Abstract
Traditionally, type 1 diabetes (T1D) has been thought of as a disease of cellular immunity, but there is increasing evidence that components of the innate immune system, controlled largely by Toll-like receptors (TLRs), play a significant role in T1D development. TLRs are pattern-recognition molecules on immune cells that recognize pathogens, leading to the production of cytokines such as interleukin-1β (IL1β, encoded by the IL1B gene). IL1β is increased in patients with newly diagnosed T1D and likely acts as an early inflammatory signal in T1D development. Because hyperglycemia is a hallmark of T1D, the effects of hyperglycemia on IL1β expression in peripheral blood mononuclear cells (PBMCs) and islet cells have been examined, but with inconsistent results, and the mechanisms leading to this increase remain unknown. Fatty acids stimulate IL1β expression and may promote inflammation, causing hyperglycemia and insulin resistance. The mechanisms by which IL1β is involved in T1D pathogenesis are controversial. Overall, studies in pancreatic β-cells suggest that IL1β-mediated damage to islet cells involves multiple downstream targets. Potential therapies to decrease the progression of T1D based on IL1β biology include pioglitazone, glyburide, IL1 receptor antagonists, and agents that remove IL1β from the circulation.
Similar content being viewed by others
Main
The incidence of type 1 diabetes (T1D), the most common endocrine disorder of children, is increasing at an alarming rate. There is no cure, and T1D pathophysiology is still not fully understood. Insulin, the main therapy for T1D, does not treat the underlying cause but treats only the consequence of autoimmune destruction of the insulin-producing pancreatic islet β-cells. Traditionally, T1D has been thought of as a disease of cellular immunity (1,2), but there is increasing evidence that components of the innate immune system play a significant role in its development. The innate immune system is regulated largely by Toll-like receptors (TLRs), pattern-recognition molecules that recognize specific pathogens or endogenous danger signals, activating a nonspecific yet robust inflammatory response. This response includes the production of cytokines such as interleukin-1β (IL1β) that orchestrate the recruitment of inflammatory cells to the islets and mediate direct cytotoxic effects on β-cells (3,4,5).
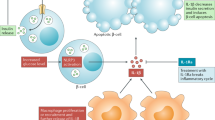
The signaling pathway from TLR activation to secretion of mature IL1β is complex. Activation of the intracellular signaling pathway via nuclear factor-κB (NFκB) increases the transcription of the IL1B gene encoding pro-IL1β and thus intracellular levels of the procytokine. Subsequent protein processing by the NLRP3 inflammasome, a complex that includes NACHT, LRR, and PYD domains-containing protein-3 (NLRP3); apoptosis-associated speck-like protein; and caspase 1 (previously known as IL-converting enzyme), cleaves pro-IL1β to the mature protein ( Figure 1 ; ref. 6). Although there are pathways other than TLR that lead to IL1β release, such as the adenosine triphosphate receptor P2X7, and there are other inflammasomes, such as IL-converting enzyme protease-activating factor and NLRP1, that activate caspase 1, this review will focus on the TLR to NLRP3 inflammasome pathway.
IL1β has long been implicated in the development of T1D, but there is increasing evidence that this cytokine acts as an early inflammatory signal in this process. IL1β has also been implicated in the pathogenesis of type 2 diabetes (T2D) (3), and much recent research involving hyperglycemia and IL1β has focused on T2D. Although there is overlap in the final pathway between these two distinct entities, and this review refers occasionally to T2D literature, it is important to remember that T1D results from autoimmune destruction of the pancreatic β-cells, whereas T2D results from a combination of insulin resistance, nonimmunological β-cell apoptosis, toxic effects of elevated glucose and fatty acids, inflammation, and other factors (3). Supplementary Table S1 is available online and it summarizes the major findings from the articles reviewed in this paper and differentiates between studies of T1D and T2D.
IL1-receptor antagonist (IL1-ra), an endogenously produced protein, blocks the interaction of IL1β with its receptor. Under homeostatic conditions, there is a balance between IL1β and IL1-ra, and no inflammatory responses are triggered. Thus, when IL1-ra content is decreased, there is unchecked activity of IL1β, resulting in downstream effects. This property of IL1-ra has been harnessed to develop synthetic IL1-ra and IL1 traps as anti-inflammatory agents that have US Food and Drug Administration approval for treatment of certain types of arthritis and cryopyrin-associated periodic syndromes. These relatively new compounds are beginning to be studied in humans with T1D. An increased understanding of the role of IL1β in T1D pathophysiology may allow improved treatment of T1D, especially because these US Food and Drug Administration–approved drugs now exist.
IL1β and T1D
Studies of IL1β in Human T1D
IL1β is secreted mainly by macrophages (7). We previously demonstrated that IL1B mRNA is increased in peripheral blood mononuclear cells (PBMCs) obtained from newly diagnosed T1D patients (8). IL1B expression decreases over the first 4 months after diagnosis, and by 2 y, IL1B mRNA levels are comparable to those of healthy controls. In addition, unstimulated monocytes from newly diagnosed patients with T1D express higher levels of IL1β, and activation of PBMCs with lipopolysaccharides (LPSs) leads to increased IL1β as compared with healthy controls (9). Others found that serum IL1β concentrations are elevated in both long-standing (10,11) and newly diagnosed T1D patients, although IL1β levels decreased after treatment of newly diagnosed T1D (11). Moreover, as determined by enzyme-linked immunosorbent spot, PBMCs from T1D patients had higher basal production of IL1β than cells from healthy controls (12). Collectively, these data suggest that IL1β is elevated early in the course of T1D. It is possible that IL1β represents an early biomarker of disease initiation that may contribute to T1D progression.
It is worth asking whether the characteristic biochemical changes of diabetes, including hyperglycemia and increased levels of glycated plasma or endothelial proteins, might affect inflammatory processes and thus create a vicious cycle that exacerbates β-cell dysfunction. There is controversy as to the effect of high glucose on IL1B expression in macrophages. High glucose induces the expression of TLR2 and TLR4 in the THP-1 human monocytic cell line (13), and TLR2 mRNA content is increased in PBMCs from patients with long-standing T1D (10). Moreover, small interfering RNA knockdown of these TLRs decreases intracellular NFκB activity and IL1β protein in the supernatant, suggesting that stimulation of IL1β secretion by hyperglycemia is modulated by TLRs via the NFκB pathway (13). However, others have found that hyperglycemia does not itself increase the production of IL1β because blood obtained from patients before the overt development of T1D had elevated IL1β expression, whereas patients with long-standing diabetes in suboptimal glycemic control did not (14). In addition, when patients with long-standing T1D were made acutely hyperglycemic, their IL1β levels were not different from those of the control group, but another proinflammatory cytokine, IL1α, was elevated in the patients with marked hyperglycemia (15). Because IL1α binds to the same receptor as IL1β, this finding would suggest that IL1α could contribute to the chronic inflammation associated with T1D.
Nonenzymatic protein glycation occurs in the presence of chronic hyperglycemia. Advanced glycation end products induce macrophages to release IL1β and tumor necrosis factor-α in some (16) but not other (17) studies. Further, stimulation of a human microvascular endothelial cell line with advanced glycation end products did not increase proinflammatory signaling molecules (17).
There is also inconsistency as to the effect of hyperglycemia on IL1β expression in islet cells, with increased IL1β in β-cells from patients with T2D in some (18) but not other (19) studies. Moreover, human islet cells from nondiabetic donors exposed to various glucose concentrations showed no differences in IL1β expression in one study (19), whereas others found that roughly half of islet cell preparations from nondiabetic donors secrete IL1β when exposed to high glucose (18,20).
In vivo, short-term glucose exposure alone does not appear to increase IL1β expression. When glucose/insulin clamp studies were performed on healthy adults, hyperinsulinemia led to increased IL1β expression as compared with baseline, and hyperglycemia increased IL1β expression further. However, hyperglycemia alone did not increase IL1β expression (21). These data may help explain increased IL1β levels in patients with T2D who have elevated insulin levels, but cannot explain elevated IL1β levels in T1D patients because they have islet cell failure.
As mentioned, IL1-ra is an endogenous protein that blocks the interaction between IL1β and its receptor. IL1-ra expression was observed in pancreatic sections from patients with T2D. Antagonizing IL1-ra using small interfering RNA in healthy human islets led to decreased glucose-mediated insulin release, whereas adding exogenous IL1-ra restored glucose stimulation. Decreased IL1-ra expression leads to β-cell dysfunction and apoptosis. Additionally, this scenario can also induce IL1β release, further damaging β-cells (22). Although the mechanisms are not yet clear, human islets from nondiabetic donors exposed to T1D sera downregulate the expression of IL1-ra relative to islets exposed to autologous or allogeneic sera (23).
IL1β Action on β-Cells
The mechanisms by which IL1β contributes to the pathogenesis of diabetes are controversial. Islet cells demonstrate autocrine increases in IL1β when exposed to IL1β or glucose (24). It is possible that IL1β acts in concert with other cytokines to cause islet cell death. For example, whereas no apoptosis was observed in nondiabetic human islet cells incubated with either IL1β or interferon-γ alone, exposure to both cytokines simultaneously resulted in increased apoptosis as a result of death protein 5/harikari activation (25), which in turn is associated with an NFκB-regulated increase in expression of the p53 upregulated modulator of apoptosis (26). These cytokines, through a currently unidentified pathway, also activate p38, which has a direct role in β-cell death (27). Another group found that IL1β activates p38, extracellular signal-regulated kinases-1/2, and c-Jun amino-terminal kinase, but only inhibition of c-Jun amino-terminal kinase protected cultured cells from apoptosis (28). In addition to signaling through mitogen-activated protein kinase, IL1β plus interferon-γ also create endoplasmic reticulum stress in rat islet cells, but this stress is not necessary for β-cell death (29).
In β-cells, increased IL1β resulting from hyperglycemia was associated with an increase in NFκB activity and eventually impaired β-cell function, as determined by acute glucose-stimulated insulin release. In the same studies, β-cells pretreated with IL1-ra were protected from apoptosis (20). Treatment with IL1-ra decreased nitric oxide production and inducible nitric oxide synthase in both human and Harlan Sprague–Dawley rat islet cells, suggesting that IL1β-induced β-cell damage was secondary to inducible nitric oxide synthase expression and NO production (30).
Culturing mouse islet cells with IL1β decreases insulin secretion and reduces intracellular insulin content (7). IL1β may decrease insulin secretion in part by reducing levels of mRNA and protein for glucokinase, a key component of the glucose sensor in islet cells (31). Moreover, IL1β inhibits insulin release from previously docked granules in the β-cells of rats. Thus, there is impaired first-phase release of insulin (32), mirroring observations in humans in the early stages of T1D.
Although in vitro experiments suggest that IL1β is detrimental to insulin secretion, mouse studies from the 1980s suggest that IL1β is protective against chemically induced diabetes development. IL1β injected into alloxan-treated mice decreased glucose levels within 30 min. IL1β was more effective in reducing blood glucose when given in the early stages of diabetes. In streptozotocin-treated mice (another chemical model), the decrease in glucose levels was more modest and required multiple IL1β injections, whereas there was improvement in diabetic db/db mice with one injection of IL1β (33). Although the mechanism by which IL1β decreases blood glucose in these situations is not clear, one possible explanation for these results is that a systemic injection of IL1β may not increase local pancreatic levels of IL1β.
Taken together, these studies suggest that IL1β-mediated damage to islet cells likely involves multiple downstream targets.
Possible Mechanisms for Increased IL1β in Patients with Newly Diagnosed T1D
TLRs
TLRs, important regulators of the innate immune system, are evolutionarily conserved receptors that recognize pathogen fragments or endogenous molecules known as pathogen-associated or danger-associated molecular patterns, respectively. TLRs are type 1 transmembrane glycoprotein receptors that contain leucine-rich repeat motifs that mediate ligand binding and a highly conserved cytoplasmic domain that binds downstream adaptor molecules. Upon ligand binding, the TLRs homodimerize or heterodimerize, and intracellular signaling is mediated via adaptors such as myeloid differentiation factor 88 or Toll-receptor–associated activator of interferon (34). Activation of TLRs triggers a signaling cascade producing inflammatory cytokines that recruit components of the adaptive immune system to kill the pathogen. TLRs activate transcription factors that increase expression of a variety of inflammatory markers (35). Although TLRs are most often associated with infectious diseases, they have more recently been associated with noninfectious conditions such as asthma (36), inflammatory bowel disease (37), rheumatoid arthritis (38), and T1D. There are 12 known TLRs with various specificities; TLR2 and TLR4 have been implicated in T1D. They signal through NFκB to prime the NLRP3 inflammasome, resulting in increased IL1β mRNA and protein expression.
We previously found that, relative to healthy controls, PBMCs from patients with newly diagnosed T1D have increased mRNA expression of TLR4 (ref. 8). In a study by Devaraj et al. (39), monocytes isolated from the blood of patients with T1D had higher mRNA expression and surface expression of TLR2 and TLR4 in both the basal and activated states than those from healthy controls. Moreover, protein concentrations of NFκB, MyD88 TIR-domain-containing adapter-inducing interferon-β, and IL1-receptor–associated kinase were also increased, suggesting that TLR-dependent pathways were upregulated in T1D (39). In contrast, a study by Du et al. (40) demonstrated no differences in surface expression of TLR2 or TLR4 in mononuclear cells obtained from patients with T1D and healthy controls. This difference could be because the patients in Du’s study were diagnosed with T1D in their 30s as compared with patients in Devaraj’s study, who were diagnosed with T1D before the age of 18 years. Although Du et al. found no difference in surface expression of TLRs at baseline, they did report increased surface expression of TLR4 after stimulation with LPS (an agonist for TLR4), and T1D monocytes secreted more IL1β than healthy controls when stimulated by LPS (40).
Although TLRs are often thought to be proinflammatory, TLR2 may have both pro- and anti-inflammatory properties, depending on ligand binding (41). Stimulation of antigen-presenting cells simultaneously with zymosan, a fungal cell wall ligand of TLR2, and dectin-1, a lectin that recognizes fungi, results in the expression of anti-inflammatory growth factors such as transforming growth factor-β. Treating nonobese diabetic (NOD)-severe combined immunodeficiency mice (a model of spontaneous T1D) with zymosan decreases hyperglycemia and results in higher rates of regulatory T cells (42), suggesting that activation of TLR2 can protect against T1D. However, TLR2 knockout mice (TLR2−/−) mice crossed with NOD mice have a 50% reduction in the development of T1D as compared with NOD-TLR2+/+ mice. Further, TLR2−/− mice treated with streptozotocin, a chemical toxic to pancreatic β-cells, are also relatively resistant to the ensuing diabetes. Interestingly, TLR4−/− mice were not resistant to this chemically induced diabetes, suggesting that TLR2 plays a more important role in T1D initiation, at least in mice (43).
Hyperglycemia may be a consequence rather than a cause of TLR2 or TLR4 activation. TLR2−/− mice fed a high-fat diet are protected from developing insulin resistance and have improved insulin signaling as compared with wild-type mice, raising the possibility that fatty acids signaling through TLR2 may promote inflammation (44) and, in turn, insulin resistance and hyperglycemia. In addition to fatty acids signaling through TLR2, they may also signal through TLR4. Indeed, monocytes from healthy humans fed cream had increased TLR4 expression as compared with those fed orange juice or water (45). These data suggest that fat may be responsible for TLR activation in humans. Fatty acids may also be important contributors to IL1β production. Saturated fatty acids activate TLR2 dimers in the murine macrophage cell line RAW264.7, resulting in NF-κB activation, whereas polyunsaturated fatty acids inhibit TLR2 dimerization and, hence, downstream signaling pathways (46). Saturated fatty acids also induce the expression of cyclooxygenase via TLR4 and activation of NF-κB (47). In INS-1 β cells treated with palmitate, a saturated fatty acid, TLR4 interacts with MyD88 to increase c-Jun amino-terminal kinase activation, leading to cell death (48). In addition, palmitate leads to an increased percentage of apoptosis in the INS-1E cell line as compared with oleate (49). Fatty acids can also stimulate IL1β production in islets, and, according to one study, oleate elicits the greatest IL1β response in human islets, whereas stearate induces the greatest response in mouse islet cells (50). Because fatty acid levels are elevated in children with T1D (51), it is possible that fatty acids may contribute to increased IL1β levels as well as islet cell death.
NLRP3 Inflammasome
As mentioned, the NLRP3 inflammasome is a protein complex including NLRP3 (also known as cryopyrin), apoptosis-associated speck-like protein, and caspase 1. Activation of NLRP3 leads to oligomerization and recruitment of apoptosis-associated speck-like protein and pro-caspase-1, with autocleavage and activation of caspase 1 (52). Active caspase 1 cleaves pro-IL1β to active IL1β, which, when secreted, can exert direct cytotoxic effects as well as recruit other inflammatory cells. Pathogen-associated and damage-associated molecular pattern molecules and environmental irritants can activate NLRP3. Because the TLRs and IL1β are upregulated in T1D, it is likely that the NLRP3 inflammasome is activated in this condition. However, the NLRP3 inflammasome is the least studied component of this pathway. Additionally, there are inflammasome-independent means to activate IL1β such as neutrophil- and macrophage-derived serine proteases that process pro-IL1β into active fragments (53,54), and these inflammasome-independent pathways could play a potential role in T1D.
Of note, population-based studies in Brazil have identified a single-nucleotide polymorphism in NLRP3 that is associated with T1D (55). Although this interesting observation should be studied in other populations, the mechanisms by which this single-nucleotide polymorphism, rs10754558, affects NLRP3 function are unknown.
A number of NLRP3 activating cofactors have been described. Thioredoxin-interacting protein (TXNIP) may activate the inflammasome via oxidative stress in pancreatic β-cells (56) but not in bone marrow–derived macrophages in the context of T2D (56,57). According to Zhou et al., NLRP3 activators generate reactive oxygen species such as H2O2 that cause TXNIP to interact with NLRP3. This interaction is necessary for inflammasome activation and IL1β production in THP-1 cells. Further, TXNIP and NLRP3 knockout mice both show improved glucose tolerance as measured by oral glucose tolerance tests (56). The involvement of TXNIP in the development of T1D is less clear. TXNIP knockout mice have larger β-cell mass relative to controls at baseline. After treatment with streptozotocin, the TXNIP knockout mice lost as much or more β-cell mass than the control mice but remained relatively resistant to the development of diabetes, likely because of their initially increased β-cell mass (58).
Islet amyloid polypeptide (IAPP) is secreted with insulin by the β-cell. IAPP aggregates cause β-cell dysfunction in T2D and contribute to the recurrence of hyperglycemia after islet cell transplantation in T1D (59). IAPP activates the NLRP3 inflammasome to act as a second signal to produce IL1β in macrophages (57). Diabetic mice transplanted with human IAPP transgenic islets had an increased number of macrophages that were inhibited by exogenously administered IL-ra. In addition, IL1-ra reduced hyperglycemia associated with IAPP expression in diabetic mice that received human IAPP transgenic islets as compared with controls (59).
Free fatty acids such as palmitate not only activate TLRs but, along with an additional signal such as LPS, induce activation of the NLRP3 inflammasome in bone marrow–derived macrophages. This activation leads to caspase 1 and IL1β production, which in turn leads to impaired insulin signaling in vitro and insulin resistance in vivo in mice fed high-fat diets (60). Therefore, high levels of palmitate could potentially impair insulin signaling and cause insulin resistance in humans. However, not all evidence supports the involvement of the NLRP3 inflammasome in T1D. For example, crossing caspase 1 knockout mice with NOD mice resulted in the expected decrease in IL1β expression, but these mice did not have lower rates of spontaneous or streptozotocin-induced diabetes as compared with NOD mice with wild-type caspase 1 (61). More research is necessary to better understand the role of the NLRP3 inflammasome in the development and exacerbation of T1D.
Potential Therapies for T1D Based on IL1β Biology
An improved understanding of T1D pathophysiology may identify novel treatment options for T1D. TLRs represent one important potential therapeutic target in the treatment or prevention of this disease. Pioglitazone, a peroxisome proliferator–activated receptor-γ agonist used to treat T2D, decreased mRNA and protein expression of TLR2 and TLR4 in human monocytes in vitro in a dose-dependent manner. Additionally, db/db mice (a model of obesity-induced T2D) given pioglitazone had decreased expression of TLR2, TLR4, and downstream inflammatory markers, as well as improved glucose tolerance (62), suggesting the importance of the TLR pathway in diabetes pathophysiology. In addition, pioglitazone decreases apoptosis of human β-cells in vitro (24).
The sulfonylurea glyburide inhibits the inflammasome upstream of NLRP3, resulting in decreased IL1β levels, and mice treated with glyburide have delayed death when treated with LPS as compared with controls (63). These effects are independent of glyburide’s effects on insulin secretion via the sulfonylurea receptor because another potent sulfonylurea, glipizide, had no effect on inflammasome activation (63).
Direct blockade of IL1β has been studied extensively as a therapeutic strategy for T1D at the preclinical level. Treating rat islet cells with IL1-ra protects against proinflammatory cytokine (IL1β, tumor necrosis factor-α, and interferon-γ)–induced cell death. In addition, mice given rat islets pretreated with proinflammatory cytokines were unable to restore glycemic control, whereas mice treated with IL1-ra under the same conditions were able to restore normoglycemia for the 28-d experiment (64). Further, although another group found that pretreatment of NOD mice with IL1-ra prevented hyperglycemia but not insulitis (65), another group reported that previously diabetic NOD mice that received an islet transplantation and were then treated with IL1-ra remained euglycemic while they received the antagonist, whereas hyperglycemia recurred in control animals within 6 d after transplantation (66). These promising preclinical studies may have an impact on how patients who receive islet cell transplants are treated and have prompted currently ongoing trials of IL1-ra in humans with T1D (ClinicalTrials.gov identifier NCT00947427 and NCT00711503). Indeed, we recently reported that patients with T1D treated with a 1-mo trial of the IL1-ra had decreased insulin requirements with similar hemoglobin A1c levels relative to historical controls (67). In addition to the interest in treating T1D patients with IL1-ra, there is an interest in using agents that remove IL1β from the circulation. Rilonacept is a fusion protein that binds to IL1β and prevents it from occupying its receptor, whereas canakinumab is a monoclonal antibody that binds to IL1β. There are ongoing clinical trials studying these drugs in patients with T1D (ClinicalTrials.gov identifier NCT00962026 and NCT00947427).
Collectively, these data provide promise for the development of new inflammasome-based therapeutics for diabetes.
Conclusions
IL1β, a cytokine long associated with T1D, may play an important role in disease pathogenesis and possibly represents an early inflammatory marker of the disease. IL1β can be induced by various mechanisms. Because TLRs have been implicated in T1D pathogenesis, and activation of TLRs induces IL1β NLRP3 via the inflammasome, it is likely that TLR effects on T1D are mediated by IL1β. Better understanding of this complex regulatory pathway may provide new therapeutic targets to prevent or limit the severity of disease. Current US Food and Drug Administration–approved drugs that act on various parts of the TLR–inflammasome–IL1β pathway should be studied further in the context of T1D.
Statement of Financial Support
The authors are financially supported by grant UL1 RR024982 from the National Institutes of Health.
References
Atkinson MA, Maclaren NK . The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med 1994;331:1428–36.
Bach JF . Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev 1994;15:516–42.
Donath MY, Størling J, Maedler K, Mandrup-Poulsen T . Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J Mol Med 2003;81:455–70.
Mathis D, Vence L, Benoist C . beta-Cell death during progression to diabetes. Nature 2001;414:792–8.
Shimabukuro M, Koyama K, Lee Y, Unger RH . Leptin- or troglitazone-induced lipopenia protects islets from interleukin 1beta cytotoxicity. J Clin Invest 1997;100:1750–4.
Martinon F, Gaide O, Pétrilli V, Mayor A, Tschopp J . NALP inflammasomes: a central role in innate immunity. Semin Immunopathol 2007;29:213–29.
Leiter EH . Murine macrophages and pancreatic beta cells. Chemotactic properties of insulin and beta-cytostatic action of interleukin 1. J Exp Med 1987;166:1174–9.
Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White PC . Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab 2007;92:3705–11.
Meyers AJ, Shah RR, Gottlieb PA, Zipris D . Altered Toll-like receptor signaling pathways in human type 1 diabetes. J Mol Med 2010;88:1221–31.
Ururahy MA, Loureiro MB, Freire-Neto FP, et al. Increased TLR2 expression in patients with type 1 diabetes: evidenced risk of microalbuminuria. Pediatr Diabetes 2012; 13:147–54.
Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK . Serum IL-1β, IL-2, and IL-6 in insulin-dependent diabetic children. Mediators Inflamm 2006;2006:59206.
Bradshaw EM, Raddassi K, Elyaman W, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 2009;183:4432–9.
Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I . High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 2008;57:3090–8.
Wang X, Jia S, Geoffrey R, Alemzadeh R, Ghosh S, Hessner MJ . Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. J Immunol 2008;180:1929–37.
Rosa JS, Flores RL, Oliver SR, Pontello AM, Zaldivar FP, Galassetti PR . Sustained IL-1alpha, IL-4, and IL-6 elevations following correction of hyperglycemia in children with type 1 diabetes mellitus. Pediatr Diabetes 2008;9:9–16.
Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A . Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science 1988;240:1546–8.
Valencia JV, Mone M, Koehne C, Rediske J, Hughes TE . Binding of receptor for advanced glycation end products (RAGE) ligands is not sufficient to induce inflammatory signals: lack of activity of endotoxin-free albumin-derived advanced glycation end products. Diabetologia 2004;47:844–52.
Böni-Schnetzler M, Thorne J, Parnaud G, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab 2008;93:4065–74.
Welsh N, Cnop M, Kharroubi I, et al. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 2005;54:3238–44.
Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–60.
Stegenga ME, van der Crabben SN, Dessing MC, et al. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med 2008;25:157–64.
Maedler K, Sergeev P, Ehses JA, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA 2004;101:8138–43.
Jackson A, McWilliams C, Kaizer E, et al. Gene expression profiling of human pancreatic islets undergoing a simulated process of instant blood-mediated inflammatory reaction. Transplant Proc 2008;40:430–2.
Zeender E, Maedler K, Bosco D, Berney T, Donath MY, Halban PA . Pioglitazone and sodium salicylate protect human beta-cells against apoptosis and impaired function induced by glucose and interleukin-1beta. J Clin Endocrinol Metab 2004;89:5059–66.
Gurzov EN, Ortis F, Cunha DA, et al. Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic β-cell apoptosis. Cell Death Differ 2009;16:1539–50.
Gurzov EN, Germano CM, Cunha DA, et al. p53 up-regulated modulator of apoptosis (PUMA) activation contributes to pancreatic beta-cell apoptosis induced by proinflammatory cytokines and endoplasmic reticulum stress. J Biol Chem 2010;285:19910–20.
Makeeva N, Myers JW, Welsh N . Role of MKK3 and p38 MAPK in cytokine-induced death of insulin-producing cells. Biochem J 2006;393(Pt 1):129–39.
Ammendrup A, Maillard A, Nielsen K, et al. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes 2000;49:1468–76.
Akerfeldt MC, Howes J, Chan JY, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes 2008;57:3034–44.
Heitmeier MR, Arnush M, Scarim AL, Corbett JA . Pancreatic beta-cell damage mediated by beta-cell production of interleukin-1. A novel mechanism for virus-induced diabetes. J Biol Chem 2001;276:11151–8.
Ma Z, Landt M, Bohrer A, Ramanadham S, Kipnis DM, Turk J . Interleukin-1 reduces the glycolytic utilization of glucose by pancreatic islets and reduces glucokinase mRNA content and protein synthesis by a nitric oxide-dependent mechanism. J Biol Chem 1997;272:17827–35.
Ohara-Imaizumi M, Cardozo AK, Kikuta T, Eizirik DL, Nagamatsu S . The cytokine interleukin-1beta reduces the docking and fusion of insulin granules in pancreatic beta-cells, preferentially decreasing the first phase of exocytosis. J Biol Chem 2004;279:41271–4.
del Rey A, Besedovsky H . Antidiabetic effects of interleukin 1. Proc Natl Acad Sci USA 1989;86:5943–7.
Ospelt C, Gay S . TLRs and chronic inflammation. Int J Biochem Cell Biol 2010;42:495–505.
Krishnan J, Lee G, Choi S . Drugs targeting Toll-like receptors. Arch Pharm Res 2009;32:1485–502.
Chun E, Lee SH, Lee SY, et al. Toll-like receptor expression on peripheral blood mononuclear cells in asthmatics; implications for asthma management. J Clin Immunol 2010;30:459–64.
Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK . The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology 2008;125:145–53.
Huang QQ, Pope RM . The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep 2009;11:357–64.
Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I . Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 2008;93:578–83.
Du T, Zhou ZG, You S, et al. Regulation by 1, 25-dihydroxy-vitamin D3 on altered TLRs expression and response to ligands of monocyte from autoimmune diabetes. Clin Chim Acta 2009;402:133–8.
Kiura K, Kataoka H, Yasuda M, Inoue N, Shibata K . The diacylated lipopeptide FSL-1 induces TLR2-mediated Th2 responses. FEMS Immunol Med Microbiol 2006;48:44–55.
Karumuthil-Melethil S, Perez N, Li R, Vasu C . Induction of innate immune response through TLR2 and dectin 1 prevents type 1 diabetes. J Immunol 2008;181:8323–34.
Kim HS, Han MS, Chung KW, et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 2007;27:321–33.
Ehses JA, Meier DT, Wueest S, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 2010;53:1795–806.
Deopurkar R, Ghanim H, Friedman J, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 2010;33:991–7.
Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem 2004;279:16971–9.
Lee JY, Sohn KH, Rhee SH, Hwang D . Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001;276:16683–9.
Lee SM, Choi SE, Lee JH, et al. Involvement of the TLR4 (Toll-like receptor4) signaling pathway in palmitate-induced INS-1 beta cell death. Mol Cell Biochem 2011;354:207–17.
Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL . Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 2004;145:5087–96.
Böni-Schnetzler M, Boller S, Debray S, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 2009;150:5218–29.
Azad K, Parkin JM, Court S, Laker MF, Alberti KG . Circulating lipids and glycaemic control in insulin dependent diabetic children. Arch Dis Child 1994;71:108–13.
Schroder K, Zhou R, Tschopp J . The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010;327:296–300.
Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA . IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog 2010;6:e1000661.
Wittmann M, Kingsbury SR, McDermott MF . Is caspase 1 central to activation of interleukin-1? Joint Bone Spine 2011;78:327–30.
Pontillo A, Brandao L, Guimaraes R, Segat L, Araujo J, Crovella S . Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity 2010;43:583–9.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J . Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010;11:136–40.
Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1ß in type 2 diabetes. Nat Immunol 2010;11:897–904.
Masson E, Koren S, Razik F, et al. High beta-cell mass prevents streptozotocin-induced diabetes in thioredoxin-interacting protein-deficient mice. Am J Physiol Endocrinol Metab 2009;296:E1251–61.
Westwell-Roper C, Dai DL, Soukhatcheva G, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 2011;187:2755–65.
Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–15.
Schott WH, Haskell BD, Tse HM, et al. Caspase-1 is not required for type 1 diabetes in the NOD mouse. Diabetes 2004;53:99–104.
Dasu MR, Park S, Devaraj S, Jialal I . Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology 2009;150:3457–64.
Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 2009;187:61–70.
Schwarznau A, Hanson MS, Sperger JM, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol 2009;220:341–7.
Nicoletti F, Di Marco R, Barcellini W, et al. Protection from experimental autoimmune diabetes in the non-obese diabetic mouse with soluble interleukin-1 receptor. Eur J Immunol 1994;24:1843–7.
Sandberg JO, Eizirik DL, Sandler S . IL-1 receptor antagonist inhibits recurrence of disease after syngeneic pancreatic islet transplantation to spontaneously diabetic non-obese diabetic (NOD) mice. Clin Exp Immunol 1997;108:314–7.
Sumpter KM, Adhikari S, Grishman EK, White PC . Preliminary studies related to anti-interleukin-1ß therapy in children with newly diagnosed type 1 diabetes. Pediatr Diabetes 2011;12:656–67.
Acknowledgements
We thank Michele Hutchison for assistance in creating the figure.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Table S1.
(DOC 123 kb)
Rights and permissions
About this article
Cite this article
Grishman, E., White, P. & Savani, R. Toll-like receptors, the NLRP3 inflammasome, and interleukin-1β in the development and progression of type 1 diabetes. Pediatr Res 71, 626–632 (2012). https://doi.org/10.1038/pr.2012.24
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.24
This article is cited by
-
High glucose enhances the activation of NLRP3 inflammasome by ambient fine particulate matter in alveolar macrophages
Particle and Fibre Toxicology (2023)
-
Prenatal and early life factors and type 1 diabetes
Endocrine (2022)
-
Visualization and modeling of inhibition of IL-1β and TNF-α mRNA transcription at the single-cell level
Scientific Reports (2021)
-
Long-term exposures to ethion and endotoxin cause lung inflammation and induce genotoxicity in mice
Cell and Tissue Research (2019)
-
NLRP3 inflammasome is expressed and regulated in human islets
Cell Death & Disease (2018)