Abstract
Background:
Patients with Rett syndrome (RTT) are at risk of having low bone mass and low-energy fractures.
Methods:
We characterized bone metabolism by both bone formation and resorption markers in blood in a RTT population of 61 girls and women and 122 well-matched healthy controls. Levels of N-terminal propeptides of collagen type 1 (P1NP), C-terminal telopeptide cross links (CTX), osteocalcin (OC), and bone-specific alkaline phosphatase (B-ALP) were compared between RTT patients and controls in regression models adjusted for BMI, vitamin D status, volumetric bone mineral apparent density of the lumbar spine (vBMADspine), and femoral neck (vBMADneck). We examined biochemical bone marker levels overall and stratified to persons younger than age 25 y or equal to or older than age 25 y.
Results:
The RTT patients had reduced levels of all biochemical bone markers (P < 0.05), which remained significant in persons younger than 25 y (P ≤ 0.001) regarding P1NP, CTX, and OC. Bone marker levels were not significantly associated to methyl-CpG-binding protein 2 (MECP2) mutation group, walking ability, or previous low-energy fractures.
Conclusion:
Our findings of a low bone turnover state in girls with RTT suggest critical attention to medical treatment of low bone mass in young RTT patients.
Similar content being viewed by others
Main
Rett syndrome (RTT) is a severe neurodevelopmental disorder mainly caused by de novo mutations in the methyl-CpG-binding protein 2 gene (MECP2) at Xq28 (1,2,3). Previously, dual-energy x-ray absorptiometry (DXA) studies have reported low bone mass in patients with RTT (4,5,6,7,8), and increased occurrence of low-energy fractures in these patients has also been shown (9). Patients with RTT are often growth retarded (10). However, bone metabolism in patients with RTT is sparsely described. Overall, bone metabolism can be characterized by biochemical markers of bone formation, resorption, mineralization, and turnover (11,12). Previous studies have only analyzed biochemical markers of bone formation in RTT children and young adults less than 25 y of age. None of these studies adjusted their analyses of bone marker values for age or pubertal status (5,13,14). Furthermore, biochemical bone markers have not been associated with measures of bone mineral density or low-energy fractures in RTT. Studies on biochemical bone markers in healthy persons have reported high levels in early childhood, peaking in puberty, and decreasing to stable levels in the mid-20s (12,15,16,17,18,19), but the level of biochemical bone markers in children and adolescents with RTT is unknown.
The aim of this study was to characterize bone metabolism in RTT, by comparing biochemical bone marker levels in RTT patients with healthy controls. Both markers of bone formation, the N-terminal propeptides of collagen type 1 (P1NP); of bone resorption, the C-terminal telopeptide cross links (CTX); of bone turnover, osteocalcin (OC); and of bone mineralization, bone-specific alkaline phosphatase (B-ALP) were analyzed in relation to age and puberty. The influence of BMI, vitamin D status, volumetric bone mineral apparent density of the lumbar spine (vBMADspine), and femoral neck (vBMADneck) on the biochemical bone markers was also analyzed. Furthermore, the association of bone markers with vBMADspine or vBMADneck was studied in both patients and control individuals. We also compared the ratios of bone formation (P1NP) to bone resorption (CTX) between patients and controls, as the balance of bone formation and resorption could be skewed in RTT. Finally, the association of MECP2 mutation group, walking ability or low-energy fractures with the bone marker levels was analyzed in patients.
Results
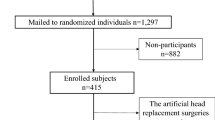
Table 1 presents descriptive clinical characteristics of RTT patients and controls. It is seen that biochemical bone markers were analyzed in most of the patients and controls, ranging from a total of 51 to 59 of the 61 patients and 102 to 118 of the 122 controls (specified in the upper and lower parts of Table 1 as a total number of each bone marker of patients and controls). Figure 1a – d shows scatter plots of the biochemical bone markers related to age in patients and controls. All the scatter plots were at a clear plateau at approximately age 25.
(a–d) Scatter plots of biochemical bone markers related to age of npatients|ncontrols, white fill = patients, black fill = controls. a: N-terminal propeptides of collagen type 1 (P1NP) (μg/l) 59|118, b: C-terminal telopeptide cross links (CTX) (μg/l) 59|118, c: osteocalcin (OC) (μg/l) 51|102, and d: bone-specific alkaline phosphatase (B-ALP) (U/l) 57|114.
Overall, patients with RTT had significantly lower values of all biochemical bone markers compared to controls, when adjusted for age and pubertal status ( Table 2 , upper part). For persons younger than age 25, RTT patients had significantly lower bone marker values compared to controls, except for B-ALP, where no significant difference was seen, in models adjusted for age and pubertal status ( Table 2 , middle part). In contrast, B-ALP was significantly higher in patients compared to controls in the group older than age 25 ( Table 2 , lower part). The BMI, vitamin D, vBMADspine, and vBMADneck were not included in the final models as no significant influence on bone markers was seen.
Figure 2a – d presents the levels of log-transformed P1NP, CTX, OC, and B-ALP in patients and controls according to pubertal status. It is seen that the biochemical bone marker levels tended to decrease from pre-early-puberty, through puberty until postpubertal ages in both patients and controls, although less for B-ALP.
(a–d) Box-and-whisker plots displaying median, interquartile (box ends), and 5%, 95% percentiles (whiskers) of levels of log-transformed biochemical bone markers according to pubertal status: pre-early puberty (Tanner breast developmental stages 1–2), puberty (Tanner breast developmental stages 3–5), and postpuberty (20–25 y of age) of npatients|ncontrols, dotted fill = patients and white fill = controls. a: N-terminal propeptides of collagen type 1 (P1NP) (μg/l) 20|40, 7|14, 5|10, b: C-terminal telopeptide cross links (CTX) (μg/l) 20|40, 7|14, 5|10, c: osteocalcin (OC) (μg/l) 17|34, 6|12, 5|10, and d: bone-specific alkaline phosphatase (B-ALP) (U/l) 18|36, 7|14, 5|10.
We analyzed associations between volumetric bone mineral density, rather than areal bone mineral density, and biochemical bone marker levels to minimize the effect of different sizes of patients and controls. There was no significant association between bone markers and vBMADspine or vBMADneck, when adjusted for age, neither in patients nor in controls (P > 0.05 in all 16 regression analyses).
Analysis of the balance of bone formation and resorption showed that the levels of log-transformed P1NP and log-transformed CTX were significantly positively associated, regardless of patient-control status: Exp(β) (95% CI) = 7.26 (4.88–9.51), P < 0.001. Moreover, the ratio of P1NP and CTX did not differ between patients and controls, as there was no significant interaction between log CTX and patient-control status: Exp(β) (95% CI) = 1.10 (0.84–1.44), P = 0.482.
The MECP2 mutation groups ( Table 3 ) and the walking ability ( Table 4 ) were not associated with significantly different levels of P1NP, CTX, OC nor B-ALP in RTT patients.
Furthermore, there was no significant difference in biochemical bone marker levels between RTT patients with or without earlier low-energy fractures in logistic regression models, adjusted for current age (data not shown). Recent fractures were not sustained in the low-energy fracture group.
Screening of parameters for general health status including liver and thyroid biochemistry, estradiol and follicle-stimulating hormone, as well as red and white blood cell counts were normal in both patients and controls. Renal biochemistry was normal in controls. RTT patients had slightly reduced levels of creatinine feasibly due to a reduced muscle mass. Further, no abnormalities in ionized calcium and parathyroid hormone were found ( Table 1 ).
Discussion
The most important finding of this study was that patients with RTT had lower values of the markers of bone metabolism in childhood compared to controls. This suggests a bone metabolism with reduced turnover, as both markers of formation and resorption were reduced in young patients. As shown previously in young populations (20,21,22) markers of formation (P1NP) and resorption (CTX) were significantly positively associated, regardless of patient-control status and age. The ratios of P1NP and CTX overall did not differ between patients and controls. This can be explained by a balanced, coupled bone formation, and resorption through age (12). In contrast, RTT patients 25 y or older had bone marker levels regarding three (P1NP, CTX, and OC) of the four bone markers similar to controls. There may be a selection bias, as the eldest RTT patients are the best survivors. As most patients in the latter older group were premenopausal, this may indicate that premenopausal adult females with RTT are not in a state of increased resorption and bone loss. This is in contrast to a previous study showing that total body BMC and BMD z scores in RTT patients decrease by age (6) indicating a possible (as biochemical bone markers were not analyzed) increased bone turnover (increased resorption to formation) by age. Only two patients and four controls were postmenopausal. Therefore, bone metabolism in the course of postmenopausal changes of sex hormones in RTT could not be examined separately in this study.
The age span of our RTT population is large and therefore different processes in bone metabolism are reflected. In adult patients with RTT, as in healthy adults, the remodeling process is assumed to dominate (23) and bone turnover cannot be shown to differ from controls. In children and adolescents with RTT, the pattern of bone turnover is characterized by a balanced decrease of both low formation and resorption activities, presumably reflecting overall decreased bone modeling, including both linear growth, circumferential growth and bone mineral accrual (12). In accordance with this, patients with RTT have smaller bones with low bone mass (8).
Our results showed that the levels of all biochemical bone markers in both patients and controls were highest in the pre-early-pubertal years, followed by an overall decrease through puberty and postpubertal years, in accordance with other studies of bone markers in children, adolescents, and adults (12,15,16,17,18,19). Previously, bone marker values have been shown to increase during early-mid puberty (Tanner breast developmental stages 2 and 3), corresponding to increasing height velocity (15,17,18). However, our material did not allow for examination of the influence of specific Tanner stages on bone marker levels due to small numbers of patients and control individuals at Tanner stage 2–5. Further studies on biochemical bone markers in RTT should focus on a large group of girls at each Tanner breast developmental stage to be followed before, through and shortly after puberty to describe a more detailed course of biochemical bone markers in relation to puberty.
B-ALP did not turn out to be significantly different between patients and controls in the group under age 25, although there was a trend towards lower values in the pre-early-pubertal group of RTT patients. In contrast, a case study of a young patient with RTT showed reduced bone matrix mineralization of the L1 vertebra. However, B-ALP was measured in the mid reference value (24). Therefore, blood measures of B-ALP may not be fully indicative of a possible altered mineralization process in bones in young patients with RTT.
In contrast, B-ALP was significantly higher in adult premenopausal females with RTT compared to controls. Known conditions where B-ALP can be increased (23) are states of increased parathyroid hormone, reduced TSH, and vitamin D deficiency. However, only two patients showed moderate vitamin D deficiency (13–25 nmol/l) with slightly elevated parathyroid hormone. Thyroid status was normal in our patients. Furthermore, vitamin D did not influence the ratio of bone markers in the regression models. Measures of total alkaline phosphatase (T-ALP) were within normal reference levels, except in the oldest patient (60 y of age) who had slightly elevated T-ALP and multiple vertebral fractures of unknown age. No other patient older than 25 y had recent fractures. Thus, the causes of elevated B-ALP in particular in adult females with RTT cannot be explained within the limits of our analysis.
RTT patients with or without previous low-energy fractures did not show differences in bone marker levels. After a recent fracture bone markers will be increased, but neither patients nor controls had recent fractures. Levels of bone markers are known predictors of fracture risk in elderly, postmenopausal women (23,25), but the relation between bone markers and fractures in young premenopausal women (26), adolescents, and girls is not well known. Prospective studies are needed to evaluate the clinical usefulness of biochemical bone markers to predict fracture risk in RTT.
The vBMADspine or vBMADneck levels had no influence on the ratio of bone marker levels in patients and controls through all ages. The rationale of adjustment for volumetric bone mineral densities was that a difference in bone mass between RTT patients and controls (8) was presumed to produce differences in levels of bone markers (27). However, no associations between bone markers and vBMADspine and vBMADneck in patients and controls were shown in our study, as also previously reported in healthy children and adolescents (20). Generally, bone markers are known to be related to changes in bone remodeling and in bone mass in elderly postmenopausal women (23,25) but not in young premenopausal women (28). Studies of the association between markers of bone turnover and subsequent bone gain in healthy girls have shown conflicting results (15,17,22,29). An association between bone markers and bone gain should be examined prospectively in a larger group of patients with RTT of different ages and pubertal stages.
We adjusted our analysis of biochemical bone markers for age and puberty, which may be part of the explanation for the difference between our and earlier results in RTT studies (5,13,14). However, limitations of the cross sectional design made it impossible to evaluate the influence on previous shown determinants of bone markers in children and adolescents, as height velocity and bone mineral accrual (15). It is feasible that those determinants are also relevant for bone marker levels in RTT.
In cerebral palsy (CP) patients with motor deficiencies the examination of biochemical bone markers (OC, B-ALP, and N-telopeptides) have shown a wide variety in serum levels (30,31) and no significant association with measures of bone mass as BMD z scores of the lumbar spine (30,31) and distal femur region (31). Furthermore, a study comparing children with CP and healthy children reported no significant differences in both bone formation (B-ALP) and (urinary) resorption markers (32). Thus, the reduced level of bone turnover in children and adolescents with RTT could be a direct effect of the mutated MECP2 gene, although levels of bone markers did not differ between different mutation groups in patients with RTT. This could be due to an overall general effect of MECP2 on regulation of growth and bone turnover. This is corroborated by findings in a mouse model of RTT including growth plate abnormalities, decreased femoral trabecular and cortical bone volume and decreased bone mineral apposition rates (33) as well as by histomorphometric examinations of bone biopsies in young RTT patients which indicate low bone volume and formation rate (34). Further, the low bone turnover in RTT fits the general downsizing of body mass in RTT (10,35,36).
The walking ability did not seem to influence the levels of biochemical bone markers in RTT patients. In accordance, bone marker levels (P1NP, CTX, OC, and B-ALP) did not differ in peripubertal girls with different load-bearing activity (gymnasts, runners, and controls) (22). However, in general, studies have reported conflicting associations of the effects of physical activity on bone turnover in different populations (37). Also, DXA studies of the association between bone mass and ambulant ability in RTT have shown diverging results (4,6,7,13,38).
Our findings are relevant when considering medical treatment of low bone mass in RTT. In a balanced low turnover state of bone metabolism, antiresorptive treatment seems less useful by further decreasing activities of the osteoclasts and the osteoblasts. In contrary, anabolic bone treatment stimulating bone formation may be more relevant, but caution should be taken due to possible medical side effects regarding risk of inducing uncontrolled bone formation in childhood (39).
In conclusion, the major findings of our study were that patients with RTT seem to have a low bone turnover in the modeling period of childhood and youth, but in premenopausal adulthood are similar to controls in bone remodeling with the exception of higher levels of B-ALP in RTT patients. Our finding of a low bone turnover state in children and adolescents with RTT warrants critical attention to medical treatment of low bone mass in young patients with RTT.
Methods
Participants
We included 61 females with RTT and a known MECP2 mutation aged 6–60 y and 122 matched controls with respect to gender, age, pubertal- and menopausal status (9). The controls were recruited from Hvidovre Municipality, Copenhagen by telephone interviews (9). Pubertal status was examined according to Tanner breast development stages 1–5 (40) as categories of three groups, a pre-early-pubertal group of Tanner stages 1 and 2, a pubertal group of Tanner stages 3, 4, and 5 and a postpubertal group of persons older than 20 y, due to differences in menarche status. The patients and controls in the pre-early pubertal group had not experienced menarche. All the patients and controls in the pubertal and the postpubertal groups were past menarche. Menopausal status was defined as cessation of menstruation, supported by levels of measured follicle-stimulating hormone in the postmenopausal reference range (22–138 IU/l).
All participants, parents of healthy children, and parents or guardians of cognitively impaired patients gave informed consent to study participation. The study was approved by the Danish Data Protection Agency and by the local ethics committee and carried out according to the Helsinki Declaration.
Blood Samples
Participants had over night fasting venous samples collected between 8 and 10 am. Plasma was frozen at −80 °C. Plasma levels of P1NP, CTX, and OC were measured by the electrochemiluminescence immunoassays as total P1NP, β-CrossLaps, and N-MID Osteocalcin (Roche Diagnostics GmbH, Mannheim, Germany). Plasma levels of B-ALP were measured by the enzyme immunoassay, Metra BAP (Quidel, San Diego, CA). The coefficients of variation (CVs) (%) were: 1.6–2.3% for P1NP, 1.5–3.0% for CTX, 1.0–1.1% for OC, and 4.2–6.7% for B-ALP at differentiated plasma levels.
P-25-hydroxy-vitamin D2 + D3 (vitamin D) was analyzed by the chemiluminescence immunoassay, DiaSorin (DiaSorin, S.p.A, Sallugia, Italy). The CV was: 11% at the level of 13 nmol/l and 6.8% at the level of 64 nmol/l. A vitamin D value above 50 nmol/l was accepted as normal (41). Fasting blood samples were analyzed to determine red and white blood cell counts, renal and liver functions, estradiol, follicle-stimulating hormone, thyroid status and calcium metabolic parameters as ionized calcium and parathyroid hormone as part of a screening of general health.
BMI and DXA Parameters
Calculated BMI and DXA results for patients and control individuals regarding vBMADspine and vBMADneck were obtained as previously described (8). All DXA scans were critically evaluated, due to the known limitations of positioning in growing children and disabled patients.
Classification of MECP2 Mutations
The MECP2 mutations of patients were classified according to assumed functionality of methyl CpG binding protein 2 (MeCP2): mutations leading to early truncation before the nuclear localization signal, mutations leading to truncation after the nuclear localization signal, C-terminal mutations, and missense mutations. The C-terminal and late truncating mutations were analyzed as one group as they both occur after the site of the nuclear localization signal. The specific MECP2 mutations have been described previously (9).
Walking Ability and Low-Energy Fractures
Data regarding walking ability (independently, lightly assisted, massively assisted/not able to) and low-energy fractures (spontaneous fractures, no known trauma or falls within the person’s height) in RTT patients have previously been described (8,9).
Statistical Analyses
Because of limited access to patients with RTT, we included a patient-control ratio of 1:2 to increase the power of the study. Scatter plots provide an overview of biochemical bone markers related to age. Differences in biochemical bone marker data between patients and control individuals overall were analyzed in linear regression models adjusted for age, pubertal status, calculated BMI, vitamin D, vBMADspine, and vBMADneck. However, nonsignificant covariates were not included in the final analyses to enhance the power of the study. Biochemical bone marker values were log-transformed (log) as they were highly skewed. Further, interaction between patient-control status and the other covariates as well as a nonlinear association of age were tested. Subgroup analysis was performed for the group of persons under 25 y of age and for the group 25 y of age or older, as biochemical bone markers have been shown to decrease by age until the mid twenties and then stabilize in early adulthood (16).
Trends in biochemical bone marker levels according to pubertal status in patients and control individuals are illustrated descriptively in box-whiskers-plots of log-transformed bone marker data.
To test the association between biochemical bone markers and vBMADspine and vBMADneck, each bone marker was analyzed in linear regression models separate for patients and controls, adjusted for age and BMI. A nonlinear association of age and interactions between vBMADspine or vBMADneck and age were tested.
The difference between patients and controls regarding the ratio of bone formation (P1NP) and resorption (CTX), was investigated in a linear regression model with log P1NP as a dependent variable, log CTX as an independent variable, and patient-control status, age, pubertal status and the interaction: log CTX * patient-control status as covariates.
Differences in biochemical bone marker data between patients owing to MECP2 mutation group or walking ability were analyzed in linear regression models adjusted for age and calculated BMI, again with the bone markers log-transformed. Further, a nonlinear association of age was tested. Furthermore, levels of log-transformed bone markers in RTT patients with and without previous low-energy fractures were compared in logistical regression models adjusted for age.
The results from the linear regression models of the log-transformed biochemical bone markers are shown as back transformed beta coefficients (exp (β)) and should therefore be interpreted as rates. Two-sided levels of P values less than 5% were considered significant. Calculations were performed with commercial software (SPSS, version 17.0, Chicago, IL).
Statement of Financial Support
The study was funded by Bevica Fonden, Danske Banks Fond, and Fonden til Lægevidenskabens Fremme. Financial support was provided by the Danish Association of Rett Syndrome.
Disclosure
There are no disclosures; none of the authors has a conflict of interest.
References
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY . Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999;23:185–8.
Hagberg B, Hanefeld F, Percy A, Skjeldal O . An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol 2002;6:293–7.
Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann Neurol 2010;68:944–50.
Haas RH, Dixon SD, Sartoris DJ, Hennessy MJ . Osteopenia in Rett syndrome. J Pediatr 1997;131:771–4.
Motil KJ, Schultz RJ, Abrams S, Ellis KJ, Glaze DG . Fractional calcium absorption is increased in girls with Rett syndrome. J Pediatr Gastroenterol Nutr 2006;42:419–26.
Motil KJ, Ellis KJ, Barrish JO, Caeg E, Glaze DG . Bone mineral content and bone mineral density are lower in older than in younger females with Rett syndrome. Pediatr Res 2008;64:435–9.
Jefferson AL, Woodhead HJ, Fyfe S, et al. Bone mineral content and density in Rett syndrome and their contributing factors. Pediatr Res 2011;69:293–8.
Roende G, Ravn K, Fuglsang K, et al. DXA measurements in Rett syndrome reveal small bones with low bone mass. J Bone Miner Res 2011;26:2280–6.
Roende G, Ravn K, Fuglsang K, et al. Patients with Rett syndrome sustain low-energy fractures. Pediatr Res 2011;69:359–64.
Schultz RJ, Glaze DG, Motil KJ, et al. The pattern of growth failure in Rett syndrome. Am J Dis Child 1993;147:633–7.
Szulc P, Seeman E, Delmas PD . Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int 2000;11:281–94.
Jürimäe J . Interpretation and application of bone turnover markers in children and adolescents. Curr Opin Pediatr 2010;22:494–500.
Cepollaro C, Gonnelli S, Bruni D, et al. Dual X-ray absorptiometry and bone ultrasonography in patients with Rett syndrome. Calcif Tissue Int 2001;69:259–62.
Gonnelli S, Caffarelli C, Hayek J, et al. Bone ultrasonography at phalanxes in patients with Rett syndrome: a 3-year longitudinal study. Bone 2008;42:737–42.
Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB . Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr 2008;153:484–90.
Walsh JS, Henry YM, Fatayerji D, Eastell R . Lumbar spine peak bone mass and bone turnover in men and women: a longitudinal study. Osteoporos Int 2009;20:355–62.
van Coeverden SC, Netelenbos JC, de Ridder CM, Roos JC, Popp-Snijders C, Delemarre-van de Waal HA . Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin Endocrinol (Oxf) 2002;57:107–16.
Eastell R . Role of oestrogen in the regulation of bone turnover at the menarche. J Endocrinol 2005;185:223–34.
Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab 2007;92:443–9.
van der Sluis IM, Hop WC, van Leeuwen JP, Pols HA, de Muinck Keizer-Schrama SM . A cross-sectional study on biochemical parameters of bone turnover and vitamin d metabolites in healthy dutch children and young adults. Horm Res 2002;57:170–9.
Mora S, Pitukcheewanont P, Kaufman FR, Nelson JC, Gilsanz V . Biochemical markers of bone turnover and the volume and the density of bone in children at different stages of sexual development. J Bone Miner Res 1999;14:1664–71.
Lehtonen-Veromaa M, Möttönen T, Irjala K, Nuotio I, Leino A, Viikari J . A 1-year prospective study on the relationship between physical activity, markers of bone metabolism, and bone acquisition in peripubertal girls. J Clin Endocrinol Metab 2000;85:3726–32.
Seibel MJ . Biochemical markers of bone remodeling. Endocrinol Metab Clin North Am 2003;32:83–113.
Hofstaetter JG, Roetzer KM, Krepler P, et al. Altered bone matrix mineralization in a patient with Rett syndrome. Bone 2010;47:701–5.
Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tankó LB . An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol 2006;62:781–92.
Glover SJ, Garnero P, Naylor K, Rogers A, Eastell R . Establishing a reference range for bone turnover markers in young, healthy women. Bone 2008;42:623–30.
Rauch F . The growing skeleton is a busy place–can biochemical bone markers keep track of the action? J Pediatr 2008;153:454–5.
Bennell KL, Malcolm SA, Khan KM, et al. Bone mass and bone turnover in power athletes, endurance athletes, and controls: a 12-month longitudinal study. Bone 1997;20:477–84.
Cadogan J, Blumsohn A, Barker ME, Eastell R . A longitudinal study of bone gain in pubertal girls: anthropometric and biochemical correlates. J Bone Miner Res 1998;13:1602–12.
Henderson RC, Lin PP, Greene WB . Bone-mineral density in children and adolescents who have spastic cerebral palsy. J Bone Joint Surg Am 1995;77:1671–81.
Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 2002;110(1 Pt 1):e5.
Chen CL, Ke JY, Wang CJ, Wu KP, Wu CY, Wong AM . Factors associated with bone density in different skeletal regions in children with cerebral palsy of various motor severities. Dev Med Child Neurol 2011;53:131–6.
O’Connor RD, Zayzafoon M, Farach-Carson MC, Schanen NC . Mecp2 deficiency decreases bone formation and reduces bone volume in a rodent model of Rett syndrome. Bone 2009;45:346–56.
Budden SS, Gunness ME . Possible mechanisms of osteopenia in Rett syndrome: bone histomorphometric studies. J Child Neurol 2003;18:698–702.
Schultz R, Glaze D, Motil K, Hebert D, Percy A . Hand and foot growth failure in Rett syndrome. J Child Neurol 1998;13:71–4.
Armstrong DD, Dunn JK, Schultz RJ, Herbert DA, Glaze DG, Motil KJ . Organ growth in Rett syndrome: a postmortem examination analysis. Pediatr Neurol 1999;20:125–9.
Maïmoun L, Sultan C . Effects of physical activity on bone remodeling. Metabolism 2011;60:373–88.
Shapiro JR, Bibat G, Hiremath G, et al. Bone mass in Rett syndrome: association with clinical parameters and MECP2 mutations. Pediatr Res 2010;68:446–51.
Tashjian AH Jr, Goltzman D . On the interpretation of rat carcinogenicity studies for human PTH(1-34) and human PTH(1-84). J Bone Miner Res 2008;23:803–11.
Tanner JM . The Development of the Reproductive System. In: Tanner JM, ed. Growth at Adolescence. Oxford, UK: Blackwell Scientific Publications, 1962: 28–39.
Mosekilde L, Nielsen LR, Larsen ER, Moosgaard B, Heickendorff L . Vitamin D deficiency. Definition and prevalence in Denmark. Ugeskr Laeger 2005;167:29–33.
Acknowledgements
We sincerely thank the Danish patients with RTT and their families and caregivers as well as all control persons and their families for participation in the project. Thanks to the Danish Association of Rett Syndrome for advertising the project. We thank Pernille Strøm and Mette Lisa Jørgensen at the Kennedy Center for handling of the project logistic. We also acknowledge the work of Anne Mette Rasmussen at the Endocrinological Department, Hvidovre Hospital for controlling the flow of examinations at Hvidovre Hospital for patients and controls. Gitte Roende is responsible for conducting the study and for the integrity of data. All authors have made contributions to the concept and design, analysis and interpretation of data, and drafting or revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Roende, G., Petersen, J., Ravn, K. et al. Low bone turnover phenotype in Rett syndrome: results of biochemical bone marker analysis. Pediatr Res 75, 551–558 (2014). https://doi.org/10.1038/pr.2013.252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.252
This article is cited by
-
Clinical and biological progress over 50 years in Rett syndrome
Nature Reviews Neurology (2017)