Abstract
Background:
Specific probiotic bacteria have proven to be effective in the prevention and treatment of infectious diseases in early life in at-risk populations. The impact of administration of Bifidobacterium animalis subsp. lactis BB-12 (BB-12) on the risk of acute infectious diseases was studied in healthy children.
Methods:
In this double-blind, placebo-controlled study, 109 1-mo-old infants were assigned randomly to a probiotic group receiving a BB-12–containing tablet (n = 55) or a placebo (n = 54). Test tablets were administered to the infants twice a day (daily dose of BB-12 10 billion colony-forming units) until the age of 2 y with a novel slow-release pacifier or a spoon. Breastfeeding habits, pacifier use, dietary habits, medications, and all signs and symptoms of acute infections were registered in diaries by parents and in questionnaires by trained professionals.
Results:
The infants receiving BB-12 were reported to have experienced fewer respiratory tract infections (RTIs; 87 vs. 100%; risk ratio: 0.87; 95% confidence interval: 0.76, 1.00; P = 0.033) than the controls. No significant differences between the groups were observed in reported gastrointestinal symptoms, otitis media, or fever. The baseline characteristics of the two groups were similar, as was the duration of breastfeeding.
Conclusion:
Administration of BB-12 in early childhood may reduce RTIs.
Similar content being viewed by others
Main
Respiratory tract infections (RTIs) and viral gastroenteritis are the most significant illnesses in early childhood. In developed countries, infants experience several RTIs during the first year of life and one quarter suffer from recurrent or prolonged infections (1,2). RTIs often coincide with or precede acute otitis media (AOM), the most common cause for antibiotic use in children (3). Antibiotic treatments may lead to the development of antibiotic resistance and disturb the normal balance of microbiota, which further facilitates the colonization of pathogens (4). On the other hand, common RTIs early in life assist in shaping immunological development, and consequently may both protect children and predispose them to repeated infections, and inflammatory and allergic diseases later in life (5,6).
Indigenous intestinal microbiota, the primary source of microbial exposure, is considered instrumental in providing maturational stimuli to the immature immune system (7). This creates the basis for the concept of probiotics, defined as “live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host” (8). Probiotics have several beneficial effects, including normalization of the gut microbiota composition and interaction with the innate and adaptive immune system of the child, which may promote resistance against pathogens (9,10,11).
The most widely used probiotic species, which belong to the genera Lactobacillus and Bifidobacterium, have shown clinically significant benefits in treatment and prevention of childhood diarrheal and allergic diseases in at-risk populations such as allergic families, hospitalized patients, or children in day-care centers (12,13). Recently, the effects of probiotics in the prevention of RTIs have received increasing attention, with conflicting results in 15 randomized trials (14).
There are many sources of confounders in probiotic interventions in children. First, mode of probiotic administration in the general child population is challenging. Second, the selection of specific probiotic strain or a probiotic mixture is crucial for the possible beneficial effects. Moreover, the duration of breastfeeding and use of infant formulas affect the outcome.
We have earlier introduced a controlled administration method for probiotic supplementation via a novel slow-release pacifier (15). We delivered to infants a probiotic tablet containing BB-12. BB-12 is a thoroughly studied, safe, and well-tolerated probiotic (16). Furthermore, at the time of the trial, BB-12–containing products were not available for children in Finland and very few such products were available for adults. The delivery of BB-12 significantly reduced the occurrence of RTIs during the first 8 mo of life (17). Here, we report common infectious symptoms and diseases that occurred in the study during the first 2 y of a child’s life. We hypothesized that administration of BB-12 to young healthy children could reduce the risk of acute infectious diseases.
Results
Baseline Characteristics
Background information on the families has been published earlier (18). The groups did not differ with regard to baseline characteristics. The age of the mothers and fathers, the educational background of the parents, the number of parents following special diets, or occurrence of reported diseases in the families did not differ between the groups. The mean duration of exclusive breastfeeding was 3.4 mo (SD 1.7) in the probiotic and 3.8 mo (SD 1.9) in the control group (P = 0.692). In both groups, more than 70% (23/32 vs. 25/35) of mothers continued to breastfeed for at least 6 mo and, respectively, more than 30% (11/32 vs. 12/35) for at least 12 mo. The diet histories of the children in the two groups were similar. Of the children, 50% (16/32) in the BB-12 group and 66% (23/35) in the control group used commercial probiotic products irregularly, mainly between the ages of 1 and 2 y.
Participant Flow
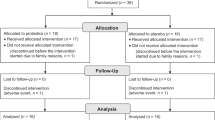
Of the randomized infants, 69% (75/109) started the novel administration method, receiving either BB-12 (n = 38) or the xylitol control (n = 37). The rest of the infants, 31% (34/109), did not accept the pacifier before the age of 2 mo (one of the inclusion criteria) and the parents were not motivated to crush the tablet. Only those who completed the follow-up (n = 67) were included in the analysis, as seen in Figure 1 . A daily diary of the symptoms of infections, doctor-diagnosed illnesses, and the use of medications was available for 96% (64/67) of the children (see Table 1 ). Compliance during the study was good: 81% (26/32) in the probiotic group and 80% (28/35) in the control group reported administering test tablets two times daily, the rest of the participants once a day. The mean duration of tablet delivery was 14.9 mo in the probiotic group and 14.8 mo in the control group (P = 0.780).
Clinical trial profile.
Acute Infections and Symptoms at the 2-y Follow-up
Intervention with delivery of BB-12 significantly reduced the occurrence of RTIs reported by parents during the first 2 y of life (see Table 1 ). At least one episode of RTI was experienced in 87% (27/31) of the children in the BB-12 group and in 100% (33/33) of the children in the control group (P = 0.033). There was a trend of lower frequency of recurrent RTIs in children receiving the probiotic, 39% (12/31), as compared with 61% (20/33) (P = 0.080) in those receiving the placebo (see Table 2 ).
As seen in Table 1 , we did not find any reduced tendency in the occurrence of AOM episodes at the 2-y follow-up. At least one doctor-diagnosed AOM was detected in 61% (19/31) of the children in the probiotic group and in 64% (21/33) of the children in the control group (P = 0.846). No association between the occurrence of AOM and pacifier usage (sucking time per day, duration of usage) could be found (not shown). Furthermore, no differences between the BB-12 and the control group were found in the occurrence of fever episodes (P = 0.562) or gastrointestinal infections (P = 0.332). Also no significant differences were detected between the two groups in antibiotic treatments prescribed by doctors (not shown).
Fecal Recovery of BB-12
Fecal samples were available for 65 children. BB-12 was recovered in the feces of 71% (22/31) of the children receiving the BB-12 tablet and in 56% (19/34) of the children receiving the control tablet at the age of 2 y. This difference was not statistically significant.
Adverse Effects
No serious adverse effects were detected during the administration period. Two children receiving BB-12 withdrew from the study as a result of gastrointestinal complaints. One infant in the control group was diagnosed with atopic eczema and his physician recommended that the family discontinue the intervention.
Discussion
We found in healthy children a significant reduction in the prevalence of RTIs during the first 2 y of life as a result of controlled supplementation of BB-12, even though the reduction was more prominent during the first 8 mo (17). We also found a trend of reduced occurrence of recurrent RTI episodes in the BB-12 group. Such infections are the most significant illnesses in this age group regardless of type of day care.
Several studies demonstrate that Lactobacillus rhamnosus GG and other probiotic lactobacilli may reduce the occurrence of RTIs in children (14). However, little information exists on the effects of other probiotics, such as bifidobacteria, on RTIs in early childhood. BB-12 did not have any effect on respiratory illnesses in infants aged 4–10 mo (19). In another study, the combination of BB-12 and L. rhamnosus GG reduced the risk of recurrent respiratory infections in 1-y-old children (20). Previous studies have often focused on at-risk populations such as allergic families or hospitalized children. In that respect, our healthy children comprise a highly interesting study population. RTIs often coincide with or precede AOM, the most common cause for antibiotic use in children. In the study by Rautava et al. (20), a reduction in the incidence of AOM was detected at the age of 7 mo, but not at 12 mo. We could not find any probiotic-associated effects on AOM either at the age of 8 mo (17) or 2 y. The low prevalence of AOM may explain the result at 8 mo (17), but not in the 2-y-old children.
It has been suggested that probiotics, including BB-12, reduce the risk of acute diarrhea (11). L. rhamnosus GG, among others, is recommended in the prevention and treatment of diarrhea (13). We found no probiotic-associated effects on gastrointestinal infection occurrence either at the age of 8 mo (17) or 2 y. The Finnish rotavirus vaccine program started only in 2009, so it could not have affected our results. Also a reduction in the occurrence of fever in children has repeatedly been reported for probiotics (11,14,21). We could, however, find no effect by BB-12 administration on occurrence of fever episodes.
The diagnoses of infections and other illnesses during the follow-up were mainly based on the subjective evaluation of symptoms by parents. Infants are unable to communicate their own symptoms, and thus, it is possible that the prevalence of infections may have been over- or underestimated. Parents also recorded in the diaries respiratory infections with complications, i.e. AOM, bronchitis, pneumonia, and sinusitis, diagnosed by a doctor (municipal or private) unrelated to the study. The accuracy of these diagnoses was impossible to standardize. Measuring can be problematic, for example in the case of allergic rhinitis, which can sometimes mimic common cold. However, it can be assumed that reporting of these infections occurred equally in both study groups and was based on careful instructions for parents when the child was 1 mo of age.
At 8 mo of age, the children in the BB-12 group showed a fecal colonization percentage of 62, while in the control group, the percentage was only 17 (17). Probiotics consumed by the mother influences the microbiota of the child, for example via breastfeeding, which may explain why BB-12 could be detected in the feces of the 8-mo-old control children (22,23). At 2 y of age, more than half of the children of both groups showed BB-12–positive fecal samples. In the probiotic group, the fecal recovery of BB-12 was higher than in the control group, but the difference was not statistically significant. The assay method that we used is subspecies but not strain specific. There is a large variety of dairy products with undefined mixtures of bifidobacteria on the Finnish market. The use of such products by the children would be reflected in the fecal BB-12 colonization we found in the 2-y-old children. In addition, maternal breast milk and intestinal bifidobacteria, including species belonging to the Bifidobacterium animalis group, guide the compositional development of the Bifidobacterium microbiota in children (23).
It could be hypothesized that xylitol could act as a prebiotic substance consequently influencing gut colonization. In theory, xylitol is able to fulfill all classification criteria for a prebiotic food ingredient. In our study, most children received a daily dose of 200 mg xylitol. Only about 10% of the children received the larger dose, 600 mg, after the age of 6 mo. Thus, the amounts of xylitol ingested by the infants should be too small by far for xylitol to function as a prebiotic substance.
Our study examined the long-term effects of probiotic bacteria on infections in healthy, normally breastfed children. The intervention lasted through the first 2 y of the child’s life. The evidence concerning the safety of probiotics in healthy newborns and young infants with immature defense systems is limited. We did not find any serious adverse effects in the BB-12 group during the administration period. This finding is in accordance with earlier BB-12 studies in infants (16,20,24). Our findings support the safe use of BB-12 even in early infancy.
The group size (54–55) obtained in the randomization should have been high enough to result in approximately 40 children starting the intervention. However, there was no way of estimating how many children would accept the novel pacifier and how many parents were prepared to deliver the tablet with a spoon in case the child did not accept the pacifier. Even though our subject numbers in the two groups were below 40 (37 and 38), we found a both clinically and statistically significant reduction in the occurrence of respiratory infections but not even trends in the occurrence of other infectious diseases. The infant number was estimated to be high enough to detect a 17% absolute risk reduction statistically significant.
Within the limitations of this study, controlled administration of BB-12 to young healthy children significantly reduced the occurrence of RTIs, but not of other acute infectious diseases. In view of the fact that in this age group, infectious diseases are common and may even lead to hospitalization, our results are of clinical importance to young families with children.
Methods
Subjects
The families participating in this randomized, double-blind, placebo-controlled clinical trial (NCT00638677, http://www.clinicaltrials.gov) were recruited from Muurame and Korpilahti, Finland, between September 2004 and February 2007.
Sample size was calculated on the basis of a previous study examining the incidence of AOM and RTIs during the first year of life. AOM occurs at least once in 40% of children (3) and RTIs occurs in most Finnish children before the age of 1 (1). Up to 80% of Finnish children use a pacifier. We calculated that with a group of 40 children or more starting the intervention, we would be able to obtain not only statistically but also clinically significant results. The population in the Korpilahti and Muurame areas is rather stable and attrition of children as a result of moving out of the area could be estimated to be low. The local housing areas are similar and the local air quality is good. Thus, it is unlikely that these conditions could have been affected to the outcome.
Pregnant mothers (n = 479) received a leaflet explaining the trial at well-baby clinics. The families interested in participating (n = 189) then contacted the research nurse. After detailed information, 175 families gave their preliminary consent to participate in the study. Finally, 135 newborn infants were assessed for eligibility and 109 were randomized, as seen in Figure 1 . One of the authors (K.P.) created a randomization list, which was computer generated in blocks of three. This two-armed trial is a substudy of a three-armed trial originally aiming to study the effect of BB-12 and xylitol on oral health (18).
The inclusion criteria of the trial were that: (i) the child was healthy, (ii) the parents agreed to use the novel slow-release pacifier, and (iii) the child started to use the pacifier before the age of 2 mo. In cases where the child did not start using the pacifier but the parents were motivated to remain in the study, they were offered the possibility of delivering the crushed tablet to the child using a spoon. Reasons for not participating in the trial included moving out of the area, miscarriage, and lack of interest in the trial.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human patients were approved by the Hospital District of Southwest Finland.
Interventions
The probiotic bacterium used was B. animalis subsp. lactis BB-12 (BB-12, DSM 15954; Chr. Hansen A/S, Hoersholm, Denmark). Each tablet contained 5 billion colony-forming units of BB-12 in addition to bulking agent xylitol. The smaller tablet contained 100 mg xylitol (Danisco, Kotka, Finland), the larger tablet 300 mg xylitol. The placebo tablet contained xylitol, in respective amounts. All tablets were manufactured by Oy Karl Fazer Ab (Vantaa, Finland) and packed in white plastic bottles with color codes. Freshly made tablets were delivered to the families biannually and stored in a refrigerator. In these conditions, the viability of the BB-12 remained unaltered. The viability was verified in all tablet batches from tablets crushed in saline and plate cultured on Difco Lactobacilli MRS (Becton Dickinson and Company, Sparks, MD) agars.
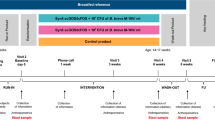
Test tablets were administered from the age of 1 to 2 mo with a novel slow-release pacifier (Alanen and Söderling, US Patent 6,203,566, 20 March 2001). The pacifier contained a pouch in which the tablet was inserted. The children received the tablets twice a day via a small pacifier (volume 120 µl) until 6–8 mo of age, thereafter via a larger pacifier (volume 250 µl) until the age of 2 y. The families were encouraged not to use any other pacifier besides the slow-release pacifier. If, however, they wanted to use a regular pacifier as well, they were offered the pouch-free version of the slow-release pacifier (Alanen and Varrela, US Patent 5,922,010, 13 July 1999). At the first study visit, the guardian received hands-on instructions on the use of the slow-release pacifier. The pacifiers were manufactured by Mekalasi Oy, Konnevesi, Finland.
Compliance, i.e. the daily usage of the tablet, was assessed during study visits. The parents reported the daily frequency and duration of tablet administration in questionnaires. In addition, fecal recovery of BB-12 can also be considered as a measure of compliance.
Study Design and Data Collection
The study design and data collection have been described in detail earlier (17). Infants and parents visited the healthcare centers at the age of 1 mo, 8 mo, and 2 y. At these appointments, a trained professional, a dental nurse, or a dental hygienist interviewed the guardian using structured questionnaires validated in earlier studies (5). At the 1-mo (baseline) visit, the parents were interviewed for background information regarding the child’s birth and the family. The guardians provided information about parents’ and siblings’ medical history, parents’ smoking habits, and special diets. At this 1-mo visit, parents also received detailed instructions on the use of study diaries concerning the child’s nutrition and health and the use of medications.
At the 2-y visit, parents were interviewed for information on breastfeeding status, use of the tablet and the pacifier, use of complementary foods in the infant’s diet, dairy products, and products containing probiotics. The use of commercial probiotic-containing products was not restricted, since, at the time of the study, BB-12 was found only in a few products for the adult population containing undefined mixtures of bifidobacteria. All infections and other health problems had been recorded in special diaries kept by parents during the first 2 y of the children’s life. Also all adverse effects had been recorded in detail.
All study personnel and participants were blinded to treatment assignment for the duration of the study. Only one of the authors (E.M.S.) had the code, since she was responsible for checking the BB-12 viability of the tablet batches. She did not, however, participate in clinical assessments, producing or analyzing the data at any stage of the trial, or have any contact with study participants.
Outcome Measures
The primary outcome measure for the study was the prevalence of overall acute infections occurring before the age of 2 y, such infections consisting of RTIs, AOM, gastrointestinal infections, and fever episodes. The symptoms of acute RTIs included runny nose, cough, and shortness of breath. AOM occurrences reported by parents included all diagnoses made by municipal or private doctors on the basis of commonly accepted criteria. Gastrointestinal infections included every episode with watery diarrhea or vomiting. Fever episodes included elevated body temperatures (>37 °C) lasting for at least 1 d. For infectious diseases and use of antibiotics and other prescription drugs, frequency of episodes/usage was registered. Four or more episodes of infectious disease was considered recurrent. Parents also reported any occurrence of atopic diseases or allergic sensitizations. Successful intestinal passage of BB-12 was chosen as the secondary outcome measure.
Quantification of BB-12 in Feces by quantitative PCR
At the 2-y visit, fresh fecal samples collected by the families were delivered to the healthcare centers. The fecal samples were stored at the healthcare centers at −20 °C for up to 2 mo, and then delivered on dry ice to Turku. Storage in Turku took place at −70 °C before transportation on dry ice to Chr. Hansen for analysis of BB-12.
BB-12 DNA was quantified by a B. animalis subsp. lactis–specific quantitative PCR assay. BB-12 is a member of this subspecies, which is rarely encountered in humans who have not ingested strains of it recently. Assay design was based on partial bifidobacterial 23S rDNA sequences (GenBank accession numbers GQ340897–GQ340908). Specificity of the assay was tested on DNA purified from reference cultures. DNA was extracted in duplicate from each feces sample. Total DNA was extracted from approximately 200 mg of feces with the help of the QiaAmp DNA Stool Mini Kit (Qiagen, Hilden, Germany) with the following modifications: in a screw cap tube, 200 mg of feces were mixed with 1 ml of buffer and 0.1 mm zirkonia beads and treated for 5 min in a Mini-Beadbeater (BioSpec, Bartlesville, OK) at highest speed. Thereafter, purification was continued according to the manufacturer’s instructions. Extraction efficiency and detection limit were determined to be 5% resp. 105 colony-forming unit/g using feces samples spiked with known amounts of BB-12 colony-forming units.
For the quantitative PCR reactions, fecal DNA was diluted 10-fold. Each DNA sample was analyzed in triplicate. Each PCR reaction contained 25 µl 2× Probe Mastermix (Eurogentec, Seraing, Belgium), 300 nM each BAL-23S-F (5′-CAGGTGGTCTGGTAGAGTATACCG-3′) and BAL-23S-R (5′-ACGGCGACTTGCGTCTTG-3′), 250 nM BAL-23S-P (5′-FAM-CGCCCACGACCCGCAAG-TAMRA-3′) and 5 µl DNA in a total volume of 50 µl. Reactions were run on an ABI Prism 7500 with the following program: 1 cycle of 10 min at 95 °C followed by 2 min at 50 °C, 45 cycles of 15 s at 95 °C followed by 1 min at 60 °C. Quantification of fecal BB-12 DNA was performed by absolute quantification against a dilution series of a pure BB-12 DNA sample.
Statistical Methods
Data were analyzed with SPSS (version 14.0; SPSS, Chicago, IL) by a blinded statistician (K.P.). In terms of baseline/background characteristics, the differences between the groups were tested for significance using the Student’s t-test and chi-square test. Additionally, differences between the groups in terms of distribution in reported acute respiratory infections, AOM, fever, and gastrointestinal infection categories were tested for significance using the chi-square test, for original frequency as well as for dichotomized data. The risk ratio and its 95% confidence interval were calculated to measure the group differences in relation to the prevalence of the studied diseases. A P value of less than 0.05 was considered statistically significant.
Statement of Financial Support
T.J.T. was supported by personal grant from Finnish Dental Society Apollonia.
Disclosures
The authors declare that there are no conflicts of interest.
References
Vesa S, Kleemola M, Blomqvist S, Takala A, Kilpi T, Hovi T. Epidemiology of documented viral respiratory infections and acute otitis media in a cohort of children followed from two to twenty-four months of age. Pediatr Infect Dis J 2001;20:574–81.
Nokso-Koivisto J, Pitkäranta A, Blomqvist S, et al. Viral etiology of frequently recurring respiratory tract infections in children. Clin Infect Dis 2002;35:540–6.
Alho OP, Koivu M, Sorri M, Rantakallio P. The occurrence of acute otitis media in infants. A life-table analysis. Int J Pediatr Otorhinolaryngol 1991;21:7–14.
Tagg JR, Dierksen KP. Bacterial replacement therapy: adapting ‘germ warfare’ to infection prevention. Trends Biotechnol 2003;21:217–23.
Kilpi T, Kero J, Jokinen J, et al. Common respiratory infections early in life may reduce the risk of atopic dermatitis. Clin Infect Dis 2002;34:620–6.
Rautava S, Arvilommi H, Isolauri E. Specific probiotics in enhancing maturation of IgA responses in formula-fed infants. Pediatr Res 2006;60:221–4.
Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy 2000;55:688–97.
Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14.
Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 1998;42:39–44.
Rautava S. Potential uses of probiotics in the neonate. Semin Fetal Neonatal Med 2007;12:45–53.
Hatakka K, Saxelin M. Probiotics in intestinal and non-intestinal infectious diseases–clinical evidence. Curr Pharm Des 2008;14:1351–67.
Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 2001;33:Suppl 2:S17–25.
Floch MH, Walker WA, Guandalini S, et al. Recommendations for probiotic use–2008. J Clin Gastroenterol 2008;42:Suppl 2:S104–8.
Lehtoranta L, Pitkäranta A, Korpela R. Probiotics in respiratory virus infections. Eur J Clin Microbiol Infect Dis 2014;33:1289–302.
Taipale T, Pienihäkkinen K, Alanen P, Jokela J, Söderling E. Dissolution of xylitol from a food supplement administered with a novel slow-release pacifier: preliminary results. Eur Arch Paediatr Dent 2007;8:123–5.
Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr 2004;79:261–7.
Taipale T, Pienihäkkinen K, Isolauri E, et al. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr 2011;105:409–16.
Taipale T, Pienihäkkinen K, Salminen S, Jokela J, Söderling E. Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res 2012;46:69–77.
Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 2005;115:5–9.
Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy–a randomised, double-blind, placebo-controlled study. Br J Nutr 2009;101:1722–6.
Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 2009;124:e172–9.
Collado MC, Delgado S, Maldonado A, Rodríguez JM. Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett Appl Microbiol 2009;48:523–8.
Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012;9:565–76.
Allen SJ, Jordan S, Storey M, et al. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J Nutr 2010;140:483–8.
Acknowledgements
The trial was designed by T.J.T., E.M.S., and J.T.J. E.I. provided the information needed to prepare the questionnaires and diaries. K.P. was responsible for data analysis. All authors participated in writing the report. The excellent assistance of the research nurses Mari Saastamoinen and Tuula Salopuro is gratefully acknowledged. Chr. Hansen A/S (Hoersholm, Denmark) donated the BB-12 and carried out the fecal analyses of BB-12. Oy Karl Fazer Ab (Vantaa, Finland) manufactured the tablets and Mekalasi Oy (Konnevesi, Finland) manufactured the pacifiers. Neither Hansen, Fazer nor Mekalasi provided financial support for this study.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Taipale, T., Pienihäkkinen, K., Isolauri, E. et al. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr Res 79, 65–69 (2016). https://doi.org/10.1038/pr.2015.174
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.174
This article is cited by
-
Effect of dietary Bifidobacterium animalis subsp. lactis BLa80 on growth, immune response, antioxidant capacity, and intestinal microbiota of juvenile Japanese seabass (Lateolabrax japonicus)
Aquaculture International (2024)
-
A microbiome case-control study of recurrent acute otitis media identified potentially protective bacterial genera
BMC Microbiology (2018)