Abstract
Background:
The homeostatic mechanisms of iron metabolism and erythropoiesis in infants are unclear. Infants synthesize both fetal hemoglobin (HbF) and adult hemoglobin (HbA), and it is not known how the hemoglobin switch is regulated. We hypothesized that iron supplements to infants affect the disappearance of HbF.
Methods:
We randomized 285 low-birth-weight infants (2,000–2,500 g) into three intervention groups receiving 0, 1, or 2 mg/kg/d of iron supplements from 6 wk to 6 mo of age. In the present secondary analysis, we analyzed iron status, total hemoglobin (Hb), and HbF fraction at 6 wk, 12 wk, and at 6 mo and calculated absolute levels of HbF.
Results:
We observed dose-dependent increased levels of Hb in iron-supplemented groups at 6 mo of age. However, for absolute HbF concentration, there was no similar effect of intervention. Mean (SD) HbF was 81.2 (16.8), 37.0 (13.8), and 8.1 (5.6) g/l at 6 wk, 12 wk, and 6 mo, respectively, similar in all groups. In linear regression analyses, postconceptional age turned out as the major predictor of HbF, independent of gestational age at birth.
Conclusion:
Our hypothesis was rejected. Instead, we confirmed a close correlation to postconceptional age, supporting a genetically programmed switch, insensitive to most environmental factors including birth.
Similar content being viewed by others
Main
Iron is an essential element in hemoglobin synthesis, and iron deficiency is the most common disorder of hemoglobin metabolism, causing iron deficiency anemia in its final stage. Due to rapid growth during the first months of life and low iron intakes, infants in general, and low-birth-weight (LBW) infants in particular, are at increased risk of iron depletion and may have an iron-restricted erythropoiesis (1). However, there is a lack of knowledge concerning homeostatic mechanisms of iron and its relation to hemoglobin synthesis during the first months of life. Due to difficulties in obtaining blood samples from infants, most of the present knowledge is based on assumptions and findings from studies in adults. We have previously showed that young infants, in contrast to older children and adults, respond to iron supplements with increased Hb synthesis, independent of iron status. Based on this finding, we suggested that regulation of erythropoiesis in infants may be different or even not completely functional (2). The mechanism behind this, and other mechanisms of iron metabolism during the first months of life, urgently requires further understanding, to better interpret interventions and develop recommendations (3).
One fundamental difference in infant erythropoiesis compared to the adults is the ongoing switch from fetal hemoglobin (HbF) to adult hemoglobin (HbA). With its greater affinity to oxygen, HbF enables maternal to fetal transport of oxygen during pregnancy. At birth, a sudden decrease occurs in hemoglobin synthesis, and total hemoglobin levels decrease rapidly in the newborn. As erythropoiesis becomes active again, mainly HbA is produced and a decrease in HbF can be observed, as old HbF-containing erythrocytes are gradually destroyed (4,5). However, also after birth, there is an ongoing synthesis of HbF, and the switch is believed to continue for several months (6,7). Environmental factors in infancy may affect HbF synthesis, e.g., stress erythropoiesis and hypoxia cause increased production of HbF (8,9,10). However, the mechanisms behind the switch are not yet fully determined, and the possible impact of maternal, perinatal, and nutritional background factors are unclear (6,11). To our knowledge, interactions between HbF and iron metabolism have not previously been studied in infants.
This was originally a randomized trial of iron supplements to marginally LBW infants (birth weight: 2,000–2,500 g), with the primary aims to study the effect on iron status and long-term neuropsychological effects. The primary outcomes are already published (12,13). Since the cohort constitutes an excellent model for further exploratory studies of infant iron metabolism and erythropoiesis, we also included other secondary analyses and hypotheses whereof some are previously published (14). This article reports data from our exploratory analyses of HbF. Based on our observations above, we hypothesized that a possible upregulation of HbF synthesis might occur if iron supplements are provided, which would partially explain the previously observed immature Hb response to iron supplements in infants. We aimed not only to investigate how iron supplements to infants at risk of iron deficiency affect synthesis and disappearance of HbF after birth but also to explore how other perinatal background factors impact the disappearance of HbF.
Results
Background Variables and Overall Trends
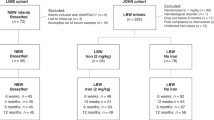
Flow chart for included infants is presented in Figure 1 . One infant diagnosed with β-thalassemia at 6 wk and one with AB0 immunization at birth were excluded from all analyses. Perinatal background characteristics for included infants analyzed for HbF at 6 wk, 12 wk, or 6 mo of age (n = 280) are presented in Table 1 . There were no significant differences between the intervention groups. Gestational ages at birth ranged from 31 to 40 wk, and the proportion of preterm infants (<37 wk of gestation) was 56%. The overall concentration of HbF and HbA, expressed both as absolute concentrations and as HbF fraction, are presented in Table 2 , together with iron status and other background data at each visit. There was a rapid decrease in mean HbF from 81.2 g/l at 6 wk to 8.1 g/l at 6 mo of age and a corresponding increase in HbA.
Trial profile of included infants. aTwo infants were excluded at 6 wk due to hematological disorders. The total number of dropouts was 25, with no significant differences between the groups. bIn 22 cases (13 at 6 wk and 9 at 12 wk), the infants discontinued the intervention as unblinded iron-supplemented cases. These unblinded cases together with another 43 infants who were considered as poor compliers due to less than 70% of iron doses given, were excluded when the intervention group effect was analyzed (per protocol) but included in other analyses. HbF, fetal hemoglobin.
Effects of Intervention
There was a dose-dependent positive effect of the iron intervention on total Hb at 6 mo of age, suggesting an increased synthesis of Hb in iron-supplemented infants between 12 wk and 6 mo of age ( Table 3 ). However, as presented in Table 3 , there was no similar effect of intervention on HbF (P = 0.429). Instead, the increase in total Hb was fully explained by changes in non-HbF hemoglobin (HbA), for which the mean concentration at 6 mo were significantly increased (P < 0.001). A nonsignificant, dose-dependent trend of decreased HbF fractions was seen in iron-supplemented groups (P = 0.213).
Associated Background Variables
In secondary univariate linear regression analyses, we further examined the correlation between absolute HbF and background variables (data not shown). At all time points, there was a strong association between HbF and postconceptional as well as postnatal age. In a bivariate model comparing the predictive value of postnatal and postconceptional ages, only postconceptional age remained significant (R2 = 0.12, P < 0.001 at 6 wk; R2 = 0.26, P < 0.001 at 12 wk; and R2 = 0.09, P < 0.001 at 6 mo), suggesting that gestational age at birth does not affect the rate of HbF disappearance. The association was further explored in Figure 2 . Regression analyses including all measures revealed that the disappearance had an exponential pattern, best fit by the equation HbF (g/l) = 13,296 × exp(−0,12 × GA(wk)).
The correlation between postconceptional age and absolute levels of HbF in 280 infants, examined at 6 wk (circles), 12 wk (triangles), and 6 mo of postnatal age (diamond symbols). At each age, there was a significant linear correlation. Regression analyses including all measures revealed that the disappearance of HbF followed an exponential pattern, best fit by the equation HbF (g/l) = 13,296 × exp(−0,12 × postconceptional age (wk)). HbF, fetal hemoglobin.
To further explore the predictive value of other baseline and background factors, we performed univariate and stepwise multivariate linear regression analyses, controlling for postconceptional age ( Table 4 ). Significant associations were found to transferrin saturation and weight at 6 wk, if the mother was European or not at 12 wk and transferrin saturation at 6 mo of life. The overall models explained 32, 27, and 12%, respectively, of the variance at 6 wk, 12 wk, and 6 mo.
Discussion
In this article, we aimed to test if iron supplements to LBW infants, a group at particular risk of iron depletion, would interact with the ongoing switch from HbF to HbA. We hypothesized that an increased iron supply would be a postnatal factor that might temporarily reawaken the synthesis of HbF and contribute to a decreased rate of disappearance. This hypothesis was rejected. We observed no significant differences or trends in absolute HbF levels between the groups at any time point. Instead, we observed, in concordance with previous publications from the present trial, an increased synthesis of total Hb in supplemented cases at least between 12 wk and 6 mo of age. This analysis showed that the increased synthesis included only non-HbF subgroups, causing a nonsignificant trend of decreased relative HbF levels, rather than the hypothesized opposite.
Furthermore, we found no association between ferritin and absolute HbF at any age ( Table 4 ), further supporting that iron availability did not predict the HbF synthesis. A limitation in these conclusions is that we have performed the first analyses at 6 wk of age and not at birth. Iron status may still interact with the switch of Hb synthesis pre- or perinatally. To further explore this, analyses on cord blood would have been helpful. Another way to improve sensitivity and to better describe the synthesis at each specific time would have been to analyze HbF fraction in reticulocytes or globin mRNA levels (15).
We found one previous trial investigating the association between iron status and HbF. Adams et al. (16) studied the proportions of hemoglobin subtypes in an iron-deficient 22-y-old man with alfa thalassemia and hereditary persistence of HbF. In contrast to our findings, they suggested that iron deficiency may alter the assembly of Hb subunits and thereby change the fractions of different Hb. However, the observations in this patient with a congenital disorder might have limited relevance for the general population.
Several previous trials have explored the HbF disappearance during the first months of life. It has been shown in cord blood that newborns already have a fraction of HbA, ranging from 5 to 35% (refs. 17,18). This confirms an already ongoing synthesis of HbA in newborns and suggests a gradual change starting before birth, rather than an abrupt switch after birth (17). Studies of reticulocyte fractions in term, newborn infants have suggested that about 50% of synthesized Hb is HbF, rapidly decreasing during the first months of life (5,6,19). Furthermore, the fraction of HbF in cord blood is inversely correlated to gestational age, decreasing with about 2.4–4% for each week of higher gestation from 34 wk. This has been interpreted as if the shift from HbF to HbA is a result of gradual developmental changes (18,20,21).
The LBW infants of this trial were born with a wide range of gestational ages, resulting in a variety of postconceptional age at blood sampling. Thereby, we could compare the correlation to postnatal age with postconceptional age and confirm the previously established correlation to the latter. The data suggest that the rate of disappearance is not affected by birth but proceed according to a prenatally programmed pattern.
The disappearance of HbF has been described as a linear decrease by several previous researchers (4,22). In contrast, Gahr and Herlemann (23) suggested a sudden reactivation of HbF production at about 2 mo of age. As illustrated in Figure 2 , the rate of decrease in the present trial was better approximated to an exponential function. This corroborates the observations of Terrenato et al. (5), who suggested that the relative synthesis of HbF decreased linearly but since the erythropoiesis suddenly drops at birth and reactivates fully at about 10 wk of age, the absolute levels of HbF rather follows an exponential decrease, very similar to what we observed.
Hemoglobinopathies, e.g., β-thalassemia and sickle cell disease, are causing great negative effect on public health worldwide (24). Interestingly, it has been shown that the severity of these diseases is correlated to the levels of HbF and that inducing or reawakening HbF production is a promising future treatment strategy (7,11). In that perspective, understanding the mechanisms of the hemoglobin switch could possibly contribute to improved diagnosis and treatment of a major public health burden (7).
Even though postconceptional age turned out to be the strongest predictor of HbF disappearance, our multivariate models suggested that it did only explain 12–26% of the variance in absolute HbF, suggesting other important contributors. Our data showed minor correlations to transferrin saturation and body weight at 6 wk of age, but the relevance of those findings is unclear and could be a type 1 error. None of the other perinatal background factor analyzed, remained significant in our models, suggesting that the hemoglobin switch is insensitive to environmental circumstances. This is in concordance with Shiao et al. (25) who analyzed HbF disappearance in preterm infants and with Bard and Prosmanne (26) who concluded that eight preterm infants born at <1,000 g and requiring prolonged intensive care and repeated blood transfusions had similar levels of HbF synthesis at term as controls.
Except for variance in laboratory analyses, other important predictors of the rate of disappearance may be genetical differences. Several genes have previously been identified, explaining differences of HbF levels in adults, however their relevance in infants is not yet determined (7,27). In trisomy 13, there is a delayed switch of hemoglobin, and two genes have been identified as the reason for this phenotype (28). This trial is limited by the fact that no genetic analyses are available, and we conclude that to further explore the predictors of the hemoglobin switch, with the goal to find future interventions in hemoglobinopathies, genetic studies should be prioritized.
Conclusion
In this trial, we explored the HbF disappearance and its possible predictors in LBW infants with a wide range of gestational ages. Our hypothesis in this trial was that iron availability during the first months of life would affect the synthesis or disappearance of HbF. The hypothesis was rejected. We found no effects of iron supplementation on the concentration of HbF. Instead, we confirmed the previously established correlation to postconceptional age, supporting a genetically programmed switch, insensitive to most environmental factors.
Methods
Study Design
This was originally a randomized, double-blinded, controlled trial of iron supplementation given to marginally LBW infants from 6 wk to 6 mo of age. There was previously a lack of data concerning benefits and harm of iron supplements to this relatively large subgroup of LBW infants, and evidence-based recommendations have been missing. The primary aims were to investigate the effect on iron status at 6 mo and the long-term effects on cognition (12,13). The trial was performed between March 2004 and June 2007 at two Swedish tertiary care hospitals: Umeå University Hospital, Umeå, and Karolinska University Hospital, Stockholm. Eligible infants were identified from delivery records, and parents accepting participation gave written informed consent. We enrolled 285 infants based on the following inclusion criteria: birth weight 2,000–2,500 g, no disease symptoms at inclusion, no chronic disease, no previous blood transfusion, and never received iron supplements. There were no dietary inclusion criteria and dietary habits are presented in detail elsewhere (12). In brief, 91.5% were breastfed at inclusion and 54.3% were exclusively breastfed.
Included infants were stratified by sex and study center and randomized into three intervention groups receiving the following doses of iron supplementation: 0 mg/kg/d (placebo), 1 mg/kg/d, or 2 mg/kg/d. To keep the randomization blinded, all participants received two bottles, one for the morning dose and one for the evening dose. The iron supplement was ferrous succinate mixture (Ferromyn S; Astra Zeneca, Södertälje, Sweden), containing 3.7 mg/ml of iron. All investigators and parents were blinded to the intervention assignment as described in detail elsewhere (12). The dose was adjusted for actual weight at 12 and 19 wk. Compliance to the intervention was monitored using a daily checklist, where parents were asked to register all doses given, and by weighing the bottles of iron/placebo before and after use. Poor compliance was defined as <70% of doses given.
Data Collection
At inclusion, background data were collected from parents and from delivery records including: gestational age at birth, sex, anthropometric data, neonatal diagnoses, and maternal birth country. The infants visited the study center at approximately the following ages: 6 wk, 12 wk, 19 wk, and 6 mo, and the exact postnatal and postconceptional ages were calculated at each visit.
At 6 wk, 12 wk, and 6 mo, phlebotomy was performed. From each blood sample, EDTA blood was sent for complete blood count, including hemoglobin (Hb) and mean cell volume, using an automated blood counter at each hospital laboratory. Blood was also drawn into serum separator tubes, centrifuged, and serum frozen at −70 °C until analyzed for ferritin, transferrin, iron, and transferrin receptor concentration as described previously (12). Transferrin saturation was calculated from serum iron and transferrin. HbF was analyzed at Karolinska University Laboratory, using ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA). HbA was approximately calculated as the difference between total Hb and HbF.
Dropouts, Unblinded Cases, and Poor Compliers
As a part of the original study design, infants with anemia at 6 wk (defined as hemoglobin less than 90 g/l) or 12 wk (less than 95 g/l) were called back to the study center for a confirmative blood sample and analysis of serum ferritin (using standard hospital routines and methods) and then referred to a pediatrician for evaluation of possible iron deficiency anemia. In this procedure, which was used to avoid untreated severe cases of iron deficiency anemia, 22 cases (13 at 6 wk and 9 at 12 wk) were prescribed iron supplementation due to suspected iron deficiency anemia, and these infants discontinued the intervention but remained in the study as unblinded cases.
Statistical Analyses
Sample size was based on estimated differences in the main outcomes, as described elsewhere (12). Because ferritin showed skewed distribution, data were log transformed in all calculations. Group comparison was performed using χ2 test or ANOVA whenever applicable. Relationships between HbF and possible contributing/predicting factors were explored with univariate and stepwise multivariate linear regression models. The following factors were examined: HbA, ferritin, transferrin saturation, transferrin receptor, iron, anthropometry at time of analysis (length and weight), age at examination (postnatal and postconceptional) and perinatal and social background factors (preterm birth, small for gestational age at birth, sex, maternal birth country, gestational age at birth, birth weight, birth length, and neonatal morbidity). Total Hb and mean cell volume were not included in the regression analyses due to expected intercorrelation. All subjects were included in the correlation analyses. However, when the intervention group effect was analyzed (main outcome), we excluded the 22 unblinded cases together with another 43 infants who were considered as poor compliers due to less than 70% of iron doses given. The rational for this per-protocol approach was that this paper includes only secondary, exploratory analyses of the molecular effects of iron supplements and not any clinical intention-to-treat hypothesis.
Statistical analyses were performed using IBM SPSS statistics 19.0 (IBM, Armonk, NY). This trial was approved by the Ethical Review Boards at Umeå University and the Karolinska Institute and registered with ClinicalTrials.gov, number NCT00558454.
Statement of Financial Support
This work was supported by grants from the Swedish Research Council Formas (222-2005-1894), Västerbotten County Council (ALF), the Jerring Foundation, the Oskar Foundation, and the Medical Faculty, Umeå University.
Disclosure
The authors declare no conflict of interest.
References
World Health Organization. Iron deficiency anaemia: assessment, prevention and control A guide for programme managers, 2001. (http://whqlibdoc.who.int/hq/2001/WHO_NHD_01.3.pdf).
Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B . Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138:679–87.
Lipiński P, Styś A, Starzyński RR . Molecular insights into the regulation of iron metabolism during the prenatal and early postnatal periods. Cell Mol Life Sci 2013;70:23–38.
Colombo B, Kim B, Atencio RP, Molina C, Terrenato L . The pattern of fetal haemoglobin disappearance after birth. Br J Haematol 1976;32:79–87.
Terrenato L, Bertilaccio C, Spinelli P, Colombo B . The switch from haemoglobin F to A: the time course of qualitative and quantitative variations of haemoglobins after birth. Br J Haematol 1981;47:31–41.
Amoyal I, Fibach E . Hemoglobin switch in the newborn: a flow cytometry analysis. Neonatology 2007;91:61–8.
Sankaran VG, Orkin SH . The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 2013;3:a011643.
Bard H, Gagnon C, Peri KG . HbF synthesis during stress erythropoiesis as determined by gamma-mRNA/non-alpha-mRNA quantification. Pediatr Res 1999;45(5 Pt 1):684–6.
Jane SM, Cunningham JM . Understanding fetal globin gene expression: a step towards effective HbF reactivation in haemoglobinopathies. Br J Haematol 1998;102:415–22.
Bard H, Fouron JC, Prosmanne J, Gagnon J . Effect of hypoxemia on fetal hemoglobin synthesis during late gestation. Pediatr Res 1992;31:483–5.
Bauer DE, Kamran SC, Orkin SH . Reawakening fetal hemoglobin: prospects for new therapies for the β-globin disorders. Blood 2012;120:2945–53.
Berglund S, Westrup B, Domellöf M . Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics 2010;126:e874–83.
Berglund SK, Westrup B, Hägglöf B, Hernell O, Domellöf M . Effects of iron supplementation of LBW infants on cognition and behavior at 3 years. Pediatrics 2013;131:47–55.
Berglund S, Lönnerdal B, Westrup B, Domellöf M . Effects of iron supplementation on serum hepcidin and serum erythropoietin in low-birth-weight infants. Am J Clin Nutr 2011;94:1553–61.
Peri KG, Gagnon C, Bard H . Quantitative correlation between globin mRNAs and synthesis of fetal and adult hemoglobins during hemoglobin switchover in the perinatal period. Pediatr Res 1998;43(4 Pt 1):504–8.
Adams JG 3rd, Coleman MB, Hayes J, Morrison WT, Steinberg MH . Modulation of fetal hemoglobin synthesis by iron deficiency. N Engl J Med 1985;313:1402–5.
Bard H, Makowski EL, Meschia G, Battaglia FC . The relative rates of synthesis of hemoglobins A and F in immature red cells of newborn infants. Pediatrics 1970;45:766–72.
Felicetti L, Novelletto A, Benincasa A, Terrenato L, Colombo B . The HbA/HbA2 ratio in newborns and its correlation with fetal maturity. Br J Haematol 1984;56:465–71.
Bard H . The postnatal decline of hemoglobin F synthesis in normal full-term infants. J Clin Invest 1975;55:395–8.
Cook CD, Brodie HR, Allen DW . Measurement of fetal hemoglobin in newborn infants; correlation with gestational age and intrauterine hypoxia. Pediatrics 1957;20:272–8.
Cottom DG . Foetal haemoglobin and postmaturity. J Obstet Gynaecol Br Emp 1955;62:945–8.
Garby L, Sjolin S, Vuille JC . Studies on erythro-kinetics in infancy. II. The relative rate of synthesis of haemoglobin F and haemoglobin A during the first months of life. Acta Paediatr 1962;51:245–54.
Gahr M, Herlemann G . Reactivation of fetal erythropoiesis during the postnatal period. Pediatr Res 1984;18:178–80.
Weatherall DJ . The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010;115:4331–6.
Shiao SY, Ou CN . Accurate measurements of fetal hemoglobin for neonates with different gestational ages. Hemoglobin 2006;30:419–35.
Bard H, Prosmanne J . Postnatal fetal and adult hemoglobin synthesis is preterm infants whose birth weight was less than 1,000 grams. J Clin Invest 1982;70:50–2.
Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008;322:1839–42.
Sankaran VG, Menne TF, Šćepanović D, et al. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc Natl Acad Sci USA 2011;108:1519–24.
Acknowledgements
We thank all participating families and our dedicated research nurses Kerstin Andersson in Stockholm, Åsa Sundström, Ruth-Gerd Larsson, and Margareta Bäckman in Umeå. The iron drops were unconditionally provided from Astra Zeneca, Sweden.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Berglund, S., Lindberg, J., Westrup, B. et al. Effects of iron supplements and perinatal factors on fetal hemoglobin disappearance in LBW infants. Pediatr Res 76, 477–482 (2014). https://doi.org/10.1038/pr.2014.116
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2014.116