Abstract
Introduction:
Protein intake in fetal life or infancy may play a key role in determining early growth rate, a determinant of later health and disease. Previous work has indicated that hair isotopic composition is influenced by diet and protein intake.
Methods:
This study analyzes the isotopic composition of hair obtained from 239 mother/newborn pairs randomly selected within a larger cohort enrolled in a study of pre- and postnatal determinants of the child’s development and health. The isotopic compositions in nitrogen (δ15N) and in carbon (δ13C) were determined by isotope ratio mass spectrometry.
Results:
Mother and newborn hair δ15N were tightly correlated (Pearson r = 0.88). The mean δ15N and δ13C values of hair from newborn infants were significantly higher than those for the mothers: 9.7 ± 0.7 vs. 8.8 ± 0.6‰ (P < 0.0001) for δ15N and −20.0 ± 0.4 vs. −20.4 ± 0.4‰ (P < 0.0001) for δ13C. Maternal hair δ15N at parturition was slightly and positively correlated with estimates of protein intake (r = 0.14, P = 0.04).
Discussion:
Hair δ15N of the fetus is both highly dependent on and systematically higher than that of the mother. Whether quantitative and qualitative protein intake, disease, or hormonal status alter hair δ15N at birth remains to be determined.
Similar content being viewed by others
Main
Early life programming plays a major role in determining health and disease in later life (1). Unsatisfactory size at birth and/or weight gain velocity in childhood are linked to increased risk of onset of coronary events in adulthood (2). Birth weight for gestational age (GA) is the main variable for fetal growth and adequacy of fetal nutrient intake (i.e., used to measure intrauterine growth retardation of placental origin, for instance). However, although several studies have reported an association between protein intake in infancy, growth velocity, and fat mass development in children (3,4,5), there are currently no reliable simple indexes for fetal protein intake. We know from animal studies that decreasing protein intake during gestation decreases birth weight and shortens life span in mice (6) and that increasing protein intake during gestation results in lower birth weight and higher fat mass at 3 mo in rats (7). Furthermore, in intrauterine growth retardation caused by an experimentally low protein intake, amino-acid transporter expression is affected before growth restriction, suggesting a causative effect of maternal nutrition (8). In humans, intrauterine growth retardation led to increased plasma amino acid concentrations in the mother coupled with decreased levels in the fetus (9), apparently due to impaired amino-acid transfer from mother to fetus (10,11). Protein intake might modulate epigenetic modification of gene expression in the offspring (12,13,14).
What we seek therefore is a rapid and robust indirect method by which the protein transfer between mother and infant during gestation can be easily assessed. Isotopic values in hair may fulfill this need. It has been shown that the isotopic content of hair is quantitatively correlated with protein source (animal or vegetable) and intake (15,16) in animals and humans including pregnant women (17) and that the δ13C and δ15N values of hair and fingernail tissue taken from newborn babies are related to those of their mothers (18). However, not only was there a considerable degree of variability in the mother/infant pairs but also the small population size makes it difficult to establish whether this tendency would be valid over a wide population, as would be required were the relationship to be exploitable as a potential marker of nutritional status. Furthermore, δ13C and δ15N of hair were followed in only one subject.
Considering that hair is the easier tissue to sample from newborn infants, we have, within the context of the EDEN mother–child cohort (prenatal and early postnatal determinants of child development and health), analyzed δ13C and δ15N for hair samples randomly taken from 239 newborn babies and their mothers in order to assess correlations between isotopic values of mothers and children in a larger population than in previous publications and to study whether there is a relationship between maternal diet and the isotopic values.
Results
Population
Mother and child characteristics did not differ between the Poitiers and Nancy populations ( Table 1 ). Diet before pregnancy did not differ between these two centers. During the last 3 mo of pregnancy, (i) carbohydrate intake expressed in g/d or % of daily energy intake (%DEI) was slightly higher in Poitiers than in Nancy; (ii) lipid intake (%DEI) was lower in Poitiers than in Nancy; (iii) vegetable protein intake (%DEI) decreased and lipid intake (%DEI) increased in both centers during the last 3 mo of pregnancy. No significant difference was observed for any other parameter of the dietary record ( Table 1 ).
Validation of the Protocol for the Measurement of δ15N and δ13C in Hair by Elemental Analyzer-Isotope Ratio Mass Spectrometer
The working sample mass range was determined using one maternal sample and varying the mass of prewashed hair encapsulated (duplicate samples) from 0.1 to 0.8 mg in 0.1 mg increments. Loss of precision was seen below 0.4 mg (data not shown). Therefore, the sample cutoff was set at 0.4 mg and the mass target at 0.6 ± 0.08 mg.
Feasibility was tested with three mother/infant hair pairs, with and without prewashing. Each pair was analyzed twice and the %C and %N determined. The overall means were %C=45.6 ± 0.3, %N = 14.5 ± 0.9 and C/N = 3.14 ± 0.16. No significant difference was seen for samples with or without prewashing (data not shown). However, for consistency, all samples were prewashed as described earlier.
Repeatability and precision were assessed with 10 samples of prewashed hair from one mother. Values of δ13C = −20.03 ± 0.07‰ (max, 20.56‰; min, 19.77‰) and δ15N = 9.08 ± 0.13‰ (max, 9.21‰; min, 8.95‰) were obtained. The precision for the working standard was ± 0.08‰ for both δ15N and δ13C. Therefore, an acceptable difference between two measurements of hair samples was set to 0.2‰ for δ13C and 0.3‰ for δ15N (95% confidence limit).
Values of δ15N and δ13C in Mother/Newborn Hair Pairs by Elemental Analyzer-Isotope Ratio Mass Spectrometer
From the 250 pairs abstracted from the 1,770 available samples in the EDEN cohort, 10 pairs with a technical problem and 1 with insufficient mass were not replaced, meaning that 239 pairs of hair samples were analyzed. These samples all gave measurements in the linear range of the spectrometer with homogeneous values. Samples falling outside the defined acceptable difference between two measurements were reanalyzed or replaced with another sample from the same protein-origin group.
Mean δ15N for infant hair was systematically significantly higher than the mean δ15N for the mother’s hair (P < 0.0001): δ15Ninfant = 9.65 ± 0.70‰ and δ15Nmother = 8.76 ± 0.62‰. No case was recorded in which the δ15Ninfant was inferior to that of the δ15Nmother. Mean δ13C for infant hair was systematically significantly higher than the mean δ13C for the mother’s hair (P < 0.0001): δ13Cinfant = −19.99 ± 0.40‰ and δ13Cmother = −20.36 ± 0.42‰. Only one Δδ13C was recorded in which the δ13Cinfant was significantly lower than the δ13Cmother. As a way of verifying that the samples were not contaminated, the C/N ratios were evaluated. The mean values for mothers and infants were 3.09 ± 0.16 and 2.95 ± 0.12, respectively (P < 0.0001). In contrast to the isotope values, no significant correlation was seen for the C/N ratios of mother/infant pairs.
In both mother and newborn hair, mean δ15N values were lower in the Poitiers population than in the Nancy population, whereas the opposite occurred for δ13C ( Table 1 ). We did not observe any effect of gender on the mean δ15N and δ13C in hair from newborns in either the Poitiers or Nancy groups (data not shown).
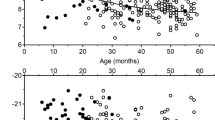
Strong correlations were observed between mother/newborn pairs of data. The stronger correlation was for δ15N ( Figure 1a : Pearson correlation coefficient r = 0.88 (0.84–0.90)), but a clear correlation was also observed for δ13C ( Figure 1b : Pearson correlation coefficient r = 0.74 (0.68–0.79)). It was particularly noticeable that the one pair seen as an outlier in δ15N still fits the mother/newborn correlation ( Figure 1a,b ). The mean δ15N for newborn hair was systematically higher than the mean δ15N for mother’s hair ( Table 1 ). Similarly, the mean δ13C for newborn hair was systematically higher than the mean δ13C for the mother’s hair ( Table 1 ). In only one case was the δ13Cnewborn significantly lower than the δ13Cmother.
Correlation between (a) δ15N (‰) values and (b) δ13C (‰) values for hair samples from mother/newborn pairs. For δ15N (‰), linear correlation is y = 0.99x + 0.99, R2 = 0.76969, Pearson correlation coefficient r = 0.88 (0.84–0.90). For δ13C (‰), linear correlation is y = 0.70x− 5.77, R2 = 0.55018, Pearson correlation coefficient r = 0.74 (0.68–0.79). Confidence limits (95%) of the measurements are 0.2‰ for δ13C and 0.3‰ for δ15N.
Isotope values were also compared for mother/infant pairs from small-for-GA (SGA), appropriate-for-GA (AGA), and large-for-GA neonate populations. The birth weight of the SGA population (n = 20) was significantly lower than that for the AGA population (n = 219): SGA birth weight 2,582 ± 301 g vs. AGA birth weight 3,412 ± 465 g (P < 10−4), but this had no impact on the δ15N value for hair (SGA 9.82 ± 0.68‰ vs. AGA 9.63 ± 0.71‰, not significant). Similarly, mothers of SGA children did not have a lower δ15N than mothers of AGA children (8.90 ± 0.75 vs. 8.78 ± 0.61‰, not significant). GA and age at birth of the mothers with SGA babies were not different from those of mothers of babies with an AGA weight. The BMI of mothers with an SGA child was lower than that of mothers of AGA children (20 ± 2 vs. 24 ± 4 kg/m2, P < 0.001), and their total energy intake during pregnancy was higher (2,851 ± 1,125 kcal/d vs. 2,396 ± 704 kcal/d, P < 0.05). No significant differences were observed between isotope values (δ13C or δ15N) of hair in mother/infant pairs for large-for-gestational age infants vs. the AGA population.
In mothers but not in newborns, δ15N was positively associated with estimates of intake of protein, specifically animal protein, and lipids during the last 3 mo of the pregnancy ( Table 2 ). The δ13C in mothers and newborns was negatively associated with mean energy, carbohydrate, and lipid intakes ( Table 2 ). We did not observe any correlation between δ15N and δ13C in mothers and newborns and estimates of energy, total and animal proteins, lipids, and carbohydrates before pregnancy (data not shown). Head circumference was significantly smaller in newborns in the upper quartile of hair δ15N values (P = 0.004 and P = 0.06 comparing the upper quartiles vs. the three lower quartiles of, respectively, newborn and mother δ15N values after adjustment for GA). Newborn hair δ15N showed a negative correlation with GA (−0.14; Table 2 ) and children within the upper quartile were of lower GA at birth (38.9 ± 1.9 vs. 39.5 ± 1.4 wk, P < 0.05). No significant correlation was observed with any other parameter of fetal growth, although a negative trend was found with birth weight ( Table 2 ).
Discussion
The data obtained in this study yielded three main results. First, the δ15N and δ13C values for individual mother/newborn pairs were strongly correlated. Second, the δ15N and δ13C values for newborn hair were consistently higher than for the mother. Third, a slight positive correlation between the δ15N of mother’s hair at parturition and the total protein intake in the last trimester of gestation was observed.
The δ15N and δ13C values of hair from newborns were strongly correlated with those of their mothers in the entire population tested. That the correlation is less strong for δ13C is to be expected because the carbon pool is influenced by glucose and lipid metabolism as well as protein, whereas the nitrogen pool is specific to protein metabolism. Because the fetus obtains its entire nutrition from the mother, this might at first sight not be surprising. However, the linear aspect of the correlation surprised us. The common belief is that fetal metabolism is the priority. If that were the case, we would expect a stronger relationship between maternal and fetal values for low values of δ15N than for high values. A linear relationship suggests that fetal protein metabolism might be directly determined by the mother.
Whether the mother’s protein intake may affect fetal protein metabolism and growth remains to be tested. It must be emphasized that this study aimed at establishing whether there exists a robust correlation between maternal protein intake and δ15N from the hair of mothers and newborns within the normal population. The correlation was not found for newborn δ15N. Many factors (level of protein intake, protein percentage derived from vegetal or animal sources, energy intake, nutritional status, whole-body protein turnover in each mother and child pair) were not controlled in this cross-sectional observational study and could have their own effect on fetal protein metabolism and 15N transfer to hair protein. In addition, we were not able to detect an association between fetal growth restriction and δ15N. This warrants further studies in pregnant animals to determine how maternal nutrition and intrauterine growth retardation determine 15N transfer from mother to fetus.
Less obvious, however, is why the δ15N values for hair were consistently higher in newborns than in their own mothers. Because the amino-acid pool of the fetus comes entirely from that of the mother, it might be expected that these two pools would be at equilibrium. However, as pointed out by several authors (16), this observation is compatible with the well-described trophic effect: the δ15N value for protein increases through the food chain from plants to herbivores to omnivorous species such as humans (15,16).
The factors leading to differential 15N/14N retention in omnivorous species need to be further studied, but a number of mechanisms have been put forward. It has been suggested that hepatic transamination favors 15N retention in body proteins(19). Studies using amino acids labeled with stable isotopes showed that leucine (20,21) or glutamine (22,23,24) turnover was higher in premature babies than in term babies as well as adults. It can be speculated that the high protein turnover of the fetus may augment transamination, thus favoring 15N retention.
In mothers, an increase in nitrogen retention in the later stages of gestation, a decrease in urea excretion and synthesis (25,26), and a decrease of branched-chain amino-acid transamination (27) will all act to lower the δ15N in hair of mothers and increase the differential between a mother and her offspring. This is consistent with a study showing a decrease in δ15N of maternal hair throughout pregnancy (17) and with studies of eating disorders (28) and digestive malfunction (29), both of which lead to depletion in 15N.
Head circumference at birth was smaller in babies within the upper quartile of δ15N. This intriguing observation persists when data are adjusted to GA. The effect of maternal protein intake on brain development is poorly documented. A recent article showed that a 30% reduction in total energy intake resulted in structural abnormalities of the brain without any change in brain mass (30). On the other hand, a 10-g/d increase in protein intake led to an 18-g reduction in birth weight without any observed change in head circumference (31). While clearly observational studies such as ours cannot test the effect of protein intake on brain development, they do support a need for further investigations, initially through animal studies. Others nutrients, such as fatty acids, are associated with food proteins and their impact needs to be taken into account.
The slight correlation between stable isotope ratios (δ15N)in mothers’ hair and dietary protein intake during the last 3 mo of pregnancy is, in contrast, readily explained. Such correlations exist because the δ15N values of the food intake are influenced by the nature of the protein source consumed. Thus, vegans (people who do not eat any animal-derived food) have a low δ15N relative to the average for the population, whereas vegetarians (people who do not eat any animal food) have values midway between vegans and omnivores (15). In accordance, we found a significant correlation mainly with proteins of animal origin. The value of the correlation coefficient (0.14) is not very high because food frequency questionnaires do not allow a precise measurement of nutrient intake and this finding needs further investigation. Our study is observational and establishes that the correlation previously indicated (15,16,17) is robust within a large population. Thus, thanks to the large cohort available, this study suggests that hair δ15N values show promise as a nutritional biomarker in European postpartum mothers and their newborn babies, for example, in potentially estimating the impact of maternal protein intake on fetal growth in normal and obese women (32). Controlled studies are now necessary to test the direct impact of protein intake on δ15N of mothers’ hair and newborns.
Methods
Ethics Statement
The study was conducted according to the principles expressed in the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Bicêtre hospital (Kremlin-Bicêtre, France) on 12 December 2002. Written consent was obtained from the mother for herself at inclusion and for her newborn child after delivery.
Subjects
The EDEN mother–child cohort is a study of the prenatal and early postnatal determinants of child development and health from birth to 5 y ( Figure 2 ). Pregnant women seen for a prenatal visit between February 2003 and January 2006 at the Departments of Obstetrics and Gynecology of the University Hospitals of Nancy (France) and Poitiers (France) before 24 wk of amenorrhea were invited to participate. Exclusion criteria were twin pregnancies; known diabetes mellitus before pregnancy; French illiteracy, plan to move out of the district.
Dietary Data
A food frequency questionnaire was completed twice by each mother: once at recruitment (on average 15 wk of amenorrhea) and a second time during the first few days postparturition. These allowed an estimation of food intake in the first and third trimester of pregnancy, respectively (33). Energy and nutrient intakes were computed from questionnaires with portion size determined using pictures (34) and the SU.VI.MAX nutrient composition database (35).
Clinical Data
At a first visit performed between 24 and 28 wk of amenorrhea by midwife research assistants, maternal height was measured with a wall stadiometer (Seca 206; Seca, Hamburg, Germany) to the nearest 0.2 cm and maternal weight was measured using electronic scales (TerraillonSL 351; Hanson, Hemel Hempstead, UK) to the nearest 0.1 kg. Weight before pregnancy was obtained by interview. Pre-pregnancy BMI was computed by the standard formula. By reference to the International Obesity Task Force, overweight was defined as a BMI ≥ 25 kg/m2 and obesity as a BMI ≥ 30 kg/m2. GA at delivery (determined from the date of the last menstrual period and early ultrasound assessment), newborn admission to an intensive care unit or neonatal unit, birth weight, length, and head circumference were extracted from clinical records, and mother’s weight after delivery was obtained with the same protocol as above.
In the two obstetric departments, electronic Seca scales (Seca 737 in Nancy and Seca 335 in Poitiers) were used to measure newborn weight and a wooden somatometer (Testut, Béthune, France) to measure newborn length. Large-for-gestational-age and SGA neonates were defined as babies with a birth weight over the 90th percentile and below the 10th percentile, respectively, of French GA and gender-specific reference curves (36). AGA neonates are defined as between the 90th and 10th percentile.
Hair Sample Collection and Preparation
Hair samples were collected from 1,770 pairs within the cohort. On the basis of the dietary data, the population was divided into 10 equal groups according to protein intake; 250 pairs were randomly taken (125 from each center), with 25 pairs being included from each protein group. The choice of number of pairs ≈250 was calculated so as to show a correlation coefficient ≈0.2 with a power of 90% and an α risk of 5%. Eleven pairs were not included in the final data set due to technical problems with analysis.
Mother and baby hair samples were taken 3 d after birth. A tuft of hair was cut in the occipital area as close as possible to the scalp. We deliberately chose to analyze a “random” sample of maternal hair so as not to bias the data toward a particular period of pregnancy. The longest hair present was selected and the hair root was not included. Samples were blind-coded with an identification number for each pair and labeled “M” or “E” to indicate mother or newborn baby, respectively. The complete hair sample of infants and a representative hair sample of mothers were transferred to a small glass bottle and cut into small sections (1 mm or less). This sample was washed in cyclohexane (20 min) to remove sebum (lipids) and residue (e.g., shampoo). Residual traces of solvent were removed by evaporation at 45°C under a stream of pure nitrogen gas until completely dry. An aliquot of each sample (~0.6 mg, giving ~0.08 mg N) was weighed with 10−5 g precision (balance; Ohaus Discovery DV215CD, Pine Brook, NJ) into two tin capsules (solids “light” 5 ×9 mm, Thermo Fisher Scientific, Bremen, Germany, http://www.thermo.com). Each hair sample was analyzed in duplicate (with a few exceptions where the infant sample mass was insufficient to prepare two capsules). When the total sample mass was <0.4 mg, the pair was excluded and replaced by new pair from the same protein group; 38 mother/child pairs required replacement, the substitute samples being selected by the same randomized procedure.
Isotope Analysis
The 13C/12C and 15N/14N ratios and the N and C percentage compositions were determined as described previously (14). Briefly, capsules containing hair samples were flash combusted in an oxygen atmosphere using an elemental analyzer Flash EA 1112 HT (Thermo Fisher Scientific) and the resultant gases (CO2 and N2) carried in a stream of He to a Delta V Advantage isotope ratio mass spectrometer (Thermo Fisher Scientific) coupled online via a Conflo III interface (Thermo Fisher Scientific). Following sample combustion, water was removed by a Mg(ClO4)2 water trap and N2 was completely separated from CO2 using a Porapak Q gas chromatography column (Thermo Fisher Scientific). Ion currents were measured for m/z28, 29, 30 and m/z 44, 45, 46, for N2 and CO2, respectively, from which the δ13C (‰) and δ15N (‰) values could be calculated. Stable isotope ratios were expressed as the δ13C (‰) and δ15N (‰) ratios relative to reference pulse peaks of laboratory CO2 and N2 respectively, calculated as follows

where R is the 13C/12C or 15N/14N isotope ratio of the sample and Rstd is the 13C/12C isotope ratio of Vienna Pee Dee Belemnite reference standard (Rstd = 0.0112372) or of atmospheric N2 (Rstd = 0.0036765). Normalization was made using a working standard of glutamic acid defined as having an isotopic composition of δ15N = −4.80 ± 0.08‰ and δ13C = −27.48 ± 0.05‰ relative to these standards.
The C/N ratio was calculated in hair samples from the weight percentages determined from the integrated peak areas for CO2 and N2 from the mass spectrometer ion currents and sample weights. The same working standard (glutamic acid) was used for the N and C weight percentage determinations.
Statistical Analysis
Data were initially processed using Microsoft Excel 2003. All statistical analyses were carried out using SAS 9.1.3 (SAS Institute, Cary, NC) on the AIX 5.1 platform (IBM, New York, NY). Pearson correlations and partial Pearson correlations taking into account center and GA were used to assess the strength of the association between mother or newborn δ15N and δ13C values, and anthropometry or diet, respectively. Confidence interval for correlation coefficients have been computed using the Fisher’s z transformation. To study the shape of the relationships, means of anthropometric variables were estimated by quartiles of δ15N and δ13C (adjusted for potential confounders: i.e., GA and/or center) using multivariate general linear regression models
Statement of Financial Support
The study was supported by a grant from the French Regional Hospital Program for Clinical Research. A.d.L. was supported by a grant from the Académie Nationale de Médecine and by prizes from the French Foundation for Medical Research (Fondation pour la Recherche Médicale) and the French Pediatrics Society of West France (Société de Pédiatrie de l’Ouest-SPO and Société Française de Pédiatrie-SFP).
Disclosure
The authors have no conflicts of interest to report.
References
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ . Weight in infancy and death from ischaemicheart disease. Lancet 1989;2:577–80.
Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG . Trajectories of growth among children who have coronary events as adults. N Engl J Med 2005;353:1802–9.
Rolland-Cachera MF, Deheeger M, Akrout M, Bellisle F . Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. Int J Obes Relat Metab Disord 1995;19:573–8.
Günther AL, Buyken AE, Kroke A . The influence of habitual protein intake in early childhood on BMI and age at adiposity rebound: results from the DONALD Study. Int J Obes (Lond) 2006;30:1072–9.
Koletzko B, von Kries R, Closa R, et al.; European Childhood Obesity Trial Study Group. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45.
Ozanne SE, Hales CN . Lifespan: catch-up growth and obesity in male mice. Nature 2004;427:411–2.
Daenzer M, Ortmann S, Klaus S, Metges CC . Prenatal high protein exposure decreases energy expenditure and increases adiposity in young rats. J Nutr 2002;132:142–4.
Jansson N, Pettersson J, Haafiz A, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol (Lond) 2006;576(Pt 3):935–46.
Cetin I, Ronzoni S, Marconi AM, et al. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol 1996;174:1575–83.
Paolini CL, Marconi AM, Ronzoni S, et al. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 2001;86:5427–32.
Marconi AM, Paolini CL, Stramare L, et al. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res 1999;46:114–9.
Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998;351:173–7.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL . Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73.
Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC . Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 2005;135:1382–6.
Petzke KJ, Boeing H, Klaus S, Metges CC . Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr 2005;135:1515–20.
Petzke KJ, Fuller BT, Metges CC . Advances in natural stable isotope ratio analysis of human hair to determine nutritional and metabolic status. Curr Opin Clin Nutr Metab Care 2010;13:532–40.
Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC, Hedges RE . Nitrogen balance and delta15N: why you’re not what you eat during pregnancy. Rapid Commun Mass Spectrom 2004;18:2889–96.
Fuller BT, Fuller JL, Harris DA, Hedges RE . Detection of breastfeeding and weaning in modern human infants with carbon and nitrogen stable isotope ratios. Am J Phys Anthropol 2006;129:279–93.
Macko SA, Fogel Estep ML, Engel MH, Hare PE . Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochim Cosmochim Acta 1986;50:2143–6.
Denne SC, Karn CA, Liechty EA . Leucine kinetics after a brief fast and in response to feeding in premature infants. Am J Clin Nutr 1992;56:899–904.
Beaufrere B, Putet G, Pachiaudi C, Salle B . Whole body protein turnover measured with 13C-leucine and energy expenditure in preterm infants. Pediatr Res 1990;28:147–52.
Hankard R, Goulet O, Ricour C, Rongier M, Colomb V, Darmaun D . Glutamine metabolism in children with short-bowel syndrome: a stable isotope study. Pediatr Res 1994;36:202–6.
des Robert C, Le Bacquer O, Piloquet H, Rozé JC, Darmaun D . Acute effects of intravenous glutamine supplementation on protein metabolism in very low birth weight infants: a stable isotope study. Pediatr Res 2002;51:87–93.
Hankard RG, Haymond MW, Darmaun D . Effect of glutamine on leucine metabolism in humans. Am J Physiol 1996;271(4 Pt 1):E748–54.
McClelland IS, Persaud C, Jackson AA . Urea kinetics in healthy women during normal pregnancy. Br J Nutr 1997;77:165–81.
Mojtahedi M, de Groot LC, Boekholt HA, van Raaij JM . Nitrogen balance of healthy Dutch women before and during pregnancy. Am J Clin Nutr 2002;75:1078–83.
Kalhan SC . Protein metabolism in pregnancy. Am J Clin Nutr 2000;71:Suppl 5:1249S–55S.
Hatch KA, Crawford MA, Kunz AW, et al. An objective means of diagnosing anorexia nervosa and bulimia nervosa using 15N/14N and 13C/12C ratios in hair. Rapid Commun Mass Spectrom 2006;20:3367–73.
Petzke KJ, Feist T, Fleig WE, Metges CC . Nitrogen isotopic composition in hair protein is different in liver cirrhotic patients. Rapid Commun Mass Spectrom 2006;20:2973–8.
Antonow-Schlorke I, Schwab M, Cox LA, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA 2011;108:3011–6.
Andreasyan K, Ponsonby AL, Dwyer T, et al. Higher maternal dietary protein intake in late pregnancy is associated with a lower infant ponderal index at birth. Eur J Clin Nutr 2007;61:498–508.
Mok E, Multon C, Piguel L, et al. Decreased full breastfeeding, altered practices, perceptions, and infant weight change of prepregnant obese women: a need for extra support. Pediatrics 2008;121:e1319–24.
de Lauzon B, Romon M, Deschamps V, et al.; Fleurbaix Laventie Ville Sante Study Group. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr 2004;134:2372–80.
Deschamps V, de Lauzon-Guillain B, Lafay L, Borys JM, Charles MA, Romon M . Reproducibility and relative validity of a food-frequency questionnaire among French adults and adolescents. Eur J Clin Nutr 2009;63:282–91.
Hercberg S, Galan P, Preziosi P, et al. Background and rationale behind the SU.VI.MAX Study, a prevention trial using nutritional doses of a combination of antioxidant vitamins and minerals to reduce cardiovascular diseases and cancers. SUpplementation en VItamines et Minéraux AntioXydants Study. Int J Vitam Nutr Res 1998;68:3–20.
Mamelle N, Munoz F, Grandjean H . [Fetal growth from the AUDIPOG study. I. Establishment of reference curves]. J Gynecol Obstet Biol Reprod (Paris) 1996;25(1):61–70.
Acknowledgements
We thank all members of the EDEN mother–child study group for their invaluable input to this study. We give special thanks to Dominique Darmaun for his helpful and critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Luca, A., Boisseau, N., Tea, I. et al. δ15N and δ13C in hair from newborn infants and their mothers: a cohort study. Pediatr Res 71, 598–604 (2012). https://doi.org/10.1038/pr.2012.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.3
This article is cited by
-
Isotopic analysis of formula milk reveals potential challenges in geolocating bottle-fed babies
Scientific Reports (2024)
-
Early life histories at medieval Mikulčice (ninth–tenth centuries AD, Czechia) based on carbon and nitrogen profiles of tooth dentine
Archaeological and Anthropological Sciences (2024)
-
A multi-isotope analysis of Neolithic human groups in the Yonne valley, Northern France: insights into dietary patterns and social structure
Archaeological and Anthropological Sciences (2019)
-
A Multidisciplinary Approach to Neolithic Life Reconstruction
Journal of Archaeological Method and Theory (2019)
-
Isotopic evidence for the reconstruction of diet and mobility during village formation in the Early Middle Ages: Las Gobas (Burgos, northern Spain)
Archaeological and Anthropological Sciences (2018)