics 2009;124:294–301.
Acknowledgements
We greatly acknowledge the help of Titia Brantsma-van Wulfften Palthe in Utrecht for correcting the English manuscript. This study was part of the research program of the Postgraduate School for Behavioural and Cognitive Neurosciences (BCN), University of Groningen, Groningen, the Netherlands.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Roescher, A., Timmer, A., van der Laan, M. et al. In preterm infants, ascending intrauterine infection is associated with lower cerebral tissue oxygen saturation and higher oxygen extraction. Pediatr Res 77, 688–695 (2015). https://doi.org/10.1038/pr.2015.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.20