Abstract
Introduction:
Traumatic brain injury (TBI) is a leading cause of death and disability in children. Metabolic failure is an integral component of the pathological aftermath of TBI. The oxygen extraction fraction (OEF) is a valuable parameter for characterization and description of metabolic abnormalities; however, OEF measurement has required either invasive procedures or the use of ionizing radiation, which significantly limits its use in pediatric research.
Results:
Patients with TBI had depressed OEF levels that correlated with the severity of injury. In addition, the OEF measured within 2 weeks of injury was predictive of patient outcome at 3 mo after injury. In pediatric TBI patients, low OEF—a marker of metabolic dysfunction—correlates with the severity of injury and outcome.
Discussion:
Our findings support previous literature on the role of metabolic dysfunction after TBI.
Methods:
Using a recently developed magnetic resonance (MR) technique for the measurement of oxygen saturation, we determined the whole-brain OEF in both pediatric TBI patients and in healthy controls. Injury and outcome were classified using pediatric versions of the Glasgow Coma Scale (GCS) and Glasgow Outcome Scale–Extended (GOS-E), respectively.
Similar content being viewed by others
Main
Traumatic brain injury (TBI) is a leading cause of death and disability in children (1). Current therapeutic approaches are primarily supportive or directed at reducing the effects of brain edema, and do not directly address the underlying neuropathological processes at work. Further understanding of such processes in children will improve our ability to identify novel therapeutic targets.
Shortly after TBI, the brain enters a period of metabolic crisis. There is a brief period of hyperglycolysis that is not matched by a proportionate increase in brain oxygen utilization (2). In the days that follow, a decrease in cerebral consumption of both glucose and oxygen is observed (2). Global ischemia is a relatively rare finding that does not explain the drop in the cerebral metabolic rate of oxygen (3). This prolonged failure of energy production has been attributed to mitochondrial failure after trauma and is associated with poor outcome in adult TBI patients (3,4,5,6,7,8,9).
A decrease in the cerebral metabolic rate of oxygen without a commensurate decrease in cerebral blood flow (CBF) will produce a decrease in the amount of oxygen extracted by the brain from the blood, as measured by the oxygen extraction fraction (OEF). OEF reflects the degree to which delivered oxygen can be extracted by the brain tissues and will reflect metabolic defects caused by mitochondrial damage (10). Recent advances in susceptibility-based oximetry have allowed for noninvasive measurement of the OEF using widely available magnetic resonance imaging (MRI) methods (11). OEF has the advantage of displaying relatively little variation between subjects or over time, easing the interpretation of results (12).
In this study, we investigated the relationship of OEF with the severity of injury and outcome during the subacute phase of recovery after TBI. We hypothesized that OEF would be decreased in children with TBI.
Results
Demographic information and OEF data are summarized in Table 1 . All but one of the severe TBI patients was sedated using fentanyl or midazolam for their initial scan; no sedation was applied for any other scans.
Patients with severe TBI had significantly lower OEF than did those with mild TBI. Severe and mild TBI patients had mean OEF of 21 ± 5% and 31 ± 8%, respectively. In contrast, the mean OEF among the controls was 49 ± 4%. For both mild and severe TBI patients, OEF was significantly lower than that of control patients at the initial time point. The difference between mild and severe TBI patients was also significant ( Figure 1 ). Slight recovery of OEF values was found in both the trauma groups: severe TBI patients improved to 23 ± 9%, whereas mild TBI patients improved to 36 ± 9% (P > 0.7). The mean OEF measured in the control patients remained steady at 46 ± 5%. At the 3-mo time point, OEF values were persistently different between the three study groups (P < 0.05 for all comparisons).
OEF vs. injury. Bar graph showing average OEF for each injury severity level at both time points. At both the initial time point (within 2 weeks of injury; n = 21) and at 3 months post-injury (n = 22), OEF was significantly decreased in mild TBI patients compared to controls and was significantly decreased in severe TBI compared to both other groups. Severe TBI ( ); mild TBI (
); mild TBI ( ); OEF for healthy control patients (
); OEF for healthy control patients ( ). Error bars represent the standard deviations. *P < 0.005 vs. controls, **P < 0.05 vs. controls, and †P < 0.05 vs. mild TBI. OEF, oxygen extraction fraction; TBI, traumatic brain injury.
). Error bars represent the standard deviations. *P < 0.005 vs. controls, **P < 0.05 vs. controls, and †P < 0.05 vs. mild TBI. OEF, oxygen extraction fraction; TBI, traumatic brain injury.
During the enrollment period, we recruited only two moderate TBI patients for the acute measurement and two for the 3-mo measurement. We screened the intensive care unit daily for eligible patients, and low enrollment was due to the low incidence of moderate TBI during the enrollment period. OEF values for moderate TBI patients were 27% and 16% at the initial time point and 33% and 13% at the 3-mo time point. Due to the low number of data points and wide variability in the values obtained, moderate TBI patients were not included in the analysis.
Overall, reproducibility of the OEF values among control patients between the initial scan and the 3-mo scan was excellent. One patient showed a large change (14%) in the OEF between the two time points, otherwise the maximum difference between any two points was 5%. No significant difference between the two time points was observed. Although we report a small sample size for the control group, based on our data a sample of >250 normal children would be needed to demonstrate a statistical difference between the two time points.
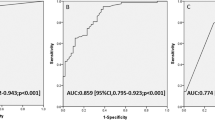
In patients with TBI, OEF measured within 2 wks of injury significantly correlated with both Glasgow Outcome Scale–Extended (GOS-E) and Functional Independence Measure for Children (WeeFIM) scores at 3 mo after injury ( Figure 2 ). For GOS-E, the correlation was 0.74 (P < 0.005) and for WeeFIM the correlation was 0.63 (P < 0.02). The correlation between the 3-mo OEF and the GOS-E outcome was 0.62 (P < 0.03). Patients were evaluated with the WeeFIM only at the 3-mo time point.
OEF vs. outcome in pediatric TBI patients. The observed relationship between outcome 3 months after injury and the OEF during the subacute period of recovery is shown. A strong, significant correlation is demonstrated between OEF measured within 2 weeks of injury and outcome at 3 months post-injury (n = 13) according to both the (a) GOS-E and (b) WeeFIM metrics. The relationship between OEF and outcome is sustained when OEF is measured at the three-month time point (n = 16) (c). GOS-E, Glasgow Outcome Scale–Extended; OEF, oxygen extraction fraction; TBI, traumatic brain injury; WeeFIM, Functional Independence Measure for Children.
Discussion
TBI is a persistent health problem for the pediatric population. After trauma, the brain enters a period of metabolic crisis that is not yet fully understood. Using an MR technique, we investigated the presence of abnormalities of cerebral oxygen metabolism in a sample of pediatric patients. Our data support the hypothesis that children with more severe TBI have lower OEF, reflecting abnormalities of brain oxygen metabolism.
Furthermore, this finding persisted for 3 mo in both mild and severe TBI patients. There was a significant decrease in the whole-brain OEF after TBI that was dependent on the severity of the injury. OEF was a predictor of outcome, with lower OEF values being associated with worse outcomes. The correlation between the two remained significant at 3 mo, solidifying the relationship between the two, although two patients displayed large improvements in outcome despite relatively poor OEF values. Due to the low number of data points and wide variability in the values obtained, moderate TBI patients were not included in the analysis. Variability in OEF values may be due to the broad spectrum of underlying pathology encompassed by a moderate Glasgow Coma Scale (GCS) injury severity classification. However, the low number of subjects limits our ability to analyze this hypothesis. Ongoing recruitment in a longitudinal study of pediatric TBI may help us characterize patients with moderate TBI.
Although only a few results are available in the literature using magnetic-susceptibility-based OEF measurements, we are encouraged by the fact that OEF values in our control patients are consistent with published results in healthy patients using positron emission tomography. Those studies cite OEF values in the range of 30–50% for healthy individuals (10,13,14). Similar values for OEF have been found using other MRI techniques. Measurements based on T2 relaxation times in healthy patients and in the same venous blood vessel reported OEF values of 30–42% (15).
Global OEF is a coarse and partial metric of cerebral metabolism. Differing levels of injury severity may be found across the injured brain. The limitations of a global measurement may explain the fact that some TBI patients in our study had OEF values that could be considered normal; these limitations may also explain the weaker correlation between OEF and WeeFIM score (which measures functional recovery) as compared to the GOS-E (which is a gross measure of global recovery). Regional measurement of OEF can be achieved with 15O-positron emission tomography, but its limited availability and use of ionizing radiation make it unsuitable for widespread studies in children. Further development of MR-based techniques may present a more suitable alternative (16). Also, known limitations of the GCS in relation to diagnosing TBI and accurately defining injury severity should also be taken into account when interpreting our results, as the degree of injury severity may have been under- or overestimated, especially in young children and mild TBI cases (17,18).
Our data may be affected by the fact that sedation was used in patients with severe TBI during the initial scan but not in patients with mild TBI and controls. Neuromuscular blocking agents were also administered to a subset of severe TBI patients. Conducting advanced MRI studies in critically ill children is challenging and such studies will often be susceptible to confounders such as the use of sedatives and neuromuscular blockade. However, based on the data provided by Hoffman et al. (19,20), administration of midazolam produces a greater decrease in the CBF than in cerebral metabolic rate of oxygen, which would lead to an overall increase in the OEF in the sedated patients. The same is true of fentanyl (21). This would result in our data being an overestimation of the initial severe OEF but does not change the interpretation of our results. Although the use of neuromuscular blocking agents can slightly affect oxygen consumption (22) and lead to overestimation of decreases in OEF, only one patient received such medication and the administration occurred under conditions of adequate sedation and normal oxygenation, which would minimize the impact of this confounding effect. Of note, the decrease in OEF was sustained at the 3-mo time point, suggesting an ongoing biological process as no sedatives or neuromuscular blocking agents were used at that time point.
Given MRI studies were conducted when all patients were clinically stable (no intracranial hypertension, hypotension or hypoxemia), no TBI patients in our study presented with ongoing global ischemia, consistent with the finding of Vespa et al. (3). This is based on the criteria for ischemia of an OEF in excess of 70% (23). This strongly argues against the presence of overall ischemia as the etiology for abnormal oxygen metabolism in the subacute phase of recovery. However, because of the global nature of our measurement it may be insensitive to small focal areas of ischemia. Future studies of OEF abnormalities in pediatric TBI patients will include concurrent regional measurements of CBF and OEF to further address the complexities of oxygen metabolism studies in diffuse brain injury (16,24,25). It is important to note that a subset of patients recovered clinically but had persistently low OEF values. This finding deserves further exploration and maybe explained in part by the fact that OEF measurements in the superior sagittal sinus (SSS) may not capture OEF abnormalities in the deep white matter (26). Consideration may be given to performing measurements in the jugular bulb. However, the SSS was preferred in light of the often severe susceptibility artifacts caused by air spaces near the jugular bulb (11). In addition, Xu et al. (27) showed that oxygen saturation levels in the SSS are comparable to those in the internal jugular vein. Phase measurement in the SSS is convenient and robust in the absence of major tissue interfaces with markedly different susceptibilities.
The relationship between outcome and OEF is consistent with the work of Sharples, who reported persistent reduction of oxygen metabolism in children with poor outcome after TBI (8). It is important to note that our mild TBI patients were all admitted to the pediatric intensive care unit for observation, suggesting that our results may not reflect most cases of mild TBI. Patients with mild TBI were also imaged earlier as compared with patients with severe TBI. This limitation was due to the fact that children with severe TBI were critically ill and the MRI study had to be delayed until patients were stable for transport and MRI data acquisition. Earlier imaging may overestimate OEF abnormalities in mild TBI as compared with severe TBI. However, the fact that the difference persisted at the 3-mo time point suggests that the effect of earlier imaging may not be significant.
For consistency across the patients studied, when calculating OEF we estimated the hematocrit to be 42% in all calculations, an approach that has been used consistently when measuring OEF with MRI (11,28,29). We recognize that, particularly in the severe TBI patients, illness and blood sampling taken as a part of regular patient care may lower the hematocrit, a source of variation that could underestimate OEF. Our initial measurement may, therefore, be an overestimation of the decrease in OEF; however, the sustained OEF deficit at 3 mo and the correlation of the OEF with outcome indicate that this is a legitimate effect. The need to sample blood exclusively for study-related hematocrit measurements may in the future be avoided by using noninvasive hemoglobin measurement devices (30).
In conclusion, this report supports the concept of persistent oxygen metabolism abnormalities in pediatric TBI. Our data are consistent with preclinical and clinical studies of brain injury supporting a role for mitochondrial dysfunction and consequent abnormalities in brain oxygen metabolism (5,31). These abnormalities are important because of the resulting neuronal dysfunction and eventual cell death (4,32,33). In addition, these abnormalities may constitute future therapeutic targets. Alternatively, trauma may induce a period of neuroprotective mitochondrial torpor, analogous to ischemic preconditioning (34,35). Our conclusions are limited by the fact that we are unable to exclude the possibility of hyperemia, which would lead to decreased OEF without requiring a pathological decrease in cerebral metabolism. The time course of CBF abnormalities after TBI is variable and heterogeneous (36,37). Both hypo- and hyperemia have been reported after TBI (38). We attempted to mitigate these effects by measuring the OEF in the subacute phase of recovery, during which time CBF is reportedly less variable (39). Concurrent CBF and OEF measurements will be necessary to refine our understanding of these important metabolic abnormalities. Advanced MRI studies are complex and can only study brain pathology at a limited number of time points. However, in addition to contributing to our understanding of brain injury, these methods may in the future prove useful for the validation of bedside technology that will allow noninvasive, continuous assessments of brain oxygen metabolism.
The use of readily available MR techniques allows for safe, noninvasive measurement of the OEF in the pediatric TBI population. OEF is persistently decreased in mild and severe TBI patients, and this abnormality correlates with injury severity and outcome. Ongoing studies will further investigate the neuropathological significance of our findings and the evolution of OEF abnormalities during long-term recovery after TBI.
Methods
Inclusion criteria were pediatric age (0–17 y) and diagnosis of TBI. Diagnosis of TBI was made by history of acute trauma to the head and clinical symptoms of brain injury on admission. Patients were excluded if they had a prior history of head trauma, preexisting neurological conditions, or had suffered cardiac arrest. Patients were classified according to their GCS at admission as having severe (GCS ≤8) or mild TBI (GCS = 14–15).
Imaging was performed as soon as possible based on clinical stability. Patients were imaged within 2 wks of injury for the initial time point and at 3 mo after injury. We also acquired data in a group of volunteer healthy controls. Controls spanned the same age range as the TBI patient sample. A total of 30 patients were enrolled, although not all patients have OEF data at both time points. OEF data were not available for analysis in eight patients and one control subject at the initial time point and eight patients at the 3-mo time point. For the initial time point, data were not available in six patients because they were enrolled in our longitudinal database before OEF data were being acquired and in one patient due to technical failure during data acquisition (head positioning error). Data are not available in one patient and one control subject due to motion. Three-mo data were not available in four patients due to loss to follow-up, in two patients due to motion, and in two patients due to technical failure during data acquisition (data not saved correctly in one patient and OEF sequence not performed during the MRI in the second patient).
MRI was performed on a 3T Siemens Trio clinical MR scanner (Siemens Healthcare, Erlangen, Germany). To obtain whole-brain OEF values, an MR-susceptibility-based oximetry technique was used (11). Field maps were obtained using a three-dimensional double-gradient echo technique with acquisition parameters: time to echo = 5.46/12.3 ms, repetition time = 25 ms, flip angle = 25°, voxel size = 2 × 2 × 1.5 mm, field of view = 256 × 224 × 64 mm. In contrast to the two-dimensional acquisition of Jain et al. (11), we used a three-dimensional acquisition because it does not require prospective identification of the straight segment of the SSS, which may be problematic if the patient moves between the localizer and the field map acquisition.
Severe TBI patients were transported to the MRI scanner by the intensive care unit team and received sedation and mechanical ventilation during the initial scan. Patients were sedated using fentanyl or midazolam to avoid motion during the study. Neuromuscular blocking agents were occasionally administered if clinically indicated. The mechanical ventilator was adjusted to maintain normal end tidal CO2 and normal oxygen saturations (fraction of inspired oxygen was maintained at 30%). No sedation was administered at the 3-mo time point. Mild TBI patients and controls did not receive sedation or mechanical ventilation at either time point. Non-sedated patients were breathing room air and were allowed the choice of listening to music or watching a projected movie.
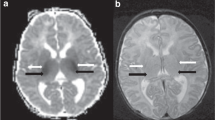
Field maps were calculated from the phase-difference images; no phase wraps were observed. To remove the background phase, a Hamming window (full width half maximum = 8 mm) was applied to the data; the resulting low-resolution image was removed, which left only the vascular contribution to the field map. A region of interest was drawn in the SSS in the sagittal plane passing through the widest portion of the vessel. The brain oxygen saturation level measured in the sagittal sinus represents a valid estimate of the whole-brain average OEF (11). The region of interest was positioned in the most posterior portion of the vessel, which was generally the straightest section available ( Figure 3 ). Regions of interest were positioned to exclude at least 1 mm from the edge of the vessel. The nominal size for a region of interest was 8 × 4 mm. The head was positioned to avoid a 54.5° angle between the SSS and the main magnetic field, which prevents OEF measurement (40).
(a) Low-resolution anatomical T1-weighted image used to co-register the region of interest (SSS; white arrows) and the magnetic field map (b) in the brain of a healthy volunteer. Low-resolution anatomical imaging allows accurate localization of the SSS while minimizing acquisition time. Short acquisition time is important in pediatric studies. (b) The magnetic field map of the same patient following filtering to remove background phase effects. The venous structures display contrast with the surrounding tissue that varies as the angle of the vessel with the magnetic field changes. The contrast between the vessel and brain tissue is proportional to the whole-brain OEF. Placement of the ROI was standardized to avoid sections where the SSS was not visible due to changes in the angle of the vessel. OEF, oxygen extraction fraction; ROI, region of interest; SSS, superior sagittal sinus.
The SSS magnetic field was converted to magnetic susceptibility using the straight cylinder approximation (41). The magnetic susceptibility was converted into OEF using the equation OEF = Δχ/(Hct × Δχ0), where χ0 is the susceptibility difference between hemoglobin and deoxyhemoglobin, Δχ is the measured susceptibility difference, and Hct is the hematocrit. We used a value for Δχ of 0.264 ppm and estimated an Hct of 45% and arterial saturation of 100% (42).
In addition, the relationship between injury severity and OEF was evaluated for the TBI patients using two-way ANOVA and Tukey’s honestly significant difference test (43). Evaluation of OEF was performed blinded to outcome measures.
Outcome was evaluated using the GOS-E (44) and the weeFIM (44). Trained research staff blinded to the OEF measurements performed evaluations at 3 mo after injury. OEF as a predictor of outcome was determined by calculating Spearman’s correlation coefficient between the initial OEF values and the 3-mo outcome measures. Statistical significance of the correlation was calculated by Fisher’s transformation (45). The relationship between OEF at 3 mo and outcome was evaluated using the same methods, as was the relationship between the weeFIM score and initial OEF.
The reproducibility of the measurement was evaluated by comparing the OEF values for the control patients at their initial scan with those at the 3-mo time point. Controls did not suffer any head injuries during the 3-mo interval and OEF was not expected to change. The maximum absolute difference between OEF values at the two time points was calculated.
Patients admitted to our pediatric intensive care unit after TBI were recruited after informed consent was obtained. The study was approved by our institutional review board (Washington University Human Research Protection Office).
Statement of Financial Support
Financial support for this study was received from the Robert Wood Johnson Foundation, the Washington University Institute of Clinical and Translational Sciences (KL2 RR024994 and UL1 RR024992), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (P30HDO62171).
References
Centers for Disease Control and Prevention. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. (http://www.cdc.gov/TraumaticBrainInjury/statistics.html.) Accessed 9 July 2011.
Bergsneider M, Hovda DA, Lee SM, et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma 2000;17:389–401.
Vespa P, Bergsneider M, Hattori N, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab 2005;25:763–74.
Clausen T, Zauner A, Levasseur JE, Rice AC, Bullock R . Induced mitochondrial failure in the feline brain: implications for understanding acute post-traumatic metabolic events. Brain Res 2001;908:35–48.
Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP . Impaired cerebral mitochondrial function after traumatic brain injury in humans. J Neurosurg 2000;93:815–20.
Robertson CS, Contant CF, Gokaslan ZL, Narayan RK, Grossman RG . Cerebral blood flow, arteriovenous oxygen difference, and outcome in head injured patients. J Neurol Neurosurg Psychiatr 1992;55:594–603.
Glenn TC, Kelly DF, Boscardin WJ, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab 2003;23:1239–50.
Sharples PM, Matthews DS, Eyre JA . Cerebral blood flow and metabolism in children with severe head injuries. Part 2: Cerebrovascular resistance and its determinants. J Neurol Neurosurg Psychiatr 1995;58:153–9.
Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW . Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. J Neurosurg 1990;72:176–82.
Diringer MN, Yundt K, Videen TO, et al. No reduction in cerebral metabolism as a result of early moderate hyperventilation following severe traumatic brain injury. J Neurosurg 2000;92:7–13.
Jain V, Langham MC, Wehrli FW . MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab 2010;30:1598–607.
Coles JP, Fryer TD, Bradley PG, et al. Intersubject variability and reproducibility of 15O PET studies. J Cereb Blood Flow Metab 2006;26:48–57.
Ito H, Kanno I, Fukuda H . Human cerebral circulation: positron emission tomography studies. Ann Nucl Med 2005;19:65–74.
Bremmer JP, van Berckel BN, Persoon S, et al. Day-to-day test-retest variability of CBF, CMRO2, and OEF measurements using dynamic 15O PET studies. Mol Imaging Biol 2011;13:759–68.
Lu H, Ge Y . Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med 2008;60:357–63.
He X, Yablonskiy DA . Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med 2007;57:115–26.
Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT; Workshop Scientific Team and Advisory Panel Members. Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008;25:719–38.
Grote S, Böcker W, Mutschler W, Bouillon B, Lefering R . Diagnostic value of the Glasgow Coma Scale for traumatic brain injury in 18,002 patients with severe multiple injuries. J Neurotrauma 2011;28:527–34.
Hoffman WE, Miletich DJ, Albrecht RF . The effects of midazolam on cerebral blood flow and oxygen consumption and its interaction with nitrous oxide. Anesth Analg 1986;65:729–33.
Hoffman WE, Albrecht RF, Miletich DJ, Hagen TJ, Cook JM . Cerebrovascular and cerebral metabolic effects of physostigmine, midazolam, and a benzodiazepine antagonist. Anesth Analg 1986;65:639–44.
Michenfelder JD, Theye RA . Effects of fentanyl, droperidol, and innovar on canine cerebral metabolism and blood flow. Br J Anaesth 1971;43:630–6.
Lanier WL, Milde JH, Michenfelder JD . The cerebral effects of pancuronium and atracurium in halothane-anesthetized dogs. Anesthesiology 1985;63:589–97.
Powers WJ, Grubb RL Jr, Darriet D, Raichle ME . Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab 1985;5:600–8.
Kim J, Whyte J, Patel S, et al. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma 2010;27:1399–411.
Mellon EA, Beesam RS, Baumgardner JE, Borthakur A, Witschey WR 2nd, Reddy R . Estimation of the regional cerebral metabolic rate of oxygen consumption with proton detected 17O MRI during precision 17O2 inhalation in swine. J Neurosci Methods 2009;179:29–39.
Patel SC, Jain R, Wagner S. The Vasculature of the Human Brain. In: Conn PM, ed. Neuroscience in Medicine. New York, Humana Press, 2008: 147–166.
Xu F, Ge Y, Lu H . Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med 2009;62:141–8.
Zhao JM, Clingman CS, Närväinen MJ, Kauppinen RA, van Zijl PC . Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med 2007;58:592–7.
An H, Lin W . Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cereb Blood Flow Metab 2000;20:1225–36.
Barker SJ, Badal JJ . The measurement of dyshemoglobins and total hemoglobin by pulse oximetry. Curr Opin Anaesthesiol 2008;21:805–10.
Robertson CL, Soane L, Siegel ZT, Fiskum G . The potential role of mitochondria in pediatric traumatic brain injury. Dev Neurosci 2006;28:432–46.
Robertson CL . Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J Bioenerg Biomembr 2004;36:363–8.
Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G . Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol 2006;197:235–43.
Zhan RZ, Fujihara H, Baba H, Yamakura T, Shimoji K . Ischemic preconditioning is capable of inducing mitochondrial tolerance in the rat brain. Anesthesiology 2002;97:896–901.
Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, Pérez-Pinzón MA . Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab 2001;21:1401–10.
Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF . Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg 1991;75:685–93.
Kelly DF, Martin NA, Kordestani R, et al. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg 1997;86:633–41.
Muizelaar JP, Marmarou A, DeSalles AA, et al. Cerebral blood flow and metabolism in severely head-injured children. Part 1: Relationship with GCS score, outcome, ICP, and PVI. J Neurosurg 1989;71:63–71.
Soustiel JF, Glenn TC, Shik V, Boscardin J, Mahamid E, Zaaroor M . Monitoring of cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma 2005;22:955–65.
Marques JP, Bowtell R. Application of a Fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts Magn Reson Part B Magn Reson Eng 2005;25B:65–78.
Sedlacik J, Rauscher A, Reichenbach JR . Quantification of modulated blood oxygenation levels in single cerebral veins by investigating their MR signal decay. Z Med Phys 2009;19:48–57.
Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ . Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med 2001;45:533–42.
TUKEY JW . Comparing individual means in the analysis of variance. Biometrics 1949;5:99–114.
Msall ME, DiGaudio K, Rogers BT, et al. The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clin Pediatr (Phila) 1994;33:421–30.
Pearson ES. The test of significance for the correlation coefficient. J Am Stat Assoc 1931 26:128–134.
Acknowledgements
We thank Tina Hicks and Tina Day for their assistance with data coordination. We also thank Beau Ances for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ragan, D., McKinstry, R., Benzinger, T. et al. Depression of whole-brain oxygen extraction fraction is associated with poor outcome in pediatric traumatic brain injury. Pediatr Res 71, 199–204 (2012). https://doi.org/10.1038/pr.2011.31
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2011.31
This article is cited by
-
Brain metabolism and severe pediatric traumatic brain injury
Child's Nervous System (2017)
-
Vascular Neural Network Phenotypic Transformation After Traumatic Injury: Potential Role in Long-Term Sequelae
Translational Stroke Research (2014)
-
Alterations in Cerebral Oxygen Metabolism after Traumatic Brain Injury in Children
Journal of Cerebral Blood Flow & Metabolism (2013)