Abstract
Background:
Ondansetron has been shown to decrease postanesthesia shivering in adults, but this effect has never been studied in children. This study aimed to determine whether ondansetron decreases postanesthesia shivering in children undergoing caudal anesthesia.
Methods:
Fifty-nine 8- to 13-y-old children undergoing both intravenous and caudal anesthesia were included. As soon as anesthetization and caudal block were complete, children were given intravenous injections of 4 mg ondansetron or an equal volume of normal saline. Heart rate, blood pressure, oxygen saturation (SpO2), and body temperature were recorded just before application of general anesthesia to children and immediately upon entry into the recovery room after awakening from anesthesia. The shivering score was assessed, using a 4-point scale of severity, immediately upon entry into the recovery room after awakening from anesthesia, 15 min after entry into the recovery room, and 30 min after entry into the recovery room.
Results:
Treatment with ondansetron was associated with decreased odds of postanesthesia shivering symptoms compared to the control group. There was also a significant decrease in shivering score with time after anesthesia.
Conclusion:
Ondansetron decreases postanesthesia shivering in children receiving caudal block after intravenous anesthesia.
Similar content being viewed by others
Main
Shivering is a common phenomenon seen during recovery from general or regional anesthesia. It is primarily a response to the hypothermia that occurs during anesthesia (1,2). This hypothermia is due to an effect of the anesthetic on the thermoregulatory center in the hypothalamus and to decreased vascular resistance if sympathetic block is also present (3). Central control of the thermoregulatory center is complex and involves a number of neurotransmitters and receptors (2). Agents acting on several pathways, for example, the α-2 adrenergic agonist clonidine, the opioid meperidine, and the anticholinergic agent physostigmine, have been shown to decrease postanesthetic shivering (4,5,6).
Ondansetron, a specific antagonist of the 5-HT3 receptor for serotonin and a drug currently used to inhibit postoperative nausea and vomiting, decreases the incidence of postanesthesia shivering after both general and regional anesthesia and has few side effects (3,4,5,7,8,9). However, all studies of its effectiveness have been performed on adults. Treatment of postanesthesia shivering has not yet been studied in children (1,10). The relatively low incidence of shivering in children may be one reason for this lack of attention (1). However, shivering is one of the most disturbing events for patients that occurs during the postoperative period (1) and therefore needs attention.
Our department is specialized for pediatric anesthesia. We have a number of patients for whom caudal block is necessary, and we have observed that many children receiving caudal block anesthesia experience shivering after awakening. Although ondansetron is well known to have an effect on postanesthesia shivering in adults, it has not been studied in children, and clinical experience has been that drugs often affect children differently from adults.
Therefore, the aim of this study was to evaluate the effectiveness of ondansetron in preventing postanesthesia shivering in school-age children receiving caudal block after general anesthesia.
Results
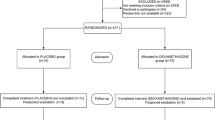
Fifty-nine children were recruited to the study, of which 28 were assigned to the ondansetron group and 31 to the control group. All patients were recruited consecutively, and there were no drop-outs. All patients completed the study ( Figure 1 ). Although we did not use gender as a selection criterion, only 4 of the 59 children were females. Demographic and clinical characteristics between ondansetron and control groups were comparable (all P > 0.05; Table 1 ). In addition, no difference between the two groups was found for vital signs assessments (all P > 0.05; Table 2 ).
CONSORT diagram.
Table 3 shows the time trend, after awakening from anesthesia, of the distribution of shivering scores in the ondansetron and control groups. The percentage in the control group with no shivering was 19.4, 35.5, and 67.7% at T0 (that is, upon awakening from anesthesia), T1, and T2. For patients treated with ondansetron, the percentage with no shivering also increased with time. Thirty minutes after entering the recovery room, almost all ondansetron patients (96.4%) had no symptoms of shivering ( Table 3 ).
Significant decreases were found in both heart rate (mean difference = −5.46, SE = 2.13, P = 0.01) and body temperature (mean difference = −0.36, SE = 0.05, P < 0.001) after anesthesia, although the numerical value of the change in body temperature was small. Treatment with ondansetron was associated with decreased odds of shivering symptoms compared to the control group (odds ratio = 0.18, 95% confidence interval = 0.07–0.45, P < 0.001). There was also a significant decrease in shivering score with time (odds ratio = 0.28, 95% confidence interval = 0.20–0.40, P < 0.001; Table 4 ).
Discussion
Ondansetron, injected after caudal block and before the initiation of surgery, significantly reduced the incidence of postanesthesia shivering in 8- to 13-y-old children undergoing general anesthesia with caudal block. A significant decrease in shivering with time after awakening was also observed. This is the first study to evaluate the effectiveness of ondansetron in preventing postanesthesia shivering in children.
Application of 4 mg intravenous ondansetron to children with caudal block after general anesthesia decreased shivering in our study. In adults, 6 and 12 mg intravenous ondansetron in one study, and 8 mg intravenous ondansetron in two other studies, decreased shivering after spinal anesthesia (3,9,11). And similar ondansetron doses (4 and 8 mg) were found to decrease shivering after general anesthesia (4,5,8). In our study, ondansetron had no effect on core temperature. Four studies in adults have also reported no effect on core temperature (4,5,9,11).
One way for a drug to reduce shivering would be to lower the shivering threshold, so that the lower temperatures seen during recovery from anesthesia would no longer trigger shivering. Several antishivering drugs, the α-adrenergic agonist clonidine, the opioid meperidine, and the 5-HT3 agonist tramadol (which also acts on other receptors), decrease the shivering threshold and reduce shivering at least partly through this mechanism (5,6,12). However, a study of ondansetron in healthy volunteers reports no decrease in the shivering threshold (6).
Active re-warming reduces the risk of postanesthetic shivering but does not completely prevent its occurrence (2). Meperidine decreases shivering but can cause nausea, vomiting, and respiratory depression (7). And of the other antishivering drugs, clonidine can cause bradycardia, hypotension, and sedation; tramadol can cause nausea, vomiting; and dizziness; and physostigmine can cause nausea, vomiting, increased heart rate, and increased blood pressure (1,7). Ondansetron, in contrast, has a good safety profile, prevents postoperative nausea and vomiting, and has no cardiovascular side effects. Therefore, it deserves further study for use in children as a drug for prophylaxis against postanesthesia shivering.
Limitations of the study were that no prior sample size calculations were performed and that almost all participants were male, although author did not intend to include male children only. Another limitation is that although we set the operating room temperature to 24 °C, we did not measure the temperature of operating room and intravenous fluids.
In conclusion, ondansetron decreases postanesthesia shivering in children given caudal block after general anesthesia.
Methods
Patient Selection
This prospective, single-center, placebo-controlled study was conducted from July to November 2014, at the Children’s Hospital of Zhejiang University School of Medicine. The approval of the Institutional Review Board of Children’s Hospital of Zhejiang University School of Medicine was obtained for the study, as well as informed written permission from the parents or legal guardians of each participant. This study had been registered and the trial number is ChiCTR-IPR-15006629.
Male or female children, 8–13 y of age, American Society of Anesthesiologists levels I–II, who were expected to undergo surgery lasting 0.5 to 2 h employing both intravenous and caudal anesthesia were included in the study. Subjects included patients needing hypospadias repair surgery, orthopedic surgery for concealed penis, lower limb orthopedic surgery, and general surgery for inguinal hernia and were assigned after inclusion (using a random number generator) to either an ondansetron group or a control group. Children with concomitant cardiopulmonary disease, emergency surgery, fever, endocrine system disease, severe trauma, or burn injuries were excluded. Other criteria for exclusion were inability to maintain spontaneous breathing, respiratory depression, or poor outcome of caudal block after anesthesia. However, no child had any of these surgical situations.
Study Protocol
After a preoperative examination using electrocardiography, pulse oximetry, noninvasive blood pressure recording, and temperature measurement using an ear thermometer was performed, a midazolam (0.1–0.2 mg/kg) and pentazocine (0.5 mg/kg) mixture was given together with propofol (1–2 mg/kg) intravenously to achieve general anesthesia. No intubation or muscle relaxants were used. Children were under sedation with spontaneous breathing, and oxygen was administered using a face mask during the entire surgical procedure. Children were then placed in a prone position, and caudal anesthesia (maximum volume 23 ml) was achieved by injecting 1 ml/kg of solution containing 0.8% lidocaine and 0.2% ropivacaine through the sacrococcygeal membrane into the sacral canal.
As soon as intravenous anesthesia and caudal block were complete, children in the ondansetron group were given an intravenous injection of 4 mg ondansetron, and children in the control group were given an intravenous injection of an equal volume of normal saline. The shivering score was assessed at three time points: immediately upon entry into the recovery room after awakening from anesthesia (T0), 15 min after entry into the recovery room (T1), and 30 min after entry into the recovery room (T2). If the children were still asleep when entering the recovery room, 0.1 mg flumazenil was injected to antagonize the effect of midazolam and cause awakening. Heart rate, blood pressure, oxygen saturation (SpO2), and body temperature were recorded at two specific time points: the time just before application of caudal anesthesia (S0) and immediately upon entry into the recovery room after awakening from anesthesia (T0). The operating room nurses who determined the shivering scores were blinded as to the group assignment of the subject and were not involved in the study.
We used methods other than giving drugs for children with severe chills and shivering: keeping warm with blankets and heating pads, verbal consolation, etc. Although serious chills and shivering in adults can be treated with drugs such as tramadol or pethidine, we do not have experience with the use of these drugs in children, and their use can sometimes cause relatively serious complications, so we did not use them during this study.
The shivering score was defined as follows: 0, no shivering; 1, piloerection or peripheral vasoconstriction but no visible shivering; 2, muscular activity in only one muscle group; 3, muscular activity in more than one muscle group but not generalized; 4, shivering involving the whole body (13). Because there is an order to the severity of shivering score, the score was treated as an ordinal variable.
Statistical Analysis
We used the power calculation from a previous study of postanesthesia shivering (5) to determine 29 patients in each group required for the study. When we performed a post hoc power calculation for the shivering score, using our study’s sample size (control, n = 31; ondansetron, n = 28) and percentage with shivering symptoms at T2 (control, 32.3%; ondansetron, 3.6%), the statistical power was 0.82 with an α level of 0.05, which appeared adequate to detect the difference in shivering between groups.
Categorical and ordinal variables (i.e., sex and shivering score, respectively) were shown by number and percent, and continuous variables (e.g., vital signs) by mean and SD. Comparisons between ondansetron and control groups were performed by Fisher’s exact test for categorical and ordinal variables and by two-sample t-tests for continuous variables. For vital signs, two-sample t-tests were used to examine differences in means between groups at each time points.
Considering repeated measurements of outcome variables, generalized estimating equations were performed to estimate group effects and time effects on vital signs and shivering. The identity link function was used for vital signs, and the mean differences between time points and groups were presented. For the shivering score, the effectiveness of ondansetron was presented by odds ratio with 95% confidence interval with the cumulative logit link function in the generalized estimating equation model. An odds ratio less than 1 with a 95% confidence interval not covering 1 indicates a significant protective effect of ondansetron.
All statistical analyses were carried out with IBM SPSS statistical software version 22 for Windows (IBM, New York, NY). A two-tailed P value less than 0.05 was considered significant.
Statement of Financial Support
We have not received any grants or financial support for this study.
Disclosures
There are no competing interests and no disclosures declared.
References
Kranke P, Eberhart LH, Roewer N, Tramèr MR. Postoperative shivering in children: a review on pharmacologic prevention and treatment. Paediatr Drugs 2003;5:373–83.
Alfonsi P. Postanaesthetic shivering: epidemiology, pathophysiology, and approaches to prevention and management. Drugs 2001;61:2193–205.
Marashi SM, Soltani-Omid S, Soltani Mohammadi S, Aghajani Y, Movafegh A. Comparing two different doses of intravenous ondansetron with placebo on attenuation of spinal-induced hypotension and shivering. Anesth Pain Med 2014;4:e12055.
Asl ME, Isazadefar K, Mohammadian A, Khoshbaten M. Ondansetron and meperidine prevent postoperative shivering after general anesthesia. Middle East J Anaesthesiol 2011;21:67–70.
Powell RM, Buggy DJ. Ondansetron given before induction of anesthesia reduces shivering after general anesthesia. Anesth Analg 2000;90:1423–7.
Komatsu R, Orhan-Sungur M, In J, et al. Ondansetron does not reduce the shivering threshold in healthy volunteers. Br J Anaesth 2006;96:732–7.
Tie HT, Su GZ, He K, Liang SR, Yuan HW, Mou JH. Efficacy and safety of ondansetron in preventing postanesthesia shivering: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2014;14:12.
Abdollahi MH, Forouzannia SK, Bagherinasab M, et al. The effect of ondansetron and meperedin on preventing shivering after off-pump coronary artery bypass graft. Acta Med Iran 2012;50:395–8.
Kelsaka E, Baris S, Karakaya D, Sarihasan B. Comparison of ondansetron and meperidine for prevention of shivering in patients undergoing spinal anesthesia. Reg Anesth Pain Med 2006;31:40–5.
Akin A, Esmaoglu A, Boyaci A. Postoperative shivering in children and causative factors. Paediatr Anaesth 2005;15:1089–93.
Safavi M, Honarmand A, Negahban M, Attari M. Prophylactic effects of intrathecal meperidine and intravenous ondansetron on shivering in patients undergoing lower extremity orthopedic surgery under spinal anesthesia. J Res Pharm Pract 2014;3:94–9.
Nakasuji M, Nakamura M, Imanaka N, Tanaka M, Nomura M, Suh SH. Intraoperative high-dose remifentanil increases post-anaesthetic shivering. Br J Anaesth 2010;105:162–7.
Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia 1994;49:205–7.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Lin, H., Wang, J., Jin, Z. et al. Preventative effect of ondansetron on postanesthesia shivering in children undergoing caudal anesthesia: a randomized double-blinded clinical trial. Pediatr Res 79, 96–99 (2016). https://doi.org/10.1038/pr.2015.185
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.185