Abstract
Background:
Nocturnal enuresis is a common developmental disorder in children, and primary monosymptomatic nocturnal enuresis (PMNE) is the dominant subtype.
Methods:
This study investigated brain functional abnormalities that are specifically related to working memory in children with PMNE using function magnetic resonance imaging (fMRI) in combination with an n-back task. Twenty children with PMNE and 20 healthy children, group-matched for age and sex, participated in this experiment.
Results:
Several brain regions exhibited reduced activation during the n-back task in children with PMNE, including the right precentral gyrus and the right inferior parietal lobule extending to the postcentral gyrus. Children with PMNE exhibited decreased cerebral activation in the task-positive network, increased task-related cerebral deactivation during a working memory task, and longer response times.
Conclusion:
Patients exhibited different brain response patterns to different levels of working memory and tended to compensate by greater default mode network deactivation to sustain normal working memory function. Our results suggest that children with PMNE have potential working memory dysfunction.
Similar content being viewed by others
Main
Nocturnal enuresis is a common developmental disorder that affects 15–20% of 5-y-old children (1). This disorder can persist into adolescence, and it negatively affects the self-image and performance of these children (2). Primary monosymptomatic nocturnal enuresis (PMNE) is diagnosed when a child exhibits enuresis without additional lower urinary tract symptoms with the exclusion of nocturia or a history of bladder dysfunction without a period of established urinary continence for more than 6 mo (3). Several factors are associated with and contribute to nocturnal enuresis, including heredity, polyuria, detrusor overactivity, sleep, and central nervous system mechanisms (4). Previous studies using electroencephalograms and event-related brain potentials indicated that maturational delays in central nervous system development are indicators of PMNE pathogenesis (5,6,7,8). Primary nocturnal enuresis children show a higher prevalence of all sleep disturbances (9). Furthermore, the prevalence of fine motor coordination and visuomotor integration were abnormal in prepubertal children with PMNE. These studies suggested that PMNE should not be considered as a voiding disorder alone (10).
Magnetic resonance imaging (MRI) techniques, such as structural MRI, functional MRI (fMRI), and diffusion MRI, provide efficient, feasible and noninvasive methods to investigate the biological mechanisms of incontinence. Several studies reported alterations in brain functions in patients with urgency and urge incontinence using fMRI (11,12). We performed a series of MRI experiments to investigate functional and structural abnormalities that are associated with PMNE. Our previous studies identified microstructural abnormalities in the thalamus, medial frontal gyrus, anterior cingulate cortex, and insular cortex of children with PMNE using diffusion MRI (13) and neurochemical abnormalities in the prefrontal cortex (PFC) and pons of children with PMNE using proton magnetic resonance spectroscopy (14). We also reported that the nature of local intrinsic activity changed in the PFC during resting states in children with PMNE (15). Furthermore, we demonstrated that functional brain networks in PMNE patients were characterized by a significantly lower clustering coefficient and global and local efficiencies, and a higher characteristic path length using graph theory-based network analysis (unpublished data). These findings suggest that children with PMNE have brain network alterations that affect global communication and integration. These studies demonstrated that children with PMNE exhibit structural, functional (under resting states), and neurochemical abnormalities in the brain, which suggest that children with PMNE have potential cognitive problems. In fact, forebrain activation in children with PMNE was altered during a response inhibition task (16).
Previous research suggests that the PFC is important for bladder control (11,12). We demonstrated previously that the PFC was abnormal in children with PMNE (13,14,15,16). The PFC is responsible for executive functions, such as mediating conflicting thoughts, making choices, predicting future events, and governing social control (17). The dorsolateral and ventrolateral PFC play important roles in working memory (18). One previous study utilized event-related fMRI in PMNE subjects and identified a dysfunction in the left cerebella of PMNE children. However, this study did not investigate PFC abnormalities during n-back working memory (19). The fMRI-testing paradigm in this previous study was a type of visual working memory task-categorical n-back paradigm of moderate difficulty without differentiation degrees. It is not clear whether impairments during different difficult n-back tasks exist in children with PMNE.
Working memory is an important cognitive function in humans that refers to the ability to transiently store and manipulate information “held online” in the service of complex cognition for further behavioral guidance (20). Working memory primarily involves a bilateral parieto-frontal network in the performance of classical n-back tasks (18). We hypothesized that children with PMNE have potential dysfunction during working memory, and they use different cognitive strategies during working memory compared to healthy children. This study investigated working memory in children with PMNE and healthy children using the classic n-back task with three levels of difficulty (0-back, 1-back, and 2-back) and fMRI.
Results
Task Performance
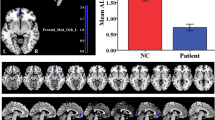
Children with PMNE exhibited slight better performance accuracy (during 1-back and 2-back) compared with control children, but no significant differences in the overall or individual n-back task means. For example, the average percentages of correct responses were 85.3 ± 7.3% for children with PMNE, and 82.9 ± 7.1% for the control children. The PMNE group exhibited a longer response times (RT) during the n-back task, and the average RT and 1-back RT were significantly longer in PMNE children. The average RT was longer in PMNE children (P < 0.05): 671.6 ± 114 ms in children with PMNE, and 588.9 ± 68.9 ms in control children. The 1-back RT was also longer in PMNE children (P < 0.05): 687.8 ± 132.7 ms in children with PMNE, and 582.3 ± 76.4 ms in control children. Additional details are shown in Figure 1 .
Working memory task performance in children with PMNE and controls. The solid line represents HC, and the dashed line represents PMNE. CR, average percentage of correct responses; HC, healthy children; RT, average response times. *P < 0.05 children with PMNE compared with healthy children.
Functional MRI Data
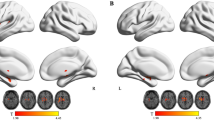
Task-positive networks across multiple cognitive domains. The control group exhibited significant right inferior parietal lobule and right lentiform nucleus activation in the “1-back” minus “0-back” contrast ( Table 1 and Figure 2 ). PMNE groups exhibited activation of bilateral middle frontal gyri, bilateral inferior parietal lobule, and left midbrain that extend to the right cerebellum anterior lobe ( Table 1 and Figure 2 ). There were no significant differences between groups.
Significant activation and deactivation within-group comparisons between 1-back vs. 0-back and 2-back vs. 0-back during working memory. Red represents positive activation, whereas blue represents negative activation. P < 0.05 with AlphaSim corrected (combined height threshold of P < 0.001 and a minimum cluster size of 49 voxels).
The control group exhibited significant activation in the right medial frontal gyrus, bilateral middle, inferior and superior frontal gyri, bilateral inferior parietal lobules extending to the superior parietal lobule, the right middle temporal gyrus, bilateral caudate, and bilateral cerebellum posterior lobes in “2-back” minus “0-back” contrast ( Table 1 and Figure 2 ). The PMNE group also exhibited similar frontal-occipital activation, including bilateral middle and inferior frontal gyri, bilateral inferior parietal lobules extending to the superior parietal lobule, and bilateral cerebellum posterior lobes ( Table 1 and Figure 2 ). There were no significant differences between groups.
The control group exhibited significant activation in the left medial frontal gyrus, bilateral middle, inferior and superior frontal gyri, bilateral inferior parietal lobules extending to the precuneus and superior parietal lobule, the right middle temporal gyrus, and the right cerebellum posterior lobe in “2-back” minus “1-back” contrast ( Table 2 and Figure 3 ). The PMNE group exhibited similar but weaker activation in the right medial frontal gyrus, bilateral middle and inferior frontal gyri, bilateral inferior parietal lobules, and the right superior parietal lobule ( Table 2 and Figure 3 ).The PMNE group exhibited significantly decreased activation in the right precentral gyrus, and the right inferior parietal lobule extending to the postcentral gyrus compared with controls ( Table 2 and Figure 3 ).
Significant activation and deactivation between 2-back vs. 1-back during low and high difficulty working memory tasks. Red represents positive activation, whereas blue represents negative activation. P < 0.05 with AlphaSim corrected (combined height threshold of P < 0.001 and a minimum cluster size of 49 voxels).
Task-related default mode network. Control children exhibited significantly reduced activation in the left anterior cingulate extending to the left medial frontal gyrus and the left anterior cingulate cortex during the n-back task compared with PMNE children ( Table 3 , Figures 2 and 3 ). Children with PMNE exhibited more n-back-related default mode network negative activation ( Table 3 , Figures 2 and 3 ) in the right medial frontal gyrus, left precuneus, left middle and posterior cingulate gyri, bilateral anterior cingulate cortex, left middle temporal gyrus, right parahippocampal gyrus, right precentral gyrus, and left cuneus extending to the lingual gyrus and insula cortex compared with controls.
Discussion
The n-back task captures the active part of working memory. Sustained attention, but not working memory, is required for the 0-back condition. Therefore, this task is typically used as a baseline condition. The 1-back task requires sustained attention and low difficulty working memory, and the 2-back task requires sustained attention and high difficulty working memory. The task involves the encoding of incoming stimuli, the monitoring, maintenance, and updating of the material, and matching the current stimulus to the n-back in the sequence (18). The task also involves decision, selection, inhibition, and interference resolution processes (18). More processes and mental effort are required in the performance of the 2-back than the 1-back task. Working memory–related activation was identified in a large cerebral network that primarily included the lateral premotor cortex; dorsal cingulate and medial premotor cortexes; dorsolateral and ventrolateral prefrontal cortexes; frontal poles; and medial and lateral posterior parietal cortexes (18).
Both groups of children exhibited activation of the fronto-parietal network during performance of the n-back task. In support of our hypothesis, different patterns of activity were observed in the working memory network in the PMNE group compared with controls. Children with PMNE exhibited more areas of activation during performance of the 1-back vs. 0-back tasks and exhibited less areas of activation during performance of the 2-back vs. 0-back tasks compared with healthy children ( Figure 2 ). Furthermore, the PMNE group exhibited significantly decreased activation in the right precentral gyrus and the right inferior parietal lobule extending to postcentral gyrus when the 2-back and 1-back conditions were compared directly ( Figure 3 ). These results suggest that children with PMNE tend to engage more brain areas during low difficulty working memory tasks and less brain areas during high difficulty working memory tasks compared to healthy children.
The PMNE group exhibited significantly decreased activation in the right precentral gyrus and the right inferior parietal lobule extending to the postcentral gyrus compared with the controls for the “2-back” minus “1-back” contrast. This result may suggest PMNE children decrease sensorimotor cortex activation to pay more attention to the task. The group difference occurred because of deactivation of the DMN in PMNE children. Children with PMNE had significantly more deactivation in the DMN compared to healthy controls. The DMN typically consists of the medial prefrontal cortex, posterior cingulate/precuneus, inferior parietal lobe, lateral temporal lobes, and hippocampal formation (21,22). The DMN is characterized by high activity when the mind is not engaged in specific behavioral tasks and low activity during focused attention on the external environment (23). Deactivation in the DMN reflects the allocation of cerebral resources to support task performance. The DMN tends to reduce activation when attention is focused on a particular task, and it tends to increase activation when attention is relaxed (21,22). Children with PMNE exhibited more deactivation in the DMN, which suggests that they paid more attention and tried their best to perform the n-back task. Anticevic et al. (23) demonstrated that DMN suppression was closely related to goal-directed cognition, possibly by a reduction in goal-irrelevant functions (e.g., mind-wandering). Previous studies demonstrated that greater DMN deactivation is associated with more successful performance in goal-directed cognitive tasks (24,25,26). Similarly, defective deactivation of DMN regions is associated with worse behavioral performance in several conditions associated with cognitive impairment (27,28,29). Children with PMNE exhibited significant deactivation during low and high difficulty working memory tasks (0-back vs. 1-back and 0-back vs. 2-back), and healthy children exhibited a few areas of deactivation during high difficulty working memory tasks (0-back vs. 2-back). McKiernan et al. (30) found that task-induced deactivation of the default mode network increased with task difficulty. Therefore, the low difficulty working memory task was not easy for PMNE children. The longer RT during 1-back working memory in children with PMNE compared with the control group also supports this hypothesis. The additional deactivation suggests that children with PMNE tend to compensate with greater DMN deactivation to sustain normal working memory function.
In addition, children with PMNE exhibited slightly reduced, but not significant, performance accuracy (during 1- back and 2-back) in the overall mean and individual n-back tasks compared with control children. Furthermore, the PMNE group exhibited longer RTs during the n-back task, and the average RT and 1-back RT were significantly longer in PMNE children. The performance data suggested that the PMNE group finished the n-back task similarly to the control group (similar accuracy) but with more difficulty (longer RT).
Our results suggest that children with PMNE have potential cognitive dysfunction. Esposito et al. (31) reported that enuretic children had a higher prevalence of mild reading difficulties than controls.
The prevalence of attention deficit hyperactivity disorder is also higher in children with PMNE compared to the normal population (32), and children with PMNE also exhibit memory/attention impairments (33). The patients in this study were diagnosed with PMNE but not attention deficit hyperactivity disorder, but they may have had potential memory/attention problems. In addition, the PMNE patients showed a higher prevalence in pathologic performance on visuomotor integration total task compared to controls (10). Previous observations suggested PMNE is complex and beyond a voiding disorder.
There is one limitation of this study. The subjects performed different task levels with different accuracies. Therefore, the incorrect responses would affect working memory-related neuronal processing. However, this factor does not affect the main difference that was identified between these two groups of children.
Conclusion
Children with PMNE exhibited decreased cerebral activation in the task positive network and increased task-related cerebral deactivation during a working memory task and longer RTs. PMNE children exhibited different patterns of brain responses to different levels of working memory, and they tended to compensate with greater DMN deactivation to sustain normal working memory function. Our results suggest that children with PMNE have working memory dysfunction and potential cognitive dysfunction.
Methods
Subjects
Forty children between 8 and 15 y of age participated in the study with the consent of the children and their guardians. There were two groups of 20 children: the PMNE group (15 males, 5 females) and the normal control group (15 males, 5 females). Children with PMNE had a mean age of 11.0 (SD = 1.8) y, and the healthy controls had a mean age of 10.8 (SD = 2.2) y. The mean duration of school education was 4.9 (SD = 1.8) y for children with PMNE and 4.7 (SD = 2.2) y for the healthy controls. All children were right handed and had an IQ > 75. All children with neurological or psychiatric diseases based on a clinical examination and structured interview were excluded. All 20 children with PMNE were outpatients of the Shanghai Children’s Medical Center. The Institutional Review Board of the Shanghai Children’s Medical Center, which is affiliated with the Shanghai Jiao Tong University School of Medicine, approved this study (No: SCMC-201014). Additional clinical data on the patient group are provided in Supplementary Table S1 online.
fMRI Paradigm: N-Back Task
This study used a similar version of the n-back task as previously described (34). Participants were instructed to perform and practice the tasks prior to the experiment. Three cognitive tasks were presented in a block-wise manner during fMRI. The paradigm consisted of a 10-s rest epoch and 12 alternating 18-s epochs of 0-back, 1-back, and 2-back conditions, followed by a 10-s rest epoch. All stimuli were shown in an IFIS-SA system (Invivo, Gainesville, FL), and the IFIS-SA system recorded the key-press responses of each subject. Subjects passively viewed a “cross” on a blank screen during the rest condition. The three experimental conditions included a red number, yellow number, and white number. Subjects responded to a single prespecified target number (e.g., 1, 2, 0) in the 0-back condition. The target in the 1-back condition was any number identical to the number that immediately preceded it (e.g., 1, 2, 2). The target in the 2-back condition was any number that was identical to the number presented two trials before the current trial (i.e., 1, 2, 1). In each block, nine numbers appeared in 2-s intervals (1 s fixation presentation and 1 s number presentation).
fMRI Image Acquisition
All imaging procedures were conducted at the Shanghai Key Laboratory of Magnetic Resonance (East China Normal University, Shanghai, China) using a Siemens 3.0 T Trio Tim MR system. Anatomical images that covered the whole brain were collected using a T1-weighted gradient echo pulse sequence (repetition time (TR) = 440 ms; echo time (TE) = 2.46 ms; flip angle = 90; 256 × 320 matrix; FOV = 22 × 22 cm2; and 32 slices). Collected images were used subsequently for exact anatomical localization. A total of 118 whole-brain volumes were collected on 32 oblique slices (3 mm thick, 33% Dist factor) using a T2*-weighted gradient echo spiral pulse sequence that was sensitive to blood oxygen level-dependent contrast with the following acquisition parameters: TE = 30 ms; TR = 2,000 ms; flip angle = 90; FOV = 22 × 22 cm2; acquisition matrix = 64 × 64; and voxel size = 3.4 × 3.4 × 3 mm3.
Analysis of Performance Data
RT and task performance accuracy were recorded for patients and control subjects for each condition. Performance data (accuracy and RT) were compared using unpaired t-tests to identify significant differences in performance between the two groups (P < 0.05).
Individual-Level fMRI Analysis
Functional images were analyzed using statistical parametric mapping software (SPM8; the Wellcome Trust Centre for Neuroimaging, London, UK) and Matlab software (Math-Works, Natick, MA) on a personal computer. Images from the first 10 TRs at the beginning of each trial were discarded to achieve steady-state signal equilibrium. Image preprocessing included motion correction and image realignment to each subject’s first image. Session images were normalized using the mean functional volume resampled to 3 × 3 × 3 mm3 voxels in Montreal Neurological Institute stereotaxic space. Spatial smoothing was performed on the functional images using a Gaussian filter (6 mm full width half-maximum). All children with head movement that exceeded 2 mm, regardless of rotation and translation, were excluded from further analyses.
We constructed six contrasts for each individual subject in the first-level analysis: 1-back minus 0-back, 2-back minus 0-back, 2-back minus 1-back, 0-back minus 1-back, 0-back minus 2-back, and 1-back minus 2-back. These contrast images were analyzed with SPM8 using a general linear model to determine voxel-wise t-statistics for each subject.
Group-Level Analysis
Con or contrast (difference in β) images of the first-level analysis were used for second-level group statistics. A random effects model was used for group analysis to determine voxel-wise t-statistics that contrasted specific conditions of interest. The contrast images of 20 PMNE subjects and 20 control subjects were used to determine within-group activation for the PMNE and control groups using one-sample t-tests. Two-sample t-tests were used to determine between-group differences. A random effects model was used for between-group analyses. Clusters of activation to display were defined as areas that surpassed a height threshold of P <0.05 with AlphaSim corrected (combined height threshold of P <0.001 and a minimum cluster size of 49 voxels) for all within- and between-group analyses.
Statement of Financial Support
This research was supported by grants from the National Natural Science Foundation of China (81201082), the Science and Technology Commission of Shanghai Municipality (14411969200), and the Shanghai Key Laboratory of Environment and Children Health (14DZ2271400). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of this manuscript. No additional external funding was received for this study.
Disclosure
The authors have no financial relationships relevant to this article to disclose.
References
Riccabona M. [Evaluation and management of enuresis. An update]. Urologe A 2010;49:861–9; quiz 870.
Theunis M, Van Hoecke E, Paesbrugge S, Hoebeke P, Vande Walle J. Self-image and performance in children with nocturnal enuresis. Eur Urol 2002;41:660–7; discussion 667.
Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol 2006;176:314–24.
Nevéus T. Diagnosis and management of nocturnal enuresis. Curr Opin Pediatr 2009;21:199–202.
Toros F, Ozge A, Bozlu M, Cayan S. Hyperventilation response in the electroencephalogram and psychiatric problems in children with primary monosymptomatic nocturnal enuresis. Scand J Urol Nephrol 2003;37:471–6.
Iscan A, Ozkul Y, Unal D, et al. Abnormalities in event-related potential and brainstem auditory evoked response in children with nocturnal enuresis. Brain Dev 2002;24:681–7.
Karlidag R, Ozisik HI, Soylu A, et al. Topographic abnormalities in event-related potentials in children with monosymptomatic nocturnal enuresis. Neurourol Urodyn 2004;23:237–40.
Freitag CM, Röhling D, Seifen S, Pukrop R, von Gontard A. Neurophysiology of nocturnal enuresis: evoked potentials and prepulse inhibition of the startle reflex. Dev Med Child Neurol 2006;48:278–84.
Esposito M, Gallai B, Parisi L, et al. Primary nocturnal enuresis as a risk factor for sleep disorders: an observational questionnaire-based multicenter study. Neuropsychiatr Dis Treat 2013;9:437–43.
Esposito M, Gallai B, Parisi L, et al. Visuomotor competencies and primary monosymptomatic nocturnal enuresis in prepubertal aged children. Neuropsychiatr Dis Treat 2013;9:921–6.
Fowler CJ, Griffiths DJ. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn 2010;29:49–55.
Griffiths D, Tadic SD. Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn 2008;27:466–74.
Lei D, Ma J, Shen X, et al. Changes in the brain microstructure of children with primary monosymptomatic nocturnal enuresis: a diffusion tensor imaging study. PLoS One 2012;7:e31023.
Zhang J, Lei D, Ma J, et al. Brain metabolite alterations in children with primary nocturnal enuresis using proton magnetic resonance spectroscopy. Neurochem Res 2014;39:1355–62.
Lei D, Ma J, Du X, Shen G, Tian M, Li G. Spontaneous brain activity changes in children with primary monosymptomatic nocturnal enuresis: a resting-state fMRI study. Neurourol Urodyn 2012;31:99–104.
Lei D, Ma J, Du X, Shen G, Tian M, Li G. Altered brain activation during response inhibition in children with primary nocturnal enuresis: an fMRI study. Hum Brain Mapp 2012;33:2913–9.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001;24:167–202.
Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59.
Yu B, Guo Q, Fan G, Ma H, Wang L, Liu N. Evaluation of working memory impairment in children with primary nocturnal enuresis: evidence from event-related functional magnetic resonance imaging. J Paediatr Child Health 2011;47:429–35.
Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci 2003;4:829–39.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 2001;98:676–82.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008;1124:1–38.
Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012;16:584–92.
Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage 2010;49:2638–48.
Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage 2004;23:921–7.
White TP, Jansen M, Doege K, et al. Theta power during encoding predicts subsequent-memory performance and default mode network deactivation. Hum Brain Mapp 2013;34:2929–43.
Fryer SL, Woods SW, Kiehl KA, et al. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front Psychiatry 2013;4:92.
Pomarol-Clotet E, Moro N, Sarró S, et al. Failure of de-activation in the medial frontal cortex in mania: evidence for default mode network dysfunction in the disorder. World J Biol Psychiatry 2012;13:616–26.
van Eimeren T, Monchi O, Ballanger B, Strafella AP. Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Arch Neurol 2009;66:877–83.
McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 2003;15:394–408.
Esposito M, Carotenuto M, Roccella M. Primary nocturnal enuresis and learning disability. Minerva Pediatr 2011;63:99–104.
Okur M, Ruzgar H, Erbey F, Kaya A. The evaluation of children with monosymptomatic nocturnal enuresis for attention deficit and hyperactivity disorder. Int J Psychiatry Clin Pract 2012;16:229–32.
Yu B, Kong F, Peng M, Ma H, Liu N, Guo Q. Assessment of memory/attention impairment in children with primary nocturnal enuresis: a voxel-based morphometry study. Eur J Radiol 2012;81:4119–22.
Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997;5:49–62.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Table S1
(DOC 49 kb)
Rights and permissions
About this article
Cite this article
Zhang, K., Ma, J., Lei, D. et al. Task positive and default mode networks during a working memory in children with primary monosymptomatic nocturnal enuresis and healthy controls. Pediatr Res 78, 422–429 (2015). https://doi.org/10.1038/pr.2015.120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.120
This article is cited by
-
Functional connectivity of thalamus in children with primary nocturnal enuresis: results from a resting-state fMRI study
Brain Imaging and Behavior (2021)