Abstract
Renal hypoplasia, defined as abnormally small kidneys with normal morphology and reduced nephron number, is a common cause of pediatric renal failure and adult-onset disease. Genetic studies performed in humans and mutant mice have implicated a number of critical genes, in utero environmental factors and molecular mechanisms that regulate nephron endowment and kidney size. Here, we review current knowledge regarding the genetic contributions to renal hypoplasia with particular emphasis on the mechanisms that control nephron endowment in humans and mice.
Similar content being viewed by others
Main
Renal hypoplasia is a common, yet poorly understood and misused term describing congenital renal anomalies. Renal hypoplasia is defined as abnormally small kidneys (<2 SD below the expected mean when correlated with age or parameters of somatic growth) with normal morphology and reduced nephron number. This definition predicts that ∼2.2% of the population exhibit renal hypoplasia, whereas epidemiologic studies suggest an estimated incidence of 1 in 400 births (1). Much confusion and the misapplication of this definition have arisen because the majority of congenitally small kidneys also exhibit evidence of tissue maldifferentiation, defined as renal dysplasia. The exact incidence of pure renal hypoplasia (without dysplasia) is difficult to define as renal dysplasia has often been incorrectly described as hypoplasia. This has predominantly been due to the lack of noninvasive diagnostic tools (i.e. Ultrasound) with resolution power adequate enough to discriminate dysplasia from hypoplasia in such settings. Severe reductions in nephron number that are characteristic of renal hypoplasia/dysplasia are the leading cause of childhood end stage renal disease. Indeed, if severe enough, these conditions can lead to significant impairment of intrauterine renal function which can in turn lead to the oligohydramnios sequence, a condition not compatible with extrauterine life. This includes severe and modest bilateral renal hypoplasia. More subtle defects in nephron number, such as those at the lower end of the normal range caused by mild bilateral renal hypoplasia, have been associated with the development of adult-onset hypertension and chronic renal failure (2–6). Here, we focus on knowledge derived from the study of human syndromic forms of renal hypoplasia and mouse mutants that provide insights into the molecular mechanisms that underlie renal hypoplasia and control nephron endowment. Congenital renal abnormalities characterized by nephron number and other renal pathologies including renal dysplasia, hydroureter, cystic dysplasia, and agenesis are reviewed in detail elsewhere (7–9).
OVERVIEW OF KIDNEY DEVELOPMENT
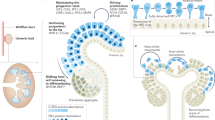
Development of the mammalian metanephric kidney is dependent on reciprocal inductive interactions between two distinct cell lineages, the ureteric cell lineage and the metanephric mesenchyme (MM) cell lineage (Fig. 1). At the onset of metanephric development, signals emanating from the mass of uninduced MM initiate the formation of an epithelial bud (the ureteric bud) from the adjacent Wolffian duct (Fig. 1A). Reciprocal inductive interactions between the ureteric bud (UB) tip and adjacent MM result in continued/successive branching of the UB, ultimately forming the mature collecting duct system of the kidney (Fig. 1B). Simultaneously, the UB tips signal to the adjacent MM cells causing them to aggregate, undergo a mesenchymal-epithelial conversion, and progress through a series of maturation steps to form a fully functional nephron (Fig. 1C and D). This iterative cycle of ureteric branching morphogenesis and nephron induction results in the formation of ∼60,000 collecting ducts and an average of ∼785,000 (range: 210,332–1,825,380) nephrons in humans (10). Human metanephric development commences at ∼5–6 wk after fertilization, and nephrogenesis is completed by 36-wk gestation. Therefore, final nephron endowment is completed during embryogenesis, after which no new nephrons can be formed, although the kidney tubules continue to mature into the postnatal period. The fetal metanephric kidney begins to function at ∼10-wk gestation when the first signs of urinary output become evident.
Morphogenesis of the metanephric kidney. A, Metanephric kidney development commences with metanephric mesenchyme (MM) induced formation of the ureteric bud (UB) from the caudal aspect of the Wolffian duct (WD). B, The UB grows toward and invades the MM and reciprocal inductive interactions between the two result in reiterative branching of the UB, ultimately forming the mature collecting duct system of the kidney. C, Simultaneously, the UB tips signal adjacent MM cells to condense (CM) and undergo a mesenchyme-epithelial transformation forming the aggregate (A), renal vesicle (RV), comma-shaped body (C), and S-shaped body (S). D, The S-shaped body undergoes further differentiation to form the functional nephron. Ascending loop of Henle (ALH), cortical stroma (CS), capillary tuft (CT), distal loop of Henle (DLH), distal tubules (DT), medullary stroma (MS), parietal cell layer (PCL), podocyte cell layer (Pod), proximal tubule (PT).
Much of the insight into the genetics of human renal hypoplasia has been obtained via studies into rare syndromic conditions (Table 1). These important genetic associations identified in human renal hypoplasia have provided a platform for many researchers to investigate the molecular mechanisms by which these genes function in both normal and abnormal kidney development. Animal model systems (predominantly murine) have provided significant insights into the molecular mechanisms underlying the development of renal hypoplasia. In turn, many of these same mechanisms have been corroborated in the human condition. Here, we discuss the most significant findings generated and our current understanding of how these genes interact with each other and within different cell lineages.
CONTRIBUTION OF THE URETERIC CELL LINEAGE TO RENAL HYPOPLASIA
Nephron formation in the MM requires reciprocal interaction with the UB tips. The proper elaboration of the UB tree and the secretion of tip-derived signals are essential for adequate nephrogenesis. Multiple processes are required within the UB for development: induction, growth and branching, survival, and differentiation. These processes are primarily regulated by receptor tyrosine kinase signaling pathways and transcription factors expressed in the UB (Table 2).
Growth and branching.
The interactions between the UB tip-specific receptor RET and its ligand GDNF are crucial for the initial UB induction from the Wolffian duct. Loss of these interactions leads to a failure of kidney development as Ret−/− mice present with renal agenesis (11). However, recent work exploring the participation of the Ret signaling axis throughout kidney development has begun to tease out a role for this pathway during all phases of UB development. Three tyrosine residues on the intracellular portion of RET, Tyr1015, Tyr1096, and Tyr1062, act as P-Tyr binding sites for multiple adaptors and effectors, with Y1062 activating the Ras/Erk, PI3 K/Akt, and JNK pathways. Mutation of the Y1062 residue in RetY1062F knockin mice abolishes this binding site. In contrast to Ret−/− mice which lack kidneys due to deficient UB induction, RetY1062F knockin mice demonstrate renal hypoplasia, characterized by normal UB induction and initial branching but decreased branching from E13.5 onwards, suggesting an additional role for Ret in the regulation of branching in late embryogenesis (12). Moreover, the observation that these knockin mice do not phenocopy Ret−/− mice indicates a role for multiple P-Tyr binding sites downstream of Ret in guiding proper kidney development throughout embryogenesis. In humans, mutations in the RET gene have been identified in some patients presenting with features of Potter Syndrome and renal anomalies, including renal hypoplasia (13). Furthermore, a common single-nucleotide polymorphism within the exon splicing enhancer of exon 7 of the human RET gene, predicting diminished function, has been identified in newborns with subtle renal hypoplasia (Table 1; 14).
Maintenance of Gdnf and Ret expression is controlled by an autoregulatory feedback loop with Wnt11. WNT11 secretion from the UB tip is responsible for the maintenance of GDNF expression in the MM. Wnt11−/− mice develop defective UB branching and failure to maintain Gdnf expression in the MM (15). Newborn mutant mice are characterized by a 64% decrease in glomerular number. Synergistic genetic interactions between Ret and Wnt11 have been demonstrated by a more severe hypoplastic phenotype in Wnt11+/−;Ret+/− mice, as well as the loss of Wnt11 in the few Ret−/− mice that develop hypoplastic kidney tissue (15).
FGF signaling via the FGFR2 receptor, expressed on UB cells, has also been shown to play a crucial role in ureteric development, with multiple knockout models resulting in renal hypoplasia. The docking protein Frs2α is classically thought of as a major intracellular docking protein for FGFR2 signaling. Conditional inactivation of Frs2α in the UB leads to mild renal hypoplasia with a reduced but normal pattern of UB growth and branching after the T-stage (16). This is in contrast to the Fgfr2−/− mice that exhibit renal hypoplasia but an abnormally thin and elongated ureteric branching pattern resulting in an abnormal mature kidney shape (17). This phenotypic difference and the lack of renal phenotype in mice that express point mutations of the Frs2α docking site of FGFR2 suggest that FGFR2 signals through other adaptor molecules in the UB and that Frs2α may transmit signals downstream of other receptor tyrosine kinases (RTKs). The finding of decreased expression of Ret and Wnt11 in Frs2α mutants suggests that this docking protein may function downstream of RET in the RET/GDNF autoregulatory pathway. Furthermore, the Ret and Fgfr2β RTK pathways converge on common transcription factors Etv4 and Etv5 (18).
Signaling from two additional RTKs, Met and Egfr, have also been shown to cooperate in the regulation of late UB branching. Met is expressed in both the UB and MM and is the receptor for Hgf. Targeted deletion of Met to the ureteric cell lineage results in a 35% decrease in nephron number by 12 wk of age (19). The Egfr was shown to be up-regulated in the collecting ducts of these mice. Elimination of Egfr on the Met deficient background causes a more severe deficit in ureteric branching suggesting that these two RTK pathways act cooperatively in directing late ureteric branching (19). Egfr null mutants alone also present with renal hypoplasia, characterized by atrophy of the renal papilla and decreased ureteric branching (20). Interestingly, conditional inactivation of Egfr in the UB lineage results in normal ureteric branching but a marked reduction in collecting duct elongation (20).
Ureteric branching and growth is also regulated at the transcriptional level. The transcription factor Lim1 is essential in the UB for growth and branching as well as Ret expression in the tip domain. Targeted deletion of Lim1 in the UB lineage also demonstrated a requirement for Lim1 in the timing of UB induction and nephric duct maintenance (21). Analysis of chimeric embryos consisting of a mix of wild type and Lim1−/− cells demonstrated a cell autonomous requirement for Lim1 in the nephric duct and UB tip domain.
Ureteric cell survival.
Renal-Coloboma syndrome (RCS) is an autosomal dominant disorder that is caused by heterozygous loss of function mutations in the human PAX2 gene (Table 1; 22). Kidneys of affected individuals are characterized by normal nephron structure but a substantial reduction in total nephron number often leading to chronic renal failure (23,24). Supporting the importance of PAX2 in human renal hypoplasia, mutations have also been detected in cases of isolated renal hypoplasia and a common variant in the PAX2 gene is associated with reduced kidney size (subtle renal hypoplasia) in newborns who lack any other phenotypic characteristic of PAX2 deficiency (25,26). Insights into the underlying mechanisms governing renal hypoplasia in RCS have been obtained via studies in mice and suggest that PAX2 in the UB lineage is crucial for ureteric growth, branching, and survival. A frameshift mutation of Pax2, identical to the G619 insertion mutation identified in some humans with RCS (Pax21Neu) in mice results in renal agenesis in homozygotes and renal hypoplasia in heterozygotes (27). Renal hypoplasia in this model is associated with a 40% reduction in nephron number, elevated apoptosis in the UB epithelium, and reduced number of ureteric branches (27,28). Constitutive expression of the proapoptotic gene, Baxα, in the ureteric cell lineage driven by the Pax2 promoter results in renal hypoplasia, elevated UB apoptosis and reduced branching, identical to the phenotype observed in the Pax21Neu/+ mice (29). Further supporting a role of ureteric cell apoptosis in renal hypoplasia, transgenic mice expressing the antiapoptotic factor, Bcl2, in ureteric cells in Pax21Neu/+ mice, prevents apoptosis and normalizes ureteric branching, nephron number, and renal function (28). The ability of Pax2 to directly activate transcription of Ret and its ligand, Gdnf, in mice, provides further evidence of the importance of these genes in renal development and the pathogenesis of human renal hypoplasia (30).
Induction of nephrogenesis.
Recently, the transcription factor Hnf1β was demonstrated to participate in a regulatory network that maintains the expression of Lim1, Pax2, and Wnt9b expression in the UB (31). Hnf1β deficient embryos develop severe renal hypoplasia with a delay in UB induction, loss of Wolffian duct maintenance, and reduced UB growth and branching. Hnf1β-regulated gene transcription is critical to the UB and MM interplay as it exhibits in vivo binding to noncoding regulatory DNA sequences of Pax2, Lim1, and Wnt9b (31). This is particularly clear given the essential requirement for WNT9b secretion from the UB stalk domain and its effect on MM nephrogenic program induction. Disruption of these events likely provides insight into the rare association of renal hypoplasia with Renal Cysts and Diabetes syndrome (RCAD) and mutations in human HNF1β (Table 1) (32).
Ureteric cell differentiation.
The transcriptional control of UB differentiation, especially of the tip domain, is essential for UB development and proper nephron formation. Ectopic overexpression of nuclear phospho protein p53 in the UB results in defective differentiation of the UB and a secondary survival defect in the MM leading to a 50% decrease in both kidney size and nephron number in adult mice (33). Normally expressed at low levels in the UB, excessive amounts of wild-type p53 in the UB resulted in defective expression and localization of Ret, a marker of the ureteric tip, as well as decreased expression of tubular differentiation markers DBA lectin and Aquaporin-2.
Hedgehog signaling effectors also regulate the differentiation of the UB tip domain. HH activity is normally restricted to the medullary domain of the kidney. Ectopic pathway activation in the cortical UB domain via targeted deletion of Ptc1 in the ureteric cell lineage results in mild renal hypoplasia characterized by abnormal UB tip morphology, decreased UB branching and glomerular number, and a severe reduction in Ret and Wnt11 expression in ureteric tip cells (34). Constitutive expression of the truncated repressor form of Gli3 in the Ptc1 deficient background rescued the expression of Ret and Wnt11 in tip cells and normalized kidney size and glomerular number. Thus, Gli3 repressor activity is required in UB tip cells for Wnt11 and Ret expression and subsequent control of ureteric growth and branching. The requirement for tight regulation of HH signaling during metanephric development is also evident in humans with Pallister-Hall syndrome (PHS) and renal abnormalities (Table 1; 35). PHS is an autosomal dominant disorder caused by frameshift/nonsense and splicing mutations that exclusively affects the second third of the GLI3 gene (nucleotides 1998–3481) predicting a truncated functional repressor form of the GLI3 protein (36). Further implicating a role for HH signaling in human renal hypoplasia is Smith-Lemli-Opitz syndrome (SLOS), an autosomal recessive disorder caused by heterozygous mutations in the DHCR7 gene (Table 1; 37). The DHCR7 locus encodes sterol delta-7-reductase that is required in mammalian sterol biosynthesis to convert 7-dehydrocholesterol into cholesterol. As HH proteins undergo cholesterol modifications, it is plausible that disruption in genes responsible for these posttranslational modifications (DHCR7) may lead to HH functional abnormalities and therefore developmental malformations associated with SLOS.
CONTRIBUTION OF THE MESENCHYMAL/STROMAL CELL LINEAGE TO RENAL HYPOPLASIA
Mesenchymal/stromal-dependent ureteric branching morphogenesis.
Because nephron formation is dependent on inductive signals from the ureteric tip, the number of ureteric tips is a considered a strong determinant of nephron endowment. However, it is the inductive signals emanating from the MM that are critical for inducing the UB tip to divide. This process of ureteric branching is primarily mediated by the GDNF/RET signaling axis. GDNF, the ligand for RET (and GFRα1), is exclusively expressed in the MM and is critical for ureteric branching morphogenesis. Although Gdnf null mice lack kidneys and ureters due to deficient UB induction (38–40), Gdnf heterozygous mice exhibit reduced ureteric branching, significantly reduced kidney size and a 30% nephron number deficit (41). Moreover, Gdnf heterozygous mice demonstrate increased mean arterial blood pressure and glomerular hypertrophy at 14 mo of age (42). The absence of known human pathogenic mutations in GDNF suggests an importance of gene dosage, as is evident from the mouse models (43). Consistent with the importance of the GDNF/RET signaling axis in ureteric branching and subsequent nephrogenesis, perturbations in mesenchymal factors that regulate Gdnf and/or modify its signaling are also associated with renal hypoplasia. Members of the Hox11 gene cluster and Gdf11 are required for Gdnf expression in the MM. Gdf11 null mice and compound Hoxa11/Hoxd11 null mice exhibit smaller kidneys, reduced branching, and a loss of Gdnf expression (44–46). Similarly, although Eya1 null mice fail to form kidneys due to absent Gdnf expression in the uninduced MM population, Eya1 heterozygote mice demonstrate a low incidence of renal hypoplasia (47). Genetic studies in mice have shown that EYA1 acts in an EYA1-SIX-PAX gene complex to regulate gene transcription. Interestingly, Eya1 heterozygous mice also display other developmental anomalies in common with human Branchio-Oto-Renal syndrome that is caused by mutations in the EYA1 gene (Table 1). Mutations in SIX1, SIX2, and SIX5 have also been identified in patients with Branchio-Oto-Renal syndrome and are predicted to disrupt formation of the EYA1-SIX complex and/or SIX-DNA binding and subsequent gene transcription (48–50). Sall1 null mice and Sall4 heterozygous mice also exhibit renal agenesis and hypoplasia associated with reduced mesenchymal Gdnf expression (51,52). Mutations in the human SALL1 and SALL4 genes result in human Townes-Brocks syndrome and Okihiro syndrome, respectively, which both demonstrate a range of renal abnormalities including hypoplasia (Table 1) (53,54).
The renal stroma is also critical for nephron endowment, primarily via regulation of ureteric branching morphogenesis by the retinoic acid-signaling axis. Retinoic acid is the active form of dietary Vitamin A that is synthesized by enzymes, including Raldh2, and signals through retinoic acid receptors (Rar). Genetic elimination of Raldh2 and compound null mutants for Rarα and Rarβ2 exhibit renal hypoplasia and reduced expression of Ret in ureteric tip cells (55,56). Interestingly, constitutive Ret expression in Rarα;Rarβ2 compound null mice normalizes kidney development, suggesting a critical role for retinoic acid in the maintenance Ret expression (57). A similar pattern of defects is observed in offspring of Vitamin A deficient mothers (58,59), discussed in more detail below. Fgf7, expressed in stromal mesenchyme, has also been implicated in renal hypoplasia. Genetic elimination of Fgf7 results in reduced ureteric branching and a 30% reduction in nephron number (60). Fgf10 null mutants also exhibit smaller kidneys with reduced branching morphogenesis (61,62). Recently, genetic analyses have demonstrated a critical role for Fgf10 in UB induction and branching morphogenesis in cooperation with Gdnf (61).
Mesenchyme survival and maintenance of nephrogenic progenitors.
The population of MM and nephrogenic precursors provides a potential limiting factor for nephron endowment. Defects in cell proliferation, survival and progenitor cell self-renewal, and commitment can result in fewer cells able to contribute to nephron formation.
Members of the Myc family of genes largely mediate cell growth, proliferation, and apoptosis. During metanephric development, expression of c-myc is restricted to early uninduced mesenchyme and n-myc to early mesenchymal aggregates. Targeted deletion of c-myc to the MM lineage or n-myc deficiency results in renal hypoplasia due to a significant decrease in mesenchymal proliferation, independent of changes in apoptosis (63,64). Interestingly, a progressive loss of nephrogenic progenitor cell marker expression, Six2 and Cited1, was also observed in the c-myc deficient kidneys suggesting that c-myc functions to modulate the proliferation, and likely self-renewal, of the nephrogenic progenitor cell population (64).
Bcl-2 is an oncogene that inhibits apoptotic cell death and is expressed in both the UB and MM (65). Bcl-2 null mutant mice develop renal hypoplasia and severe renal failure (66,67). Interestingly, these mutants exhibit a significant increase in apoptosis, predominantly in the MM, resulting in reduced ureteric branching and nephrogenesis.
Six2 is exclusively expressed in the cap mesenchyme, marking the nephron progenitor population (68,69). Genetic elimination of Six2 results in severe renal hypoplasia, characterized by premature and ectopic differentiation of nephrogenic tubules and a rapid depletion of the nephrogenic progenitor population (69). Furthermore, the Brachyrrine mouse model (Br) demonstrates a marked decrease in the embryonic expression of Six2 (70). Br heterozygous mice exhibit a severe reduction in kidney size, 88% decrease in nephron number, elevated mean arterial pressure, and declined renal function. Further implicating a requirement for progenitor cell maintenance in normal nephron endowment, Six2 mutations have recently been identified in humans with isolated renal hypoplasia (Table 1; 50).
IN UTERO ENVIRONMENT AND RENAL HYPOPLASIA
There is an increasing amount of evidence, derived from human epidemiologic studies and animal models, demonstrating an important role for the intrauterine environment and fetal programming in the pathogenesis of renal hypoplasia and predisposition to later kidney disease (Table 3) (71,72).
Low birth weight or IUGR is generally considered a clinical outcome of suboptimal in utero environment. In these instances, the fetal kidney is particularly susceptible leading to reduced nephron number. Among the most common causes of human IUGR, animal models of uteroplacental insufficiency and maternal undernutrition are known to exhibit significant reductions in nephron endowment (73,74). In particular, the association between maternal nutrition and nephron endowment is striking. Above, we have already discussed the mechanisms by which perturbations of the retinoic acid signaling axis cause renal hypoplasia. Retinoids are active metabolites of Vitamin A. Vitamin A deficiency is a global health problem, particularly in developing countries where poor nutrition commonly results in developmental anomalies of the genitourinary tract. Interestingly, these anomalies can be prevented by early maternal administration of vitamin A during the period of kidney development (59). Furthermore, vitamin A deficiency in rats results in a significant decrease in glomerular number and Ret expression, consistent with the genetic studies described above (58). In addition, maternal dietary protein restriction results in decreased nephron number, reduced renal function, and hypertension in a variety of species including rodents and sheep (75–80). Although the precise mechanisms governing this are not well defined, there is some evidence suggesting that the maternal diet programs the expression of critical genes required for embryonic kidney development, cell survival, and renal function (76,81,82). Interestingly, a single midgestation retinoic acid administration is able to normalize kidney size and nephron number in rat offspring exposed to maternal protein restriction raising the possibility of preventative approaches in humans (78).
Reduced nephron number and IUGR are not always synonymous. Maternal diabetes and in utero exposure to drugs and alcohol have all been linked to renal hypoplasia in the absence of reduced birth weight. In animal experiments, offspring of hyperglycemic or diabetic mothers demonstrate a significant nephron deficit (83). Human studies have demonstrated that infants exposed to cocaine in utero had an increased risk of renal tract anomalies including renal hypoplasia (84–86). Similarly, infants with Fetal Alcohol Syndrome also have a higher incidence of renal malformations, including small kidneys (87–89), and a recent study in sheep demonstrated that repeated alcohol exposure in late gestation leads to a mild nephron deficiency (90). Several clinical medications have also proven detrimental to fetal nephron endowment. Dexamethasone, a synthetic glucocorticoid that readily crosses the placental barrier, is commonly used in obstetrics to promote fetal lung maturation and in general therapeutic use as an anti-inflammatory. However, sheep and rodent investigations have demonstrated that the presence of maternal corticosterone elevations due to the natural stress response or exogenous fetal dexamethasone exposure are associated with a reduction in nephron endowment and subsequent development of hypertension in offspring (91–95). Thalidomide is another clinical drug associated with renal abnormalities and hypoplasia (96,97). A powerful tranquilizer and painkiller, it was widely prescribed in the late 1950s before the finding that it too could readily cross the placental barrier, resulting in severe birth defects.
Importantly, the underlying genetic and molecular mechanisms that cause renal hypoplasia in response to suboptimal in utero environment are largely unknown. A greater understanding in these cases will provide a significant opportunity for future preventative interventions.
CONCLUSION
Genetic studies in humans and mice have provided valuable insights into the genetic contribution and molecular mechanisms leading to normal nephron endowment and renal hypoplasia. Specifically, the roles of several critical parallel and interacting signaling pathways, including GDNF/Ret, FGF, PAX2, and HH, have been strongly implicated in the pathogenesis of renal hypoplasia. Furthermore, factors influencing the in utero environment are also critical in establishing sufficient nephron number, albeit by mostly unknown mechanisms. With the continual advancements in human and mouse genetic analyses, the identification of other known and novel genes in nephron endowment is sure to follow. Understanding of the precise mechanisms governing nephron endowment is required to advance the prediction, diagnosis, prevention, and treatment of renal hypoplasia and the health risks associated with low nephron endowment.
Abbreviations
- FGF(R):
-
fibroblast growth factor (receptor)
- HH:
-
Hedgehog
- MM:
-
metanephric mesenchyme
- Rar:
-
retinoic acid receptor
- UB:
-
ureteric bud
- UBM:
-
ureteric branching morphogenesis
References
Hiraoka M, Tsukahara H, Ohshima Y, Kasuga K, Ishihara Y, Mayumi M 2002 Renal aplasia is the predominant cause of congenital solitary kidneys. Kidney Int 61: 1840–1844
Brenner BM, Chertow GM 1994 Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175
Brenner BM, Garcia DL, Anderson S 1988 Glomeruli and blood pressure. Less of one, more the other?. Am J Hypertens 1: 335–347
Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF 2006 Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int 70: 104–110
Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE 2006 Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69: 671–678
Keller G, Zimmer G, Mall G, Ritz E, Amann K 2003 Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108
Sanna-Cherchi S, Caridi G, Weng PL, Scolari F, Perfumo F, Gharavi AG, Ghiggeri GM 2007 Genetic approaches to human renal agenesis/hypoplasia and dysplasia. Pediatr Nephrol 22: 1675–1684
Schedl A 2007 Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802
Woolf AS, Price KL, Scambler PJ, Winyard PJ 2004 Evolving concepts in human renal dysplasia. J Am Soc Nephrol 15: 998–1007
Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF 2003 A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 63: S31–S37
Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V 1994 Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383
Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M 2004 A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol 24: 8026–8036
Schmidt W, Schroeder TM, Buchinger G, Kubli F 1982 Genetics, pathoanatomy and prenatal diagnosis of Potter I syndrome and other urogenital tract diseases. Clin Genet 22: 105–127
Zhang Z, Quinlan J, Hoy W, Hughson MD, Lemire M, Hudson T, Hueber PA, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Houghton F, Bertram J, Goodyer P 2008 A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol 19: 2027–2034
Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP 2003 Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: 3175–3185
Sims-Lucas S, Cullen-McEwen L, Eswarakumar VP, Hains D, Kish K, Becknell B, Zhang J, Bertram JF, Wang F, Bates CM 2009 Deletion of Frs2alpha from the ureteric epithelium causes renal hypoplasia. Am J Physiol Renal Physiol 297: F1208–F1219
Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM 2004 Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415
Lu BC, Cebrian C, Chi X, Kuure S, Kuo R, Bates CM, Arber S, Hassell J, MacNeil L, Hoshi M, Jain S, Asai N, Takahashi M, Schmidt-Ott KM, Barasch J, D'Agati V, Costantini F 2009 Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat Genet 41: 1295–1302
Ishibe S, Karihaloo A, Ma H, Zhang J, Marlier A, Mitobe M, Togawa A, Schmitt R, Czyczk J, Kashgarian M, Geller DS, Thorgeirsson SS, Cantley LG 2009 Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 136: 337–345
Zhang Z, Pascuet E, Hueber PA, Chu L, Bichet DG, Lee TC, Threadgill DW, Goodyer P 2010 Targeted Inactivation of EGF Receptor Inhibits Renal Collecting Duct Development and Function. J Am Soc Nephrol 21: 573–578
Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR 2005 Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132: 2809–2823
Chung GW, Edwards AO, Schimmenti LA, Manligas GS, Zhang YH, Ritter R III 2001 Renal-coloboma syndrome: report of a novel PAX2 gene mutation. Am J Ophthalmol 132: 910–914
Ford B, Rupps R, Lirenman D, Van Allen MI, Farquharson D, Lyons C, Friedman JM 2001 Renal-coloboma syndrome: prenatal detection and clinical spectrum in a large family. Am J Med Genet 99: 137–141
Parsa CF, Silva ED, Sundin OH, Goldberg MF, De Jong MR, Sunness JS, Zeimer R, Hunter DG 2001 Redefining papillorenal syndrome: an underdiagnosed cause of ocular and renal morbidity. Ophthalmology 108: 738–749
Nishimoto K, Iijima K, Shirakawa T, Kitagawa K, Satomura K, Nakamura H, Yoshikawa N 2001 PAX2 gene mutation in a family with isolated renal hypoplasia. J Am Soc Nephrol 12: 1769–1772
Quinlan J, Lemire M, Hudson T, Qu H, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Zhang Z, Houghton F, Goodyer P 2007 A common variant of the PAX2 gene is associated with reduced newborn kidney size. J Am Soc Nephrol 18: 1915–1921
Porteous S, Torban E, Cho NP, Cunliffe H, Chua L, McNoe L, Ward T, Souza C, Gus P, Giugliani R, Sato T, Yun K, Favor J, Sicotte M, Goodyer P, Eccles M 2000 Primary renal hypoplasia in humans and mice with PAX2 mutations: evidence of increased apoptosis in fetal kidneys of Pax2(1Neu) +/− mutant mice. Hum Mol Genet 9: 1–11
Dziarmaga A, Eccles M, Goodyer P 2006 Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol 17: 1568–1575
Dziarmaga A, Clark P, Stayner C, Julien JP, Torban E, Goodyer P, Eccles M 2003 Ureteric bud apoptosis and renal hypoplasia in transgenic PAX2-Bax fetal mice mimics the renal-coloboma syndrome. J Am Soc Nephrol 14: 2767–2774
Clarke JC, Patel SR, Raymond RM Jr, Andrew S, Robinson BG, Dressler GR, Brophy PD 2006 Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15: 3420–3428
Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S 2010 vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 137: 347–357
Bingham C, Ellard S, van't Hoff WG, Simmonds HA, Marinaki AM, Badman MK, Winocour PH, Stride A, Lockwood CR, Nicholls AJ, Owen KR, Spyer G, Pearson ER, Hattersley AT 2003 Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int 63: 1645–1651
Godley LA, Kopp JB, Eckhaus M, Paglino JJ, Owens J, Varmus HE 1996 Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes Dev 10: 836–850
Cain JE, Islam E, Haxho F, Chen L, Bridgewater D, Nieuwenhuis E, Hui CC, Rosenblum ND 2009 GLI3 repressor controls nephron number via regulation of Wnt11 and Ret in ureteric tip cells. PLoS One 4: e7313
Sama A, Mason JD, Gibbin KP, Young ID, Hewitt M 1994 The Pallister-Hall syndrome. J Med Genet 31: 740
Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, Abbott MH, Aughton DJ, Aylsworth AS, Bamshad MJ, Booth C, Curry CJ, David A, Dinulos MB, Flannery DB, Fox MA, Graham JM, Grange DK, Guttmacher AE, Hannibal MC, Henn W, Hennekam RC, Holmes LB, Hoyme HE, Leppig KA, Lin AE, Macleod P, Manchester DK, Marcelis C, Mazzanti L, McCann E, McDonald MT, Mendelsohn NJ, Moeschler JB, Moghaddam B, Neri G, Newbury-Ecob R, Pagon RA, Phillips JA, Sadler LS, Stoler JM, Tilstra D, Walsh Vockley CM, Zackai EH, Zadeh TM, Brueton L, Black GC, Biesecker LG 2005 Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet 76: 609–622
Donnai D, Young ID, Owen WG, Clark SA, Miller PF, Knox WF 1986 The lethal multiple congenital anomaly syndrome of polydactyly, sex reversal, renal hypoplasia, and unilobular lungs. J Med Genet 23: 64–71
Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A 1996 Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79
Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H 1996 Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382: 73–76
Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M 1996 Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382: 70–73
Cullen-McEwen LA, Drago J, Bertram JF 2001 Nephron endowment in glial cell line-derived neurotrophic factor (GDNF) heterozygous mice. Kidney Int 60: 31–36
Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF 2003 Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension 41: 335–340
Zhang Z, Quinlan J, Grote D, Lemire M, Hudson T, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Houghton F, Bouchard M, Goodyer P 2009 Common variants of the glial cell-derived neurotrophic factor gene do not influence kidney size of the healthy newborn. Pediatr Nephrol 24: 1151–1157
Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR 1995 Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 375: 791–795
Esquela AF, Lee SJ 2003 Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol 257: 356–370
Patterson LT, Pembaur M, Potter SS 2001 Hoxa11 and Hoxd11 regulate branching morphogenesis of the ureteric bud in the developing kidney. Development 128: 2153–2161
Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R 1999 Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23: 113–117
Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten DJ, Kimberling WJ, Smith RJ, Weil D, Petit C, Otto EA, Xu PX, Hildebrandt F 2007 Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet 80: 800–804
Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F 2004 SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci USA 101: 8090–8095
Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, Knuppel T, Zurowska AM, Caldas-Alfonso A, Litwin M, Emre S, Ghiggeri GM, Bakkaloglu A, Mehls O, Antignac C, Network E, Schaefer F, Burdine RD 2008 SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol 19: 891–903
Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T 2001 Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128: 3105–3115
Warren M, Wang W, Spiden S, Chen-Murchie D, Tannahill D, Steel KP, Bradley A 2007 A Sall4 mutant mouse model useful for studying the role of Sall4 in early embryonic development and organogenesis. Genesis 45: 51–58
Becker K, Beales PL, Calver DM, Matthijs G, Mohammed SN 2002 Okihiro syndrome and acro-renal-ocular syndrome: clinical overlap, expansion of the phenotype, and absence of PAX2 mutations in two new families. J Med Genet 39: 68–71
Newman WG, Brunet MD, Donnai D 1997 Townes-Brocks syndrome presenting as end stage renal failure. Clin Dysmorphol 6: 57–60
Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M 1994 Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120: 2749–2771
Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C 2010 Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137: 283–292
Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C 2001 Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27: 74–78
Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Benichou C 1998 Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54: 1455–1462
Wilson JG, Roth CB, Warkany J 1953 An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 92: 189–217
Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D 1999 FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126: 547–554
Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D'Agati V, Licht JD, Martin GR, Costantini F 2010 Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet 6: e1000809
Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N 2000 FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277: 643–649
Bates CM, Kharzai S, Erwin T, Rossant J, Parada LF 2000 Role of N-myc in the developing mouse kidney. Dev Biol 222: 317–325
Couillard M, Trudel M 2009 C-myc as a modulator of renal stem/progenitor cell population. Dev Dyn 238: 405–414
Novack DV, Korsmeyer SJ 1994 Bcl-2 protein expression during murine development. Am J Pathol 145: 61–73
Nagata M, Nakauchi H, Nakayama K, Nakayama K, Loh D, Watanabe T 1996 Apoptosis during an early stage of nephrogenesis induces renal hypoplasia in bcl-2-deficient mice. Am J Pathol 148: 1601–1611
Sorenson CM, Rogers SA, Korsmeyer SJ, Hammerman MR 1995 Fulminant metanephric apoptosis and abnormal kidney development in bcl-2-deficient mice. Am J Physiol 268: F73–F81
Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP 2008 Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181
Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G 2006 Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228
Fogelgren B, Yang S, Sharp IC, Huckstep OJ, Ma W, Somponpun SJ, Carlson EC, Uyehara CF, Lozanoff S 2009 Deficiency in Six2 during prenatal development is associated with reduced nephron number, chronic renal failure, and hypertension in Br/+ adult mice. Am J Physiol Renal Physiol 296: F1166–F1178
Denton KM 2006 Can adult cardiovascular disease be programmed in utero?. J Hypertens 24: 1245–1247
Moritz KM, Dodic M, Wintour EM 2003 Kidney development and the fetal programming of adult disease. Bioessays 25: 212–220
Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM 2007 Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18: 1688–1696
Zohdi V, Moritz KM, Bubb KJ, Cock ML, Wreford N, Harding R, Black MJ 2007 Nephrogenesis and the renal renin-angiotensin system in fetal sheep: effects of intrauterine growth restriction during late gestation. Am J Physiol Regul Integr Comp Physiol 293: R1267–R1273
Brennan KA, Kaufman S, Reynolds SW, McCook BT, Kan G, Christiaens I, Symonds ME, Olson DM 2008 Differential effects of maternal nutrient restriction through pregnancy on kidney development and later blood pressure control in the resulting offspring. Am J Physiol Regul Integr Comp Physiol 295: R197–R205
Gilbert JS, Lang AL, Grant AR, Nijland MJ 2005 Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol 565: 137–147
Hoppe CC, Evans RG, Bertram JF, Moritz KM 2007 Effects of dietary protein restriction on nephron number in the mouse. Am J Physiol Regul Integr Comp Physiol 292: R1768–R1774
Makrakis J, Zimanyi MA, Black MJ 2007 Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr Nephrol 22: 1861–1867
Pires KM, Aguila MB, Mandarim-de-Lacerda CA 2006 Early renal structure alteration in rat offspring from dams fed low protein diet. Life Sci 79: 2128–2134
Villar-Martini VC, Carvalho JJ, Neves MF, Aguila MB, Mandarim-de-Lacerda CA 2009 Hypertension and kidney alterations in rat offspring from low protein pregnancies. J Hypertens 27: S47–S51
Abdel-Hakeem AK, Henry TQ, Magee TR, Desai M, Ross MG, Mansano RZ, Torday JS, Nast CC 2008 Mechanisms of impaired nephrogenesis with fetal growth restriction: altered renal transcription and growth factor expression. Am J Obstet Gynecol 199: 252.e1–252.e7
Welham SJ, Riley PR, Wade A, Hubank M, Woolf AS 2005 Maternal diet programs embryonic kidney gene expression. Physiol Genomics 22: 48–56
Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M 1999 Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48: 2240–2245
Battin M, Albersheim S, Newman D 1995 Congenital genitourinary tract abnormalities following cocaine exposure in utero. Am J Perinatol 12: 425–428
Chasnoff IJ, Chisum GM, Kaplan WE 1988 Maternal cocaine use and genitourinary tract malformations. Teratology 37: 201–204
Rosenstein BJ, Wheeler JS, Heid PL 1990 Congenital renal abnormalities in infants with in utero cocaine exposure. J Urol 144: 110–112
Havers W, Majewski F, Olbing H, Eickenberg HU 1980 Anomalies of the kidneys and genitourinary tract in alcoholic embryopathy. J Urol 124: 108–110
Qazi Q, Masakawa A, Milman D, McGann B, Chua A, Haller J 1979 Renal anomalies in fetal alcohol syndrome. Pediatrics 63: 886–889
Taylor CL, Jones KL, Jones MC, Kaplan GW 1994 Incidence of renal anomalies in children prenatally exposed to ethanol. Pediatrics 94: 209–212
Gray SP, Kenna K, Bertram JF, Hoy WE, Yan EB, Bocking AD, Brien JF, Walker DW, Harding R, Moritz KM 2008 Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. Am J Physiol Regul Integr Comp Physiol 295: R568–R574
Dickinson H, Walker DW, Wintour EM, Moritz K 2007 Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol 292: R453–R461
Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM 2002 Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734
Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, Moritz KM 2007 Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol 579: 503–513
Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA 2007 Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am J Physiol Renal Physiol 293: F548–F554
Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M 2003 Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935
McBride WG 1977 Thalidomide embryopathy. Teratology 16: 79–82
Smithells RW, Newman CG 1992 Recognition of thalidomide defects. J Med Genet 29: 716–723
Kanjilal D, Basir MA, Verma RS, Rajegowda BK, Lala R, Nagaraj A 1992 New dysmorphic features in Rubinstein-Taybi syndrome. J Med Genet 29: 669–670
Chitayat D, Hodgkinson KA, Chen MF, Haber GD, Nakishima S, Sando I 1992 Branchio-oto-renal syndrome: further delineation of an underdiagnosed syndrome. Am J Med Genet 43: 970–975
Slavotinek AM, Tifft CJ 2002 Fraser syndrome and cryptophthalmos: review of the diagnostic criteria and evidence for phenotypic modules in complex malformation syndromes. J Med Genet 39: 623–633
Ounap K, Laidre P, Bartsch O, Rein R, Lipping-Sitska M 1998 Familial Williams-Beuren syndrome. Am J Med Genet 80: 491–493
Grix A Jr, Curry C, Hall BD 1982 Patterns of multiple malformations in infants of diabetic mothers. Birth Defects Orig Artic Ser 18: 55–77
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants awarded by the Canadian Institute of Health research and The Canada Research Chair Program (N.D.R.).
Rights and permissions
About this article
Cite this article
Cain, J., Di Giovanni, V., Smeeton, J. et al. Genetics of Renal Hypoplasia: Insights Into the Mechanisms Controlling Nephron Endowment. Pediatr Res 68, 91–98 (2010). https://doi.org/10.1203/PDR.0b013e3181e35a88
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181e35a88
This article is cited by
-
Regulation of nephron progenitor cell lifespan and nephron endowment
Nature Reviews Nephrology (2022)
-
Premature differentiation of nephron progenitor cell and dysregulation of gene pathways critical to kidney development in a model of preterm birth
Scientific Reports (2021)
-
A single center’s experience in pediatric cystine stone disease management: what changed over time?
Urolithiasis (2020)
-
Sonographic evaluation of kidney echogenicity and morphology among HIV sero-positive adults at Lagos University Teaching Hospital
Journal of Ultrasound (2018)
-
Chronic kidney disease
Nature Reviews Disease Primers (2017)