Abstract
Background:
Preterm infants with a patent ductus arteriosus (PDA) are at risk for death or development of bronchopulmonary dysplasia (BPD). However, PDA treatment remains controversial. We investigated if PDA treatment and other clinical or echocardiographic (ECHO) factors were associated with the development of death or BPD.
Methods:
We retrospectively studied clinical and ECHO characteristics of preterm infants with birth weight <1,500 g and ECHO diagnosis of a PDA. Logistic regression and classification and regression tree analyses were performed to assess variables associated with the combined outcome of death or BPD.
Results:
Of 187 preterm infants with a PDA, 75% were treated with indomethacin or surgical ligation and 25% were managed conservatively. Death or BPD occurred in 80 (43%) infants. The results of logistic regression analyses showed that lower gestational age (odds ratio (OR): 0.5), earlier year of birth during the study period (OR: 0.9), and larger ductal diameter (OR: 4.3) were associated with the decision to treat the PDA, whereas gestational age was the only variable associated with death or BPD (OR: 0.6; 95% confidence interval: 0.5–0.8).
Conclusion:
Only lower gestational age and not PDA treatment or ECHO score was associated with the adverse outcome of death or BPD. Further investigation of PDA management strategies and effects on adverse outcomes of prematurity is needed.
Similar content being viewed by others
Main
The ductus arteriosus closes within hours of birth in most term infants. However, in 50–70% of preterm infants with birth weight <1,500 g, the ductus remains patent (1). Preterm infants with a persistently significant patent ductus arteriosus (PDA) are at risk for complications, including death and bronchopulmonary dysplasia (BPD) (2,3,4,5). An open ductus may contribute to the development of BPD by shunting blood into the lungs, resulting in pulmonary edema and the persistent need for ventilatory support. This population of preterm infants with a PDA would benefit from strategies to reduce complications and optimize outcomes.
Historically, clinicians have advocated closure of the PDA through medical treatment with indomethacin or ibuprofen and/or surgical ligation; however, recent controversy has developed over the need to treat a PDA. The ductus has a high incidence of spontaneous closure over time (6), and medical therapy may be ineffective in closing the PDA (7). Treatment is also not without risk of short- and long-term complications (8,9,10,11,12,13,14). Moreover, treatment of a persistent PDA may not reduce the risk of common morbidities, including BPD, intraventricular hemorrhage, or necrotizing enterocolitis (15,16). Although an association may exist between the presence of a PDA and complications of prematurity such as death and BPD, this association may not be causal, given the possible lack of treatment effect.
Conservative management of the PDA or “watchful waiting” has been advocated as another strategy in lieu of active treatment. This approach may entail use of fluid restriction and diuretics, higher positive airway distending pressures, inotropic support, and liberal blood transfusions to minimize pulmonary edema from the left to right shunt and maintain adequate systemic oxygenation (17). The disadvantages of such an approach include delayed feeding and nutrition as well as delayed weaning from respiratory support while the PDA remains hemodynamically significant. In addition, if a persistent PDA does indeed contribute to adverse long-term complications such as BPD and death, then missing the window of opportunity to treat a PDA by electing to manage conservatively could lead to adverse consequences for the infant.
A randomized clinical trial of PDA treatment vs. no treatment would be the ideal method to determine the benefit of treatment. However, current neonatal clinical practice has until recently been biased toward treatment of the PDA, making it difficult to maintain the equipoise needed for such a trial. We conducted a retrospective study to determine the effect of treatment on the outcome of death or BPD for preterm infants with a PDA and associations with clinical and echocardiographic (ECHO) factors.
Results
Study Population
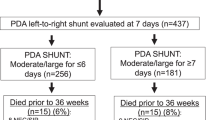
During the 5-y study period, there were 615 preterm infants with birth weight <1,500 g admitted to the Neonatal Intensive Care Unit, of whom 209 (34%) were found to have a PDA by ECHO. Twenty-two patients were excluded and 187 remaining subjects had their PDA either treated (75%) or conservatively managed (25%) ( Figure 1 ). Tocolytic indomethacin was not used antenatally, nor was prophylactic indomethacin given postnatally in any patient. The mean gestational age of the 187 subjects was 27.6 ± 2 wk and the mean age at PDA treatment was 4 ± 4 d among those treated. Mean ductal diameter at the time of treatment was 1.7 ± 0.6 mm.
Study population. Flow diagram of enrollment is shown. CHD, congenital heart disease; ECHO, echocardiographic; PDA, patent ductus arteriosus; VLBW, very-low-birth-weight.
Inter-Rater Reliability of ECHO Parameters
Twenty-seven infants (14%) did not have ECHO scores due to the limited nature of the recording impeding the reader’s ability to assess ECHO variables of interest. Moderate agreement between readers was seen for most of the ECHO parameters, with interclass correlation coefficients or κ values ≥0.6. More substantial inter-rater agreement was seen for ductal diameter, Doppler flow pattern, left pulmonary artery (LPA) diastolic velocity, and the presence of mitral regurgitation, whereas less agreement was seen for ductus proximal isovelocity surface area and the abdominal aortic flow ratio ( Table 1 ).
Characteristics and Outcomes by PDA Treatment
When comparing treated infants with those managed conservatively ( Table 2 ), significant differences included a lower birth weight, lower gestational age, lower 5-min Apgar score, larger ductal diameter, higher LPA diastolic velocity, and higher ECHO score in the treated group. However, there were no significant differences between the two groups in frequency of morbidities associated with a PDA, including death or BPD. Eighty infants (43%) died or developed BPD before hospital discharge, 46% of infants treated for a PDA and 33% of those untreated (P = 0.1).
Variables Associated With Treatment
Lower gestational age, earlier year of birth, larger ductal diameter, and higher LPA diastolic velocity were associated with the decision to treat a PDA by multivariate regression analysis ( Table 3 ). Clinical severity score and ECHO score were not associated with treatment.
Variables Associated With Death or BPD
In contrast, lower gestational age (odds ratio (OR): 0.6; 95% confidence interval (CI): 0.5–0.7) was the only variable associated with the primary outcome of death or BPD. Clinical score, ECHO score, and 5-min Apgar score were not significant in predicting outcomes. Inclusion of ECHO variables in this model demonstrated that ductal diameter and LPA diastolic velocity were also not associated with death or BPD, even though these ECHO variables were associated with the decision to treat a PDA. Lack of apparent treatment effect was confirmed by looking at logistic regression coefficients with treatment as a predictor variable in the full data set.
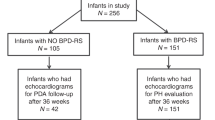
To further assess the impact of treatment, classification and regression tree (CART) analysis of the full data set was performed. CART analysis is a complementary statistical approach to logistic regression and does not make assumptions about the relative importance of predictor variables. CART analysis uses recursive partitioning and automatic selection of optimal cut points of predictor variables (e.g., gestational age or ductal diameter) to classify outcomes (death or BPD). The optimal cut points for each predictor variable are determined by the software using the available data, and the higher the variable appears in the classification tree, the more closely associated it is with the outcome. CART analysis found that among 32 infants with gestational age <25.8 wk, 26 (81%) died or developed BPD. In contrast, among 56 infants with gestational age ≥28.5 wk, 49 (87.5%) did not die or develop BPD. In infants with gestational age between 25.8 and 28.5 wk, a group where treatment of the ductus is most controversial, ductal diameter does not appear to be an important predictor. Large ductal diameter ≥1.6 mm in this group classified only 37% of infants with the primary outcome of death or BPD. A flow diagram representing the best classification scheme by CART is depicted in Figure 2 . Gestational age classification cut points did not change when all nine ECHO predictors were added into the CART analysis. Notably, treatment did not seem to make a difference in the primary outcome of death or BPD, as it did not appear in the classification tree.
Flow diagram of classification and regression tree (CART) analysis. Flow diagram of CART analysis demonstrates gestational age (GA) cutoffs of <25.8 and >28.5 wk in addition to ductal diameter of 1.6 mm in classifying subjects for the outcome of death or bronchopulmonary dysplasia (BPD).
Prediction Model
We generated receiver operating characteristic curves for a logistic regression model in which gestational age, as a continuous variable, was the sole independent variable. The area under the curve was 75.6%. Note that this estimate is biased upward by the fact that we used the same data to estimate the effects of gestational age and the area under the curve. Fitting this model to independent data would likely give a lower estimate. Categorical grouping of gestational age into tertiles did not improve the discrimination of the model (c = 74.8%).
Discussion
In this observational study of preterm infants with a PDA, with prospectively reviewed echocardiographic data, we found that only lower gestational age was associated with the adverse outcome of death or BPD. Other clinical and echocardiographic variables were not associated with the development of death or BPD, including whether or not an infant was treated with medication or surgical ligation.
Clinicians were more likely to treat an infant with a PDA if they had lower gestational age, larger ductal diameter, or higher LPA diastolic velocity (an indicator of shunting magnitude). These findings are not surprising, given the perception that a more significant PDA in a lower gestation infant would more likely be associated with adverse outcomes. Clinicians were also less likely to treat the PDA if the infant was born later in the cohort, signifying a shift in practice over this period. The ECHO score itself was not significant in determining treatment, which may be due to inconsistent availability or lack of clinician familiarity with the reviewed ECHO parameters. The clinical severity score was not significant in determining treatment, although individual components of the clinical score were not separately analyzed. Individual factors that comprise the clinical score such as degree of respiratory support or history of feeding intolerance may well have influenced the neonatologist’s decision to treat the PDA.
Despite studies associating a PDA with the development of death or BPD, no literature exists identifying specific clinical predictors of death or BPD in preterm infants with a PDA. Other investigators have developed prediction models for death or BPD in preterm infants with respiratory failure and have identified lower birth weight, male gender, and increased severity of respiratory failure as clinical risk factors for BPD (18,19). Our analysis looked at a slightly different cohort of preterm infants with a PDA; however, many of these infants also had respiratory failure. When birth weight was substituted for gestational age as a predictor variable in our analysis, similar associations between low birth weight and poor outcomes were found by logistic regression. However, severity of respiratory failure, which was incorporated as a part of the clinical severity score, was not associated with adverse outcomes in our analysis and neither was male sex a clinical predictor. Inherent differences in our cohort with a PDA may explain the apparent lack of significance for these established predictors of death or BPD. Systemic infection was also not considered in the analysis. However, the presence of infection and associated inflammation in the setting of a PDA may further contribute to the development of BPD (3).
Although we did not distinguish medical and surgical interventions in our analysis, we found that treatment of the PDA had no effect on the development of death or BPD. CART analysis cannot distinguish the bias inherent in selecting treatment for those infants with the poorest prognosis who are most likely to die or develop BPD. However, both CART analysis and logistic regression did not demonstrate a treatment effect. In retrospective analyses of large cohorts, Madan et al. (20) found that surgical ligation of a PDA was associated with an increased odds of developing BPD (OR: 2.19; CI: 1.16–4.15) compared with medical therapy alone, but the authors did not list a comparison with a supportive therapy only group. Chorne et al. (21) and Kabra et al. (22) also found increased odds of BPD (OR: 1.91; CI: 1.02–3.57 and OR: 1.8; CI: 1.09–3.03) in preterm infants treated with surgical ligation compared with those receiving only indomethacin prophylaxis or treatment (21,22). However, comparison with a conservatively managed group was again not available. Whether treatment of the PDA is detrimental to preterm infants or only not beneficial in reducing death and BPD remains to be determined. One historical cohort study suggested an unadjusted increase in chronic lung disease or mortality with watchful waiting and limited intervention for preterm infants with a PDA (23). Variability in the definition of conservative management may impact outcomes. Selective benefit of treatment to a subset of infants based on clinical factors such as gestational age could also still be possible. Our findings support the need for additional study of conservative management of the PDA and its effect on long-term outcomes.
None of the echocardiographic parameters in our cohort of subjects were significantly associated with adverse outcomes. CART analysis identified ductal diameter, left atrial-to-aortic root ratio, LPA:ductus ratio, and LPA diastolic velocity as discriminatory in classifying a subset of infants with intermediate gestational age range for death or BPD, but limitations of sample size make it difficult to ascertain the significance of these ECHO variables for select preterm infants. In fact, for ductal diameter alone, our CART analysis demonstrated the counter-intuitive classification of infants with a larger PDA (>1.55 mm) having a smaller risk. Other investigators have identified specific ECHO parameters to predict PDA treatment, failure of pharmacologic closure, or spontaneous ductal closure (24,25,26). Ramos et al. (25) identified the PDA to LPA ratio as predictive of treatment in extremely-low-birth-weight infants. Our analysis identified only larger ductal diameter and greater LPA diastolic velocity as being associated with the clinical decision to treat a PDA. Variability in clinician interpretation of echocardiogram reports and lack of standards for hemodynamic significance of a PDA may account for differences in decisions to treat. Our echocardiographic scoring system has not been validated, but our ECHO score as well as the scoring system of other investigators (27) did not identify infants at highest risk for death or BPD. Sehgal et al. (28) devised an ECHO scoring system associated with the development of chronic lung disease. However, their population was limited to a smaller group of preterm infants, already receiving ibuprofen for medical closure of a PDA. Thus, for all preterm infants with a PDA, echocardiography alone may not be sufficient for identification of those at risk for adverse outcomes.
This research is limited by sample size and retrospective observational design. Treatment efficacy cannot be assessed because of selection bias, where infants with the poorest prognosis who are most likely to die or develop BPD are inherently treated. Adjustment for clinical and ECHO factors did not yield a treatment benefit, but still there may be residual bias such that the sicker infants were more likely to be treated. Of infants <27 wk gestational age in our study, 84% (64/76) were treated for their PDA. Furthermore, death and BPD are not the only adverse outcomes associated with a PDA. Clinical and ECHO variables chosen for our analysis may instead be better predictive of outcomes such as neurodevelopmental impairment, necrotizing enterocolitis, or intraventricular hemorrhage. Interobserver reliability with some ECHO parameters was also suboptimal and may have contributed to a lack of significance of ECHO parameters in identifying infants with development of death or BPD.
Conclusion
In a population of preterm infants with a PDA, conservative management was not associated with the adverse outcome of death or BPD. Only lower gestational age was associated with death or BPD after examination of other clinical and ECHO variables. The results of this observational study provide additional justification for the need to conduct a randomized clinical trial of treatment or no treatment for a PDA to determine the best strategy for PDA management.
Methods
Subjects
Eligible infants were preterm infants with birth weight <1,500 g born between 1 January 2006 and 31 December 2010 and admitted to the Neonatal Intensive Care Unit at Lucile Packard Children’s Hospital at Stanford with a diagnosis of a PDA by echocardiogram. Infants who were outborn or those with congenital heart disease or other congenital or chromosomal anomalies were excluded. Expedited approval without consent was obtained from the Stanford University Human Subjects in Medical Research Committee.
Data Collection
Clinical variables collected included gestational age, birth weight, Apgar scores, oxygen requirement, ventilatory support, and radiographic findings. In addition, information on the degree of apnea and bradycardia, metabolic acidosis, hypotension, feeding intolerance, and oliguria at the time of PDA diagnosis was collected. A clinical severity score was calculated from these variables based on the scoring system devised by McNamara and Sehgal (29). Management of the PDA was at the discretion of the attending neonatologist. Outcome data included death, defined as death prior to hospital discharge, and BPD, defined as oxygen use at 36 wk postmenstrual age.
A cardiologist blinded to clinical outcomes reviewed echocardiograms of all subjects. A subset of 20 infants was used to determine interobserver reliability of PDA measurements between two pediatric cardiologists. ECHO variables included ductal diameter, left atrial-to-aortic dimension ratio, abdominal aorta reverse to forward flow ratio, LPA:ductus ratio, LPA diastolic velocity, ductus Doppler pattern, presence of proximal isovelocity surface area, presence of holodiastolic retrograde abdominal aorta flow, and presence of mitral valve regurgitation as previously described (30,31,32,33,34). An ECHO score was calculated using five of these variables ( Table 4 ), which were selected a priori, based on the best determinants of a clinically significant shunt by cardiologists (N.S. and R.P.) at our institution. These scoring parameters were developed to assess the ductus prior to initiation of the study.
Statistical Analysis
Inter-rater reliability of ECHO parameters between readers was determined using Cohen’s κ statistic for categorical variables and interclass correlation coefficients for continuous variables. Differences in baseline characteristics and demographics as well as outcomes were compared between subjects treated for a PDA and those untreated using Wilcoxon rank sum test for nonparametric continuous variables and χ2 or Fisher’s exact test for nominal variables. Multivariate logistic regression was used to assess the clinical and ECHO variables associated with the decision to treat a PDA. All analyses were conducted using SAS version 9.1 (Cary, NC) with statistical significance established for P < 0.05. CART analysis was used with R version 2.13.0 software (R Foundation for Statistical Computing, Vienna, Austria) to explore dependencies between clinical and ECHO variables and treatment as predictors of death or BPD. These results were compared with traditional multivariate logistic regression.
Statement of Financial Support
Financial support was received from the Stanford National Institutes of Health/National Center for Research Resources Clinical and Translational Science Awards grant number KL2 RR025743.
Disclosure
We have no conflicts of interest to disclose.
References
Clyman RI, Couto J, Murphy GM . Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol 2012;36:123–9.
Bancalari E, Claure N, Sosenko IR . Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 2003;8:63–71.
Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E . Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 1996;128:470–8.
Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O’Shea TM . Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics 1999;104:1345–50.
Oh W, Poindexter BB, Perritt R, et al.; Neonatal Research Network. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 2005;147:786–90.
Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM . The ductus arteriosus rarely requires treatment in infants > 1000 grams. Am J Perinatol 2008;25:661–6.
Chorne N, Jegatheesan P, Lin E, Shi R, Clyman RI . Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr 2007;151:629–34.
Lemmers PM, Toet MC, van Bel F . Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 2008;121:142–7.
Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF . Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr 1999;135:733–8.
Pryds O, Greisen G, Johansen KH . Indomethacin and cerebral blood flow in premature infants treated for patent ductus arteriosus. Eur J Pediatr 1988;147:315–6.
Watterberg KL, Gerdes JS, Cole CH, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 2004;114:1649–57.
Rheinlaender C, Helfenstein D, Walch E, Berns M, Obladen M, Koehne P . Total serum bilirubin levels during cyclooxygenase inhibitor treatment for patent ductus arteriosus in preterm infants. Acta Paediatr 2009;98:36–42.
Clement WA, El-Hakim H, Phillipos EZ, Coté JJ . Unilateral vocal cord paralysis following patent ductus arteriosus ligation in extremely low-birth-weight infants. Arch Otolaryngol Head Neck Surg 2008;134:28–33.
Noori S, Friedlich P, Seri I, Wong P . Changes in myocardial function and hemodynamics after ligation of the ductus arteriosus in preterm infants. J Pediatr 2007;150:597–602.
Benitz WE . Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol 2010;30:241–52.
Sosenko IR, Fajardo MF, Claure N, Bancalari E . Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr 2012;160:929–35.e1.
Benitz WE . Learning to live with patency of the ductus arteriosus in preterm infants. J Perinatol 2011;31:Suppl 1:S42–8.
Ambalavanan N, Van Meurs KP, Perritt R, et al.; NICHD Neonatal Research Network, Bethesda, MD. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J Perinatol 2008;28:420–6.
Sinkin RA, Cox C, Phelps DL . Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics 1990;86:728–36.
Madan JC, Kendrick D, Hagadorn JI, Frantz ID 3rd ; National Institute of Child Health and Human Development Neonatal Research Network. Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics 2009;123:674–81.
Chorne N, Leonard C, Piecuch R, Clyman RI . Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics 2007;119:1165–74.
Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A ; Trial of Indomethacin Prophylaxis in Preterms Investigators. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr 2007;150:229–34, 234.e1.
Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G . What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants? J Perinatol 2012;32:344–8.
Kwinta P, Rudzinski A, Kruczek P, Kordon Z, Pietrzyk JJ . Can early echocardiographic findings predict patent ductus arteriosus? Neonatology 2009;95:141–8.
Ramos FG, Rosenfeld CR, Roy L, Koch J, Ramaciotti C . Echocardiographic predictors of symptomatic patent ductus arteriosus in extremely-low-birth-weight preterm neonates. J Perinatol 2010;30:535–9.
Su BH, Peng CT, Tsai CH . Echocardiographic flow pattern of patent ductus arteriosus: a guide to indomethacin treatment in premature infants. Arch Dis Child Fetal Neonatal Ed 1999;81:F197–200.
El-Khuffash AF, McNamara PJ . The patent ductus arteriosus ligation decision. J Pediatr 2011;158:1037–8; author reply 1038–9.
Sehgal A, Paul E, Menahem S . Functional echocardiography in staging for ductal disease severity: role in predicting outcomes. Eur J Pediatr 2013;172:179–84.
McNamara PJ, Sehgal A . Towards rational management of the patent ductus arteriosus: the need for disease staging. Arch Dis Child Fetal Neonatal Ed 2007;92:F424–7.
Chen S, Tacy T, Clyman R . How useful are B-type natriuretic peptide measurements for monitoring changes in patent ductus arteriosus shunt magnitude? J Perinatol 2010;30:780–5.
Reller MD, Lorenz JM, Kotagal UR, Meyer RA, Kaplan S . Hemodynamically significant PDA: an echocardiographic and clinical assessment of incidence, natural history, and outcome in very low birth weight infants maintained in negative fluid balance. Pediatr Cardiol 1985;6:17–23.
Silverman NH, Lewis AB, Heymann MA, Rudolph AM . Echocardiographic assessment of ductus arteriosus shunt in premature infants. Circulation 1974;50:821–5.
Su BH, Watanabe T, Shimizu M, Yanagisawa M . Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch Dis Child Fetal Neonatal Ed 1997;77:F36–40.
Serwer GA, Armstrong BE, Anderson PA . Continuous wave Doppler ultrasonographic quantitation of patent ductus arteriosus flow. J Pediatr 1982;100:297–9.
Acknowledgements
We thank David Johnston for his assistance with CART analysis.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Chock, V., Punn, R., Oza, A. et al. Predictors of bronchopulmonary dysplasia or death in premature infants with a patent ductus arteriosus. Pediatr Res 75, 570–575 (2014). https://doi.org/10.1038/pr.2013.253
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.253
This article is cited by
-
Natural evolution of the patent ductus arteriosus in the extremely premature newborn and respiratory outcomes
Journal of Perinatology (2022)
-
Diagnostic and predictive value of Doppler ultrasound for evaluation of the brain circulation in preterm infants: a systematic review
Pediatric Research (2020)
-
Duration of significant patent ductus arteriosus and bronchopulmonary dysplasia in extremely preterm infants
Journal of Perinatology (2019)
-
Near-infrared spectroscopy for detection of a significant patent ductus arteriosus
Pediatric Research (2016)