Abstract
Oxygen is critical for multicellular existence. Its reduction to water by the mitochondrial electron transport chain helps supply the metabolic demands of human life. The incompletely reduced, reactive oxygen byproducts of this reaction, however, can be quite toxic. In this review, we explore the mechanisms responsible for maintaining oxygen homeostasis and the consequences of their dysfunction. With an eye toward defining clinical care guidelines for the management of critically ill neonates, we present evidence describing the role of physiologic hypoxia during development and the adverse consequences of hyperoxia in-term as well as preterm infants.
Similar content being viewed by others
Main
Oxygen is the second most abundant element in Earth's atmosphere. Although critical for aerobic respiration, it also poses significant dangers to life. The reduction of molecular oxygen to water by the mitochondrial electron transport chain enables the conversion of ADP into ATP. A consequence of this reaction, however, is the formation of toxic reactive oxygen species (ROS) that can damage various classes of biologic molecules. In the absence of oxygen, the electron transport chain is inhibited and glucose metabolism is shunted down glycolytic pathways. The resultant depression of cellular metabolism is incompatible with life in higher organisms.
Metazoan cells are exposed to a wide range of oxygen tensions. It is only under near anoxic conditions, however, that the electron transport chain becomes inhibited. At a partial pressure of ∼0.4 kPa (3 mm Hg) or ∼0.5% (as compared with atmospheric oxygen, i.e. 21%), mitochondrial electron transport of cultured cells is inhibited and apoptotic death is initiated (1,2). Mitochondria, thus, have the capacity to maintain maximal ATP levels over a large range of physiologic oxygen concentrations. In mouse embryonic fibroblasts, prolonged culture under “hypoxic” (3% O2) conditions even prevents cell senescence (3). This “physiologic hypoxia” (1-10% O2), as opposed to “pathologic hypoxia” (<1% O2) during which metabolism becomes compromised, represents the natural environment for most mammalian tissues.
All eukaryotic organisms must maintain oxygen homeostasis. A number of defense and regulatory mechanisms have been developed to protect the cell from low as well as high oxygen levels. In this review, we will describe some of the regulatory principles responsible for maintaining oxygen homeostasis. Interestingly, both increases and decreases in cellular O2 levels result in the generation of ROS. Further, some of the adverse effects of hyperoxia and hypoxia will be described and finally we will attempt to translate this information into a set of clinical practice guidelines for the handling of newborn infants.
Evolutionary Aspects
Four billion years ago, the Earth's atmosphere contained approximately one part per million (ppm) of oxygen. The first cells, thus, evolved in an oxygen-free environment generating energy via oxygen-independent pathways. As a result of the appearance of cyanobacteria that used photosynthesis as a means of energy production some 2.7 billion years ago, atmospheric oxygen levels rose to approximately 1%. Concurrently, the first eukaryotes began to appear in the geological record. These cells made sterols for their membranes—a sure sign of anoxic environment given the oxygen-dependence of this reaction (4,5). In addition, many eukaryotes began to acquire mitochondria as a result of an endosymbiotic relationship with proteobacteria (6). Endowed with oxidative phosphorylation, these organisms were uniquely poised to take advantage of the newly emerging oxygen-rich atmosphere.
As summarized by Lane (7) there has been significant variation in the Earth's oxygen environment over time. Approximately 2.2 billion years ago, the oxygen concentration of the atmosphere rose sharply to 5-18%, with significant variations thereafter, until present day levels were finally reached. Five hundred million years later, during the so-called Cambrian explosion, the oxygen level again rose to present levels. Two hundred million years later, in the late Carboniferous and early Permian period, oxygen may have reached levels as high as 35%. Several smaller peaks, approximately 100 million years ago, also occurred until the present level was once more reached. The accumulation of free oxygen in the atmosphere allowed the regeneration of water from free hydrogen, thus, preventing the loss of hydrogen to space and preserving Earth's oceans. The injection of oxygen into the atmosphere by photosynthesis may therefore have prevented the Earth from becoming as sterile as Mars or Venus (7).
Historical Aspects
Oxygen was discovered as an element in the 1770's by the Swedish apothecary Carl Scheele and the English clergyman and chemist Joseph Priestly. Priestly, who in 1774 produced oxygen by focusing sunlight onto an oxide of mercury, published his findings before Scheele. However, neither understood the full significance of their discovery. It was the French chemist, Antoine Lavoisier, who proved that oxygen was the reactive constituent of air. Interestingly, alchemists had already appreciated the significance of oxygen well before that. The Polish alchemist Michael Sendivogius suggested in 1604 that a so-called “aerial food of life” circulated between the air and earth by way of nitre. When heated above 336°C nitre decomposes to release oxygen, which Sendivogius considered the Elixir of life, “without which no mortal can live” (7). In 1798, Thomas Beddoes in Bristol founded the Pneumatic Institute using pure oxygen to treat diseases previously found incurable (7). However, more than 10 years before that oxygen had already been used to treat newborns.
Normoxia, Hypoxia, and Hyperoxia
By convention, normoxia has been defined as the level of oxygen required for normal physiologic processes to occur. Hypoxia implies an imbalance between oxygen supply and demand. However, it can also simply indicate a change in the oxygen environment that triggers a cascade of physiologic and biochemical events that are compensated (or, physiologic,) or noncompensated (or, pathologic). Although in the former scenario cellular bioenergetic status is maintained, the latter leads to specific cellular changes affecting enzyme activities, mitochondrial function, cytoskeletal structure, membrane transport, and antioxidant defenses.
During pathologic hypoxia, limited oxygen availability decreases oxidative phosphorylation resulting in a failure to resynthesize energy-rich phosphates, including ATP and phosphocreatine. Membrane ATP-dependent Na+/K+ pump is altered favoring the influx of Na+, Ca++, and water into the cell and thereby producing cytotoxic edema. Furthermore, adenine nucleotide catabolism during ischemia results in the intracellular accumulation of hypoxanthine (8). We found elevated hypoxanthine in umbilical cord blood after birth asphyxia and therefore understood hypoxanthine can be used as a sensitive marker of intrauterine hypoxia (8). In the presence of xanthine oxidase, hypoxanthine can generate toxic ROS with the reintroduction of molecular oxygen during newborn resuscitation (9,10). Increased cytosolic calcium triggers numerous metabolic pathways, including activation of phospholipases, release of prostaglandins, lipases, proteases, and endonucleases, all of which injure various structural components of cells (11). Additionally, a proinflammatory state is induced via the expression of certain proinflammatory gene products in the endothelium, such as leukocyte adhesion molecules and cytokines, and bioactive agents, such as endothelin and thromboxane A2 (12). On the other hand gene products such as prostacyclin and nitric oxide that may be protective are repressed (13).
Hyperoxia is generally thought to arise when elevated oxygen levels result in the production of toxic ROS. However, deviations from physiologic hypoxia that do not result in direct ROS-dependent injury can also have adverse consequences by inactivating physiologic hypoxia driven developmental processes. It is also important to note that the levels of oxygen that indicate hypoxia or hyperoxia may be context dependent. Brain and muscle require different levels of oxygen and physiologic as well as pathologic processes may influence whether a given amount of oxygen constitutes normoxia, hypoxia, or even hyperoxia (14).
Oxygen Toxicity and Free Radicals
It was already realized by Priestly that oxygen might be toxic; however, it took 100 years until oxygen toxicity was systematically described by Paul Bert. Some years later, the Scottish pathologist, James Smith, showed that exposure to 75% oxygen induces inflammation in the lungs (7). In the early 1950s, Gerschman et al. proposed that, similar to radiation injury, oxygen is toxic because of its ability to generate free radicals (15).
Oxygen has two unpaired electrons that help prevent it from forming new chemical bonds. The spin of these two unpaired electrons is magnetic, making them more resistant to react (7). This may be overcome by feeding oxygen with one electron at a time so that each of the unpaired electrons receives a partner, thus, generating oxygen radicals including superoxide anions (O2−, OH·), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−) (Fig. 1).
The main site of superoxide radical production occurs in mitochondria through electron leakage from the electron transport chain. It has been estimated that 1 to 2% of the total oxygen consumed by cells escape as oxygen-free radicals. A cell produces around 50 hydroxyl radicals every second and a grown up weighing 70 kg produces approximately 1.7 kg of superoxide radicals every year (7). Other potential sources include the hypoxanthine-xanthine oxidase system (8,16) and activated phagocytes (17).
Reperfusion/reoxygenation of ischemic/hypoxic tissues results in the formation of toxic ROS, and reactive nitrogen species (RNS), especially peroxynitrate (ONOO−). Normally, excess hypoxanthine is oxidized by xanthine dehydrogenase to xanthine and uric acid. However, during ischemia, xanthine dehydrogenase is converted to xanthine oxidase (18) and leaks into the circulation to be widely distributed (19). When oxygen is reintroduced during reperfusion/reoxygenation, conversion of excess hypoxanthine by xanthine oxidase results in the formation of ROS. Our understanding of this phenomenon in 1980 (9) represented the basis of understanding the oxygen paradox of hypoxia-reoxygenation injury (also called ischemia reperfusion injury). In the presence of nitric oxide, RNS form as well (11). In the brain, activation of NMDA receptors results in the influx of calcium and subsequent calmodulin-mediated activation of neuronal NOS. Both neuronal and inducible NOS are high in the developing brain. NO may lead to the formation of peroxynitrite initiating lipid peroxidation. On the other hand, NO also exhibits neuroprotective effects by inducing vasodilatation, angiogenesis, inhibiting platelet aggregation, leukocyte activation, and antiapoptotic effects through its inhibition of cytochome C release from mitochondria.
ROS and RNS are potent oxidizing and reducing agents that directly damage cellular structures. Most of them are free radicals, which are defined as any atomic or molecular species capable of independent existence that contain one or more unpaired electrons in one of their molecular orbitals. Thus, they are able to peroxidize membranes, structural proteins, enzymes, and nucleic acids. A large number of enzymatic and nonenzymatic antioxidants has evolved in biologic systems to protect cellular structures against free radical damage. The most important enzymes are superoxide dismutases, catalases, glutathione peroxidases, and glucose 6-phosphate dehydrogenase. The major nonenzymatic intracellular antioxidant is glutathione (GSH), which is able to reduce free radicals by establishing a disulfur bond with another GSH molecule forming oxidized glutathione (GSSG) and releasing one electron. GSSG is reduced again to GSH by the intervention of glutathione reductase.
Oxidative stress
Oxidative stress is defined as the imbalance of pro- and antioxidants in a biologic system in favor of the former. Different biomarkers of oxidative stress have been used in biology and medicine. They may reflect direct damage to different components of the cell; thus, for lipid peroxidation, malondialdehyde and n-aldehydes or for nucleic acid peroxidation, oxidated nucleotides such as 8-oxo-dihydroguanosine. Isoprostanes are prostaglandin-like compounds produced in vivo by noncyclooxygenase mechanism involving peroxidation of polyunsaturated fatty acids. Oxidated amino acids, such as ο-tyrosine, are indicators of hydroxyl radical activity. Increased activities of superoxide dismutases, catalases, glutathione peroxidases, or glutathione redox cycle enzymes, and increased concentration of GSSG or decreased GSH/GSSG ratio, indirectly may reflect a prooxidant status (11,14).
Oxidative stress also induces inflammation. Tumor necrosis factor (TNF)-α plays an important role in inflammatory responses causing apoptosis by signaling through death receptors. On the other hand, TNF-α generates ROS by activating NADPH oxidase enzymes that are crucial in endothelial death (20). One important defense mechanism against TNF-α induced endothelial death is heme oxygenase (HO). This enzyme metabolizes heme, a prooxidant, to biliverdin and bilirubin, both potent antioxidants. In addition, carbon monoxide is split-off. CO is a gaseous messenger molecule that also is protective against oxidative stress (12).
Although ROS are mainly known as harmful agents it is also clear they have an important role in regulating normal processes. It is almost 40 y since Babior et al. understood they play an important role in immune function (17) and 20-25 y since they were understood to be important regulators of circulation (21). ROS activate cellular growth factors, eliminate dysfunctional proteins by oxidation, and are essential for the function of cellular organelles (For review see 14).
Developmental Aspects
Early investigators noted the optimum oxygen concentration for mammalian embryonic development to be approximately 3 to 5% (22–29). Exposure of early mammalian embryos to atmospheric (21%) oxygen was consistently shown to impair development. In agreement with these studies, direct measurement of oxygen tension from the oviducts and uterine horns of various mammals have consistently shown that these structures provide preimplantation embryos with a hypoxic environment (30–35). Similarly in humans, follicular fluid aspirates exhibit oxygen tensions varying from <1 to 5.5% (36). Amazingly, the first 10 to 12 wk of human pregnancy transpires without significant maternal blood flow to the fetus. Only after this point does the placental bed become perfused with maternal blood in a pulsatile fashion (37,38). Thus, the entire process of organogenesis unfolds under hypoxic conditions. Even after access to the relatively oxygen-rich maternal vasculature, the fetal circulation provides developing tissues with hypoxic levels of oxygen. Even though fetal Hb has a greater affinity for oxygen and, thus, is able to maintain near adult levels of total blood oxygen content, direct measurements of fetal blood oxygen partial pressure (pO2) after establishment of the maternal-fetal circulation indicate a continued hypoxemia throughout the remainder of human gestation. Although human maternal arterial pO2 is normally ∼12 kPa (90 mm Hg) and venous pO2 is ∼9.3 kPa (70 mm Hg) (∼13 to 10% O2), fetal arterial and venous pO2 values rarely exceed ∼4 kPa (30 mm Hg) (∼4% O2) (39). Thus, from conception through parturition, mammalian development occurs under a physiologic hypoxia.
Regulatory Aspects
Oxygen sensing occurs at many levels and is an important process needed for normal fetal development, as well as for adaptation to abnormal oxygen levels either as a response to changes in ambient oxygen levels or because of disease processes. The carotid body represents a global oxygen-sensing mechanism in mammals. When the glomus cells in the carotid body detect hypoxia, they are depolarized and send signals to the respiratory centers in the medulla oblongata to increase respiratory rate. At the cellular level, hypoxia is quickly sensed by incompletely defined mechanisms. Mitochondria are the site of highest oxygen consumption and, thus, represent a likely site of oxygen sensing (more on this below). Additionally, molecules located close to high conductance calcium and voltage-gated potassium channels have been proposed to act in this capacity. NADPH oxidase and NO have also been suggested as possible oxygen sensing and signaling molecules.
Another factor affecting oxygen homeostasis is metabolism itself. A high rate of oxygen consumption creates a gradient of oxygen that promotes the influx of oxygen into the cell. Along these lines, other factors affecting diffusion, such as the lipid composition of cell membranes may play a critical role for oxygen entry. For instance, an increase in the cholesterol concentration of red blood cell membranes decreases the transport of oxygen across the cell perhaps by decreasing cell membrane fluidity and stiffening of the lipid bilayer (14).
Adaptation.
Long standing moderate hypoxia may lead to an adjustment of the normoxia set point so that cells perceive hyperoxia under otherwise normal oxygen concentrations. Activation of perceived hyperoxia-sensitive genes plays an important role in remodeling and healing of cardiomyocytes and fibroblasts after ischemia-reperfusion injury.
Several species, such as the turtle and brine shrimp, are capable of surviving long periods of anoxia. Roth and coworkers have discovered that anoxia in Caenorhabditis elegans and zebrafish can induce a suspended animation-like state from which full recovery can be achieved (40–42). Similarly, Haddad et al. have discovered that the fruit fly Drosophila melanogaster may survive and recover from several hours of total oxygen deprivation. With long-term experimental selection over many generations, these scientists were able to develop a D. melanogaster strain that can live in extremely low, normally lethal, oxygen tensions. These strains show a higher rate of O2 consumption in hypoxia, along with a decreased body size and a larger trachea diameter perhaps to enhance oxygen delivery. At the molecular level, it seems that the glucose dimer trehalose is important for protection against anoxic stress in Drosophila as well as in mammalian cells. These authors cloned and transfected the gene for trehalose-6 phosphate synthase into mammalian cells. These cells had higher trehalose concentration and were also more resistant to hypoxia (14,43).
Hypoxia-Inducible Factor 1 and the Transcriptional Response to Hypoxia
Investigations of cellular and the organism's responses to hypoxia led to the identification of hypoxia-inducible factor 1 (HIF-1) (44). Study of erythropoietin production under reduced oxygen tension resulted in the biochemical purification of a protein complex that specifically bound the erythropoietin gene in an oxygen-dependent fashion (45). A basic helix-loop-helix PAS transcription factor composed of two subunits, HIF-1α and HIF-1β/arylhydrocaron receptor nuclear translocator (ARNT), (46) the range of functions covered by this ubiquitous heterodimer includes the majority of cellular and organismal responses to oxygen deprivation (47). In addition to erythropoiesis, glucose uptake is increased, breathing is enhanced, and angiogenesis is promoted. HIF-1 is an important protein causing a shift from aerobic to anaerobic metabolism by inducing a variety of glycolytic enzymes and glucose transporters (48). HIF-1 also reduces mitochondrial oxygen consumption by inducing pyruvate dehydogenase kinase I and thereby slowing flux through the citric acid cycle (49,50).
Under normoxic conditions, HIF-1α is constitutively transcribed, translated and hydroxylated at multiple proline residues by a set of prolyl-4-hydroxylase (PHD) enzymes (51–54). The hydroxylated prolines are then recognized by the Von Hippel-Lindau (pVHL) E3 ubiquitin ligase complex, which targets HIF-1α for proteasomal degradation. PHD or pVHL deficiency results in constitutive HIF activity. This system is exquisitely sensitive to oxygen, such that following as little as 5 minutes of reoxygenation, most stabilized HIF-1α is degraded. In contrast, nonhydroxylated HIF-1α translocates to the nucleus, heterodimerizes with ARNT and activates hypoxia-induced genes.
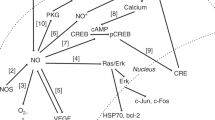
A leading model of oxygen sensing (Fig. 2) proposes that under hypoxic conditions, mitochondrially generated ROS inhibit HIF-1α prolyl hydroxylation and degradation (55–59). According to this model, ROS are generated at mitochondria as a function of decreasing oxygen tension (56,60–62). Consistent with this, free radical scavengers are able to inhibit HIF-1α accumulation (63,64). Importantly, cells devoid of mitochondrial DNA and, hence, mitochondrial electron transport fail to induce HIF-1α under physiologic hypoxia (56). Thus, it seems that the natural byproducts of oxidative phosphorylation have been incorporated as signaling molecules conveying cellular oxygen availability.
The regulation of HIF stability by oxygen tension. During normoxia, HIF-1α is rapidly degraded. Critical proline residues within HIF-1α s oxygen-dependent degradation domain are hydroxylated by a family of prolyl-4-hydroxylases (PHD) resulting in the formation of a recognition motif for the von Hippel Lindau tumor suppressor (pVHL). As this protein is an E3 ubiquitin ligase, HIF-1α is poly-ubiquitylated and targeted for proteasomal degradation. Under conditions of oxygen deprivation, mitochondria paradoxically increase their production of reactive oxygen species (ROS). Serving as signaling molecules, these ROS inhibit the hydroxylation of HIF-1α and, thus, preventing its proteasomal degradation. Stabilized HIF-1α is able to translocate to the nucleus where it forms a heterodimeric complex with HIF-1β/ARNT. This transcription factor complex activates the expression of scores of genes, such as Epo and Vegf, to increase oxygen delivery and many others including pyruvate dehydrogenase 1 (PDK1) to reduce oxygen consumption. These factors secure oxygenation to the tissues. Further, glucose uptake is increased and a shift to anaerobic metabolism occurs in an attempt to maintain energy homeostasis.
HIF and Development
The first indication that hypoxia-induced gene expression pathways are critical regulators of mammalian development followed the genetic inactivation of the murine Arnt(Hif-1β) locus (65,66). These animals died midway through gestation because of impaired blood vessel formation. It was proposed that the hypoxic environment of the developing embryo activated HIF-dependent Vegf expression and angiogenesis. Similar results were obtained with Hif-1α-null embryos (67,68). pVHL and PHD-2-deficiency also resulted in embryonic lethality indicating that constitutively active HIF is just as detrimental as its absence (69,70) and that the dynamic modulation of HIF activity is critical for embryonic development.
Stem cell function was also affected by the lack of a transcriptional response to hypoxia. Hematopoietic stem cells derived from Arnt-null embryos were impaired in their ability to proliferate in response to physiologic hypoxia both in vitro and in vivo (71). Consistent with the ability of oxygen tension to regulate human placental development (72), the placentas of Arnt-null mice were also severely defective (65,73). Derivation of trophoblast stem cells from Arnt-null embryos indicated that HIF activity determines not only stem cell numbers but also stem cell fate decisions in the placenta (73,74). This was largely due to the ability of the HIFs to interact with and modulate cellular histone deacetylase activity.
Targeted inactivation of these factors in individual organ systems has provided yet more evidence of the importance of this pathway during embryogenesis. For example, inactivation of Hif-1α gene expression in chondrocytes indicated that HIF activity is necessary for proper chondrocyte Vegf production, proliferation, extracellular matrix deposition and maturation (75–78). Similarly, neuronal HIF-deficiency results in severely diminished CNS vascularization because of diminished Vegf production and impaired neural precursor cell proliferation (79,80). Importantly, hypoxic culture conditions have been shown to be able to protect cultured sympathetic neurons from growth factor withdrawal-induced cell death (81). Additionally, hypoxic culture conditions promote the in vitro maintenance of CNS precursors (82).
The related HIF-2α is also required for murine development. HIF-2α deficient embryos died in utero because of impaired catecholamine production and depressed cardiac output (83) suggesting that hypoxia-induced dopamine production helps regulate vascular tone. In an alternate genetic background, half of the embryos died in utero, although a significant fraction survived to term, only to succumb to a condition highly reminiscent of respiratory distress syndrome (84). In this case, impaired hypoxia-induced Vegf production in the lung prevented proper maturation of the alveolar epithelium, resulting in reduced surfactant production and subsequent perinatal demise because of respiratory distress. Finally, a third group witnessed a requirement for HIF-2α early postnatally because of impaired ROS scavenging abilities and disrupted metabolism (85). HIF-2α function is thus critical for a variety of developmental processes and its inhibition by exogenous oxygen administration in premature neonates may lie at the heart of many of the pathologies associated with preterm existence in humans.
Protection against hyperoxia.
These studies suggest that oxygen should be considered a developmental morphogen. Importantly, the biologic defenses against hyperoxia may not be as robust as against hypoxia given that during evolution no organisms would have been exposed to supraphysiological oxygen tensions. Although in some periods oxygen in the atmosphere exceeded the present 21%, it never was as high as can be produced by man. Protection against hyperoxia must therefore be left to the discretion of medicine itself, while hypoxia is quickly sensed and preventive measures at the cellular level are activated.
Still, the body does have some defense mechanisms against hyperoxia. During hyperoxia, antioxidant enzymes are up-regulated by mitogen-activated protein kinases. p38 Mitogen-activated protein kinase and Sarcoma kinase have been shown to regulate activation of NADP oxidase in endothelial cells exposed to hyperoxia. A number of transcription factors have also been shown to activate antioxidant protective mediators (86). The process of childbirth is accompanied by an increase in oxidative stress and this continues the first months of life (87). Its role for development is unclear, but it is known that ROS play a role in signal transduction and are essential for development (88).
Oxygen Targets in Newborn Babies
We have provided important evidence for a careful reconsideration of oxygen therapy in-term and preterm neonates (16,89). Based on considerations mentioned above as well as clinical and experimental evidence it is clear that mammals strictly regulate their oxygen environments. Unfortunately, this has not been reflected into clinical practice in which high oxygen levels have been imposed on term and preterm infants. Some of the negative consequences of these practices have been known for almost 60 y and some have only been revealed recently. It may seem that immediately after birth hyperoxia may be more deleterious than later in life. Perhaps oxygen is a programming factor acting via epigenetic mechanisms to alter physiologic processes. Exposure to hyperoxia in sensitive periods of development may thus have unforeseen long-term consequences.
Oxygen for newborn resuscitation.
It is known that oxygen levels are low in fetal life with saturations as low as 50% or even lower. After birth, arterial oxygen saturation (SaO2) increases slowly and reaches 90% with a median time of 5 minutes in normal healthy babies. However, levels as low as 40% are reported in normal newborn babies during the first 3-4 min of life. This indicates that a newborn baby is not supposed to be pink for several minutes. Recent meta-analyses of studies investigating the effects of resuscitation of-term or near-term newborns with either 21 or 100% O2 have consistently found that 21% resuscitated infants have a lower neonatal mortality, higher heart rate at 90 s, a higher 5 min Apgar score, and take their first breath approximately 30 s earlier than those resuscitated with 100% O2 (90). The optimal FiO2 for newborn resuscitation may not be known, however, it seems clear that the use of pure oxygen should be avoided or limited to as brief a period as possible (91).
Oxygenation of extremely low birth weight infants.
The optimal oxygen saturation for extremely low birth weight infants during the first weeks of life is still not defined. However, a number of studies have recently indicated that a high saturation, defined as higher than 93% or at least higher than 95% is detrimental to these children when compared with a lower saturation, defined as 88-93%, or even as low as 85%. Consistently these studies have shown that a high saturation leads to significantly more pulmonary problems. Most studies also show significantly more severe retinopathy of prematurity (ROP) in infants nursed with high saturations. Further, it seems to be important to reduce fluctuations in SaO2. By avoiding both high SaO2 and large fluctuations inSaO2 severe ROP and thereby ROP treatment is almost completely eradicated (92). Further, long-term follow-up does not show any detrimental effects of a low saturation regime (93). On the contrary, one study indicates that maintaining high oxygen saturation targets in these infants results in a reduced mental developmental index (94).
Today, there is, therefore, a trend worldwide to try to avoid hyperoxic peaks both for newborn resuscitation and in the maintenance care of extremely low birth weight infants. Still the optimal upper and lower limits of oxygenation need to be defined. Further, even if such limits should be described better it is still a long way to identify the needs of each individual infant. This may be especially challenging because these needs may differ from individual to individual, from organ to organ, and from situation to situation. Therefore, a more thorough understanding of the regulatory mechanisms at the cellular level for both hypoxia and hyperoxia are imperative. If oxygen sensors were identified, such sensors could be used to define the optimal oxygenation of each individual. Along these lines, global analyses of cellular and organismal responses to oxygen exposure involving genetic, epigenetic, proteomic, and metabolomic approaches should prove useful in guiding clinical decision making. Given the difficulties surrounding the institution of clinical trials designed to define optimal oxygen concentrations for term and preterm neonates, such studies should be highly prioritized to help improve outcomes in the intensive care nursery.
Abbreviations
- ARNT:
-
arylhydrocarbon receptor nuclear translocator
- GSH:
-
reduced glutathione
- GSSG:
-
oxidized glutathione
- HIF:
-
hypoxia-inducible factor
- PHD:
-
prolyl-4-hydroxylase
- PO2:
-
oxygen partial pressure
- ROP:
-
retinopathy of prematurity
- RNS:
-
reactive nitrogen species
- ROS:
-
reactive oxygen species
- SaO2:
-
arterial oxygen saturation
References
Brunelle JK, Chandel NS 2002 Oxygen deprivation induced cell death: an update. Apoptosis 7: 475–482
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ 1996 Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature 379: 88–91
Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J 2003 Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5: 741–747
Brocks JJ, Logan GA, Buick R, Summons RE 1999 Archean molecular fossils and the early rise of eukaryotes. Science 285: 1033–1036
Semenza GL 2007 Life with oxygen. Science 318: 62–64
Margulis L 1975 Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. Symp Soc Exp Biol ( 29): 21–38
Lane N 2002 Oxygen: The Molecule That Made the World. Oxford University Press, Oxford, 2002
Saugstad OD 1975 Hypoxanthine as a measurement of hypoxia. Pediatr Res 9: 158–161
Saugstad OD, Aasen AO 1980 Plasma hypoxanthine concentrations in pigs. A prognostic aid in hypoxia. Eur Surg Res 12: 123–129
McCord JM, Fridovich I 1968 The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 243: 5753–5760
Blomgren K, Hagberg H 2006 Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med 40: 388–397
Basuroy S, Bhattacharaya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H 2006 HO–2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-a in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908
Douglas RM, Haddad GG 2003 Effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J Appl Physiol 94: 2068–2083
Kulkarni AC, Kuppusamy P, Parinandi N 2007 Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal 9: 1717–1730
Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO 1954 Oxygen poisoning and X-irradiation: a mechanism in common. Science 119: 623–626
Saugstad OD 1988 Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res 23: 143–150
Babior BM, Kipnes RS, Curnutte JT 1973 Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52: 741–744
Granger DN, Rutili G, McCord JM 1981 Superoxide radicals in feline intestinal ischemia. Gastroenterology 81: 22–29
Saugstad OD 1985 Oxygen radicals and pulmonary damage. Pediatr Pulmonol 1: 167–175
Yoshida LS, Tsunawaki S 2008 Expression of NADPH oxidases and enhanced H(2)O(2)-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. Int Immunopharmacol 8: 1377–1385
Clyman RI, Saugstad OD, Mauray F 1989 Reactive oxygen metabolites relax the lamb ductus arteriosus by stimulating prostaglandin production. Circ Res 64: 1–8
Bernardi ML, Flechon JE, Delouis C 1996 Influence of culture system and oxygen tension on the development of ovine zygotes matured and fertilized in vitro. J Reprod Fertil 106: 161–167
Catt JW, Henman M 2000 Toxic effects of oxygen on human embryo development. Hum Reprod 15: 199–206
Eppig JJ, Wigglesworth K 1995 Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol Reprod Dev 42: 447–456
Li J, Foote RH 1993 Culture of rabbit zygotes into blastocysts in protein-free medium with one to twenty per cent oxygen. J Reprod Fertil 98: 163–167
Pabon JE Jr, Findley WE, Gibbons WE 1989 The toxic effect of short exposures to the atmospheric oxygen concentration on early mouse embryonic development. Fertil Steril 51: 896–900
Quinn P, Harlow GM 1978 The effect of oxygen on the development of preimplantation mouse embryos in vitro. J Exp Zool 206: 73–80
Thompson JG, Simpson AC, Pugh PA, Donnelly PE, Tervit HR 1990 Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil 89: 573–578
Umaoka Y, Noda Y, Narimoto K, Mori T 1992 Effects of oxygen toxicity on early development of mouse embryos. Mol Reprod Dev 31: 28–33
Fischer B, Bavister BD 1993 Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil 99: 673–679
Kaufman DL, Mitchell JA 1994 Intrauterine oxygen tension during the oestrous cycle in the hamster: patterns of change. Comp Biochem Physiol Comp Physiol 107: 673–678
Mitchell JA, Yochim JM 1968 Intrauterine oxygen tension during the estrous cycle in the rat: its relation to uterine respiration and vascular activity. Endocrinology 83: 701–705
Mitchell JA, Yochim JM 1968 Measurement of intrauterine oxygen tension in the rat and its regulation by ovarian steroid hormones. Endocrinology 83: 691–700
Rodesch F, Simon P, Donner C, Jauniaux E 1992 Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 80: 283–285
Yochim JM, Mitchell JA 1968 Intrauterine oxygen tension in the rat during progestation: its possible relation to carbohydrate metabolism and the regulation of nidation. Endocrinology 83: 706–713
Van Blerkom J, Antczak M, Schrader R 1997 The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 12: 1047–1055
Burton GJ, Jauniaux E, Watson AL 1999 Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 181: 718–724
Jaffe R, Jauniaux E, Hustin J 1997 Maternal circulation in the first-trimester human placenta—myth or reality?. Am J Obstet Gynecol 176: 695–705
Emmanouilides G, Allen H, Riemenschneider T, Gutgesell H 1995 Moss and Adams' Heart Disease in Infants, Children and Adolescents, 5th Ed. Williams and Wilkins
Nystul TG, Goldmark JP, Padilla PA, Roth MB 2003 Suspended animation in C. elegans requires the spindle checkpoint. Science 302: 1038–1041
Nystul TG, Roth MB 2004 Carbon monoxide-induced suspended animation protects against hypoxic damage in Caenorhabditis elegans. Proc Natl Acad Sci USA 101: 9133–9136
Padilla PA, Roth MB 2001 Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc Natl Acad Sci USA 98: 7331–7335
Haddad GG 2006 Tolerance to low O2: lessons from invertebrate genetic models. Exp Physiol 91: 277–282
Semenza GL 1998 Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 8: 588–594
Wang GL, Semenza GL 1995 Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270: 1230–1237
Wang GL, Jiang BH, Rue EA, Semenza GL 1995 Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514
Semenza GL 2001 HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13: 167–171
Semenza GL, Shimoda LA, Prabhakar NR 2006 Regulation of gene expression by HIF-1. Novartis Found Symp 272: 2–8; discussion 8–14, 33–16.
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC 2006 HIF-1 mediates adaptation to hypoxia by actively down-regulating mitochondrial oxygen consumption. Cell Metab 3: 187–197
Kim JW, Tchernyshyov I, Semenza GL, Dang CV 2006 HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185
Bruick RK, McKnight SL 2001 A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ 2001 C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr 2001 HIF-alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ 2001 Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472
Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS 2005 Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414
Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT 1998 Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT 2005 Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408
Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC 2005 Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1: 393–399
Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C 2000 ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 468: 53–58
Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT 1998 Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619–11624
Killilea DW, Hester R, Balczon R, Babal P, Gillespie MN 2000 Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 279: L408–L412
Wood JG, Johnson JS, Mattioli LF, Gonzalez NC 1999 Systemic hypoxia promotes leukocyte-endothelial adherence via reactive oxidant generation. J Appl Physiol 87: 1734–1740
Bell EL, Emerling BM, Chandel NS 2005 Mitochondrial regulation of oxygen sensing. Mitochondrion 5: 322–332
Sanjuan-Pla A, Cervera AM, Apostolova N, Garcia-Bou R, Victor VM, Murphy MP, McCreath KJ 2005 A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett 579: 2669–2674
Kozak KR, Abbott B, Hankinson O 1997 ARNT-deficient mice and placental differentiation. Dev Biol 191: 297–305
Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC 1997 Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386: 403–407
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL 1998 Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162
Ryan HE, Lo J, Johnson RS 1998 HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015
Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH 2006 Placental but not heart defects are associated with elevated hypoxia-inducible factor {alpha} levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26: 8336–8346
Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM 1997 Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA 94: 9102–9107
Adelman DM, Maltepe E, Simon MC 1999 Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev 13: 2478–2483
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ 1997 Regulation of human placental development by oxygen tension. Science 277: 1669–1672
Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E 2000 Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 14: 3191–3203
Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ 2005 Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development 132: 3393–3403
Pfander D, Cramer T, Schipani E, Johnson RS 2003 HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci 116: 1819–1826
Pfander D, Kobayashi T, Knight MC, Zelzer E, Chan DA, Olsen BR, Giaccia AJ, Johnson RS, Haase VH, Schipani E 2004 Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development 131: 2497–2508
Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS 2001 Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 15: 2865–2876
Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR 2004 VEGFA is necessary for chondrocyte survival during bone development. Development 131: 2161–2171
Milosevic J, Maisel M, Wegner F, Leuchtenberger J, Wenger RH, Gerlach M, Storch A, Schwarz J 2007 Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. J Neurosci 27: 412–421
Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, Gonzalez FJ, Takahama Y 2003 Defective brain development in mice lacking the HIF-1alpha gene in neural cells. Mol Cell Biol 23: 6739–6749
Xie L, Johnson RS, Freeman RS 2005 Inhibition of NGF deprivation-induced death by low oxygen involves suppression of BIMEL and activation of HIF-1. J Cell Biol 168: 911–920
Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R 2000 Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 20: 7377–7383
Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL 1998 The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 12: 3320–3324
Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P 2002 Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710
Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA 2003 Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35: 331–340
Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Penathur A, Crdounel AJ, Zweier JL, Garcia JG, Natarajan V 2003 Hyperoxia-induced NAD(P)H oxidase activation ad regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 284: L26–L38
Friel JK, Friesen RW, Harding SV, Roberts LJ 2004 Evidence of oxidative stress in full-term healthy infants. Pediatr Res 56: 878–882
Allen RG 1991 Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med 196: 117–129
Higgins RD, Bancalari E, Willinger M, Raju TN 2007 Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics 119: 790–796
Saugstad OD 2007 Optimal oxygenation at birth and in the neonatal period. Neonatology 91: 319–322
Saugstad OD 2008 To oxygenate or not to oxygenate. That is the question. Am J Physiol Heart Circ Physiol 295: H1371–H1372
Chow LC, Wright KW, Sola A, CSMC Oxygen Administration Study Group 2003 Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants?. Pediatrics 111: 339–345
Tin W, Milligan DW, Pennefather P, Hey E 2001 Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed 84: F106–F110
Deulofeut R, Critz A, Adams-Chapman I, Sola A 2006 Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. J Perinatol 26: 700–705
Author information
Authors and Affiliations
Corresponding author
Additional information
The Role of Oxygen in Health and Disease - A Series of Reviews
This is the first article in a series of reviews focusing on the role that oxygen plays in health and disease. In this review, Drs. Maltepe and Saugstad examine mechanisms responsible for maintaining oxygen homeostasis and the consequences of dysfunction leading to oxidative stress, a disturbance in the pro-oxidant–antioxidant balance and subsequent tissue damage. Premature exposure to high oxygen levels influences many developmental events with potentially life-long effects.
Petra S. Hüppi, M.D.
European Chief Editor
Rights and permissions
About this article
Cite this article
Maltepe, E., Saugstad, O. Oxygen in Health and Disease: Regulation of Oxygen Homeostasis-Clinical Implications. Pediatr Res 65, 261–268 (2009). https://doi.org/10.1203/PDR.0b013e31818fc83f
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31818fc83f
This article is cited by
-
Generation of transient and tunable oxygen gradients in microfluidic channels utilizing the oxygen scavenging properties of thiol-ene polymers
Microfluidics and Nanofluidics (2022)
-
Aging alters gastrocnemius muscle hemoglobin oxygen saturation (StO2) characteristics in healthy individuals
European Journal of Applied Physiology (2022)
-
Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration
Stem Cell Research & Therapy (2021)
-
Cold bubble humidification of low-flow oxygen does not prevent acute changes in inflammation and oxidative stress at nasal mucosa
Scientific Reports (2021)
-
The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing
Tissue Engineering and Regenerative Medicine (2021)