Abstract
Modulation of the intestinal immune response early in life by administration of probiotic bacteria may be an effective strategy for preventing or attenuating infectious diarrhea. We preinoculated the mice early in life with the probiotic bacteria Lactobacillus acidophilus NCFM (La) at age 2 wk. Dendritic cells (DCs) were collected and purified from mesenteric lymph nodes (MLN) and spleens of the BalbC/ByJ mice. DC isolation and adoptive transfer was used to examine the function of probiotics. We demonstrated that when mice were adoptively transferred with La-primed DCs (t-LaDC) instead of oral consumption with La, there was a similar effect on fecal bacteria counts, IgA levels, and colonic histopathology, as well as cytokine levels in MLN when there was intestinal bacterial infection. The above findings suggest that DCs play a key role in probiotics attenuating Citrobacter rodentium (Cr) colitis. Moreover, the location of La-primed DC hints that there is interaction of DCs and T cells in the digestive system of the host. Up-regulated expression of a surface marker on DCs indicated that inoculation with probiotics will stimulate the function of DCs, thereby further increasing immune response triggered by DC.
Similar content being viewed by others
Main
The use of probiotics is considered a potentially important strategy to modulate infectious and inflammatory responses in the gastrointestinal tract of the host. The inhibitory effects of probiotics on intestinal pathogenic bacterial infections, such as Shigella (1), Salmonella (2), and enterohemorrhagic Escherichia coli (EHEC) (3) in the murine model, and enteropathogenic E. coli (EPEC) in the piglet model (4) has been reported. Previous studies (5–7), including our own, also provide evidence showing a beneficial effect of probiotics on Citrobacter rodentium (Cr) infection in mice.
Introduction of Lactobacillus or Bifidobacterium species into the gastrointestinal tract of mice restores mucosal adherent probiotic populations by producing immunoregulatory factors that may enhance colonization or survival in the host (8) and reduces the infectious and inflammatory responses in these animals. Lactobacillus acidophilus NCFM (La), a probiotic strain that survives gastrointestinal tract transit in both healthy and diseased populations and reduces the incidence of pediatric diarrhea (9).
C. rodentium (Cr), the only known murine attaching and effacing (A/E) pathogen, has served as a model for intestinal infections in small animals, which is similar to EPEC and EHEC in infantile diarrhea of humans. The formation of A/E lesions is central to the pathogenesis of EPEC-mediated disease in humans and Cr-mediated transmissible colonic hyperplasia in mice (10). These bacteria intimately attach to host intestinal cells, causing the effacement of brush border microvilli, induces a Th1-type immune response (11).
Although various studies have been carried out to investigate the mechanisms underlying the immunoregulatory effect of the intestinal flora, relatively little attention has been focused on their effect on dendritic cells (DCs), the most potent antigen-presenting cells (APC), and the principal stimulators of naive Th cells (12). DCs are distributed in most tissues and at sites that interface with the external environment, such as the mucosa of the gastrointestinal tract, and these cells can be modulated by intestinal normal flora and those administered orally such as probiotics. DCs reside in Peyer's patch, lamina propria (LP), and mesenteric lymph nodes (MLN) of the gastrointestinal system. It has been widely suggested that the delicate balance between Th1 and Th2 immunity, as well as tolerance (Th3), is pivotally controlled by stimulated DCs (13). It appears reasonable that the intestinal flora, including probiotics, may exert immunoregulatory effects through modulation of the Th1/Th2/Th3-promoting capacity of DCs in the gut.
Current study investigated the effects of probiotic inoculation in attenuating Citrobacter-associated colitis in the murine model, and explored the role of DCs in the modulation of host responses using an adoptive transfer approach involving transfer of La-primed DCs (LaDC). Our results show that La inoculation induces intestinal DC activation and adoptive transfer of probiotics-primed DC results in an enhancement of host protection against Cr infection and attenuation of Citrobacter-associated colitis.
MATERIALS AND METHODS
Mice and probiotics bacteria inoculation.
Six- to 8-wk-old female and male BALBc/ByJ mice were purchased from the National Animal Center (Taipei) and bred in the animal room of Chang Gung Memorial Hospital. This study was approved by Chang Gung Memorial Hospital Institutional Animal Care and Use Committee. The mice were fed autoclaved food and water and maintained in the facility. Neonatal mice were born to pregnant female Balbc/ByJ mice. Some of the neonatal mice were orally inoculated with La once per week beginning at 2 wk of age for 4 wk. Obtained from frozen stocks (−80°C), La were inoculated in deMan, Rogosa, and Sharpe broth (MRS; Difco) and grown at 37°C for 20 h, then resuspended in phosphate-buffered saline (PBS) before colonizing the mice (0.25 mL/mouse for 2-wk old mice, approximately 5 ×108 CFU of La).
C. rodentium (Cr) infection and antigen preparation.
Mice were orally inoculated with Cr (strain DBS 100, ATCC) at age 6 to 8 wk. Bacteria were grown overnight in Luria broth (LB) and resuspended in PBS before being used to infect the mice (0.5 mL/mouse, approximately 5 × 108 CFU of Cr). Cr antigen was prepared by collecting culture of Cr overnight in LB. The bacteria antigen was used for the cell culture of MLN and antibody assays with ELISA.
DCs isolation and adoptive transfer.
Spleens and MLNs from probiotics preinoculated mice (4 wk-post-La inoculation) and normal control mice (age-matched BALB/c) were collected aseptically into complete Dulbecco's modified Eagle's medium (DMEM). The tissues were digested with collegenase (type IV, Worthington Biochemical) at 200 U/mL for 30 min at room temperature. The low-density fraction of the cell suspension was obtained by gradient centrifugation in an Optiprep gradient (Invitrogen Life). DCs were purified by positive selection over a magnetic cell-sorting column (MACS) using microbeads-conjugated anti-CD11c MAb (Miltenyi Biotec). After checking the purity, the fresh purified DCs (2 × 106 cells/mouse) were adoptively transferred (via tail vein injection) into age-matched normal BALB/c recipients.
Experimental design.
Group A were infected with Cr at age 6 to 8 wk without any La inoculation. Group B were adoptively transferred with La-primed DCs (t-LaDC) at age 6 to 8 wk, and then infected with Cr the next day. Group C were adoptively transferred with normal mice DCs (t-NrDC) at age 6 to 8 wk, and infected with Cr the next day. Group D were preinoculated with La (pre-La) weekly beginning at age 2 wk and were then infected with Cr at age 6 to 8 wk. Group E were preinoculated with La weekly beginning at age 2 wk. Group F were normal controls without extra bacteria colonization. All mice were killed 14 d after Cr infection. To assess the systemic effect of Cr infection and colonization by probiotic strains, body weight and survival of the mice were measured throughout the experiment period.
Quantitation of C. rodentium.
To assess the clearance of Cr, fecal pellets were collected from each mouse weekly over the course of the experiment. The fecal pellets were weighed, homogenized, and serially diluted and plated on selective MacConkey agar. After overnight incubation at 37°C, bacteria colonies were counted as described previously (6).
Fecal IgA antibody assays.
Fecal pellets were collected from each mouse 2 wk after Cr infection. The fecal pellets were put into a protease inhibitor cocktail immediately after collection, then weighed, homogenized, and incubated at 4°C for 1 h. Immuno II plates were coated with goat anti-mouse IgA (2 μg/mL) or Cr antigen (50 μg/mL) and incubated overnight at 4°C. Total IgA and antigen-specific IgA were detected as described previously (6).
Histopathological examinations.
At necropsy, colonic tissues were removed and flushed with cold PBS to remove fecal contents. Colonic tissues were then cut into small pieces, frozen in tissue Tek OCT compound (Miles.), and stored at −80°C. Five-micrometer sections were cut on a 2800 Frigocut cryostat (Reichert-Jung) and stained with hematoxylin and eosin. The sections were analyzed without prior knowledge of the type of treatment. Colonic pathology was scored according to a modified histology scoring system based on previously published methods (6,14).
Immunofluorescence microscopy.
To analyze the location and the abundance of DCs, some mice were killed on day 10 after adoptive transfer with DCs. Colonic tissues and MLN were frozen in Tissue-Tek OCT compound as described. The tissue sections were incubated with different antibodies. To examine intestinal CD4+T cells, the sections were stained with anti-mouse CD4 fluorescein isothiocyanate (FITC) (BD Pharmingen). To study DCs, the sections were stained with biotin-labeled anti-mouse CD11c (Biolegend) or anti-mouse CD11c FITC (BD Pharmingen). After washing in PBS, tissue sections were incubated with streptavidin-Cy3 (Cedarlane Laboratories). All sections were analyzed by immunofluorescence microscopy.
Isolation and purification of lamina propria dendritic cells.
Each colon of BALB/c mice was flushed with Hanks' balanced salt solution (HBSS), cut longitudinally, and the gut epithelium removed from the LP as described (15). After filtering, tissue was digested in a solution of collagenase D and DNase. The suspension containing released lamina propria mononuclear cells (LPMC) was filtered, collected, and CD11c+LPMC were fractionated with MACS columns (Miltenyi Biotec). The numbers of LPMC and CD11c+LPMC were counted. T-cell subpopulation (CD4, CD8, and CD45RB) and activation (CD44, CD69) were analyzed by fluorescence activated cell sorter (FACS), and activation and maturation of DCs [including CD11c, CD80, CD86, CD40, and MHC-II (I-Ad)].
Measurement of cytokine production.
After mice being killed, lymphocytes were isolated from MLN of mice. These cells were cultured with Cr (50 μg/mL) and the culture supernatants were collected 48 h later. Culture supernatants were collected for the assessment of IFN-γ, IL-10, and IL-12p70 cytokine production by using ELISA as described previously (6).
Cell surface phenotype expression.
DCs were isolated from spleens and MLN from probiotics preinoculation and normal control mice. Cells were stained using a panel of monoclonal antibodies (MAb) directed against CD11c (FITC) together with one of the following: CD80, CD86, CD40, MHC-II (I-Ad), CCR6, or CCR7 [all phycoerythrin (PE) conjugated; PharMingen]. Cells were acquired (at least 10,000 events for MLN, approximately 30,000–50,000 events for spleen) using a FACScan (Becton Dickinson) and analyzed with Cell Quest software.
Analysis on mRNA expression of chemokines and chemokine receptors using RT-PCR.
Total RNA was isolated from various organs and DCs (MLN, spleen, and LP of colon) using RNAzol (Life Technologies) and was used for cDNA synthesis. The cDNAs were used as templates for PCR using specific primers for mouse CCR7 (forward:5′-ACAGCGGCCTCCAGAAGAAGAGCGC; reverse:5′-TGACGT-CATAGGCAATGTTGAGCTG), SLC (forward:5′-CAACCACAACCATGGCTC; reverse:5′-GGCGGGATCCTGGGCTAT), and CXCR4 (forward:5′-GGTCTGGAGACTATGACTCC-3′, reverse:5′-CACAGATGTACCTGTCATCC-3′). Specific primers and probes for mouse CCR6 and ß-actin were purchased from Applied Biosystems. The results were normalized to ß-actin expression.
Statistical analysis.
All the results are expressed as the mean ± standard error of the mean (SEM). n refers to the number of mice used. Statistical differences were determined using the two-tailed t test or one-way analysis of variance (ANOVA) test with GraphPad Prism (GraphPad software). A p value of <0.05 was considered significant.
RESULTS
Probiotic La preinoculation and adoptive transfer of probiotic-primed DCs reduces susceptibility of mice to Cr infection.
As expected, mice infected with Cr showed signs of Citrobacter-associated disease such as soft stool, a hunched posture, disturbed body hair, and body weight loss early during the infection. In contrast, mice that received pre-La developed less severe disease and little weight loss during the Cr infection. Furthermore, as mice with inoculation with La, t-LaDC resulted in an attenuated body weight loss during the course of infection. These results are compatible with our previous report (6).
Probiotic La preinoculation and probiotic primed-DC adoptive transfer results in attenuated Citrobacter-associated colonic pathology.
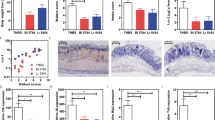
On the end of our experiments, mice from different groups were killed and the colons were examined both macroscopically and microscopically. Figure 1 shows the results of histopathological analysis of distal colon. Histologic analysis of colonic tissue of mice that were t-LaDC+Cr (Fig. 1C), or mice pre-La+Cr (Fig. 1D) showed a significantly attenuated Cr-induced colitis. These results suggest that the attenuated colonic histopathology caused by Cr may involve the effects of probiotics La on DC (Fig. 1G).
Histologic pathology of the distal part of the colon, magnification ×100. The different groups are described as Cr-alone (A), t-NrDC+Cr (B), t-LaDC+Cr (C), pre-La+Cr (D), pre-La alone (E), and normal control (F). Histologic analysis of colonic tissue in t-LaDC+Cr (C) and Pre-La+Cr (D) showed a less severe pathology compared with Cr-alone (A). The colonic pathology score (G) of mice at 2 wk after Cr infection showed measurements of individual mice pooled from three independent experiments (n = 12–15 mice, each group). The horizontal line represents the mean score of different groups. Data were analyzed using one-way analysis of variance (nonparametric) *p < 0.05, §p > 0.05.
Preinoculation with La and La-primed DC transfer results in lower Cr output in the fecal pellets.
Our results showed that the bacterial output was lower in the mice pre-La, which is in line with our previous report (6) and the mice t-LaDC (Fig. 2A) at an early stage of infection (1 wk-postinfection), indicating that La preinoculation or immune alterations in intestinal mucosa induced by La-primed DC may inhibit the colonization of Cr on the colonic epithelial surface. Furthermore, we observed that the bacterial shed was significantly lower in mice of both pre-La and t-LaDC groups at 2 wk-post-Cr infection (Fig. 2B), suggesting that t-LaDC may cause a similar effect as pre-La, resulting in an inhibition of Cr proliferation and facilitating pathogenic bacteria clearance in these mice.
Fecal Cr counts in selective MacConkey agar plate. The data shown are the number of Cr recovered from per gram fecal samples of Cr-alone (black bar), t-NrDC+Cr (white bar), t-LaDC+Cr (black bar), Pre-La+Cr (white bar), pre-La alone and healthy control (no bacteria colony was found) at 1 wk (A) and 2 wk (B) post-Cr infection. Pre-La and t-LaDC results in lower Cr output at 2-wk (B) post-Cr infection. The data are represented as the mean ± SEM (n = 8–10 at each time point), and statistical significant differences compared with Cr-alone *p < 0.05.
La preinoculation and adoptive transfer with La-primed DC provides protective immune response by stimulating the host to produce higher IgA in the intestinal lumen and enhance protective bacterial-antigen-specific immune responses.
We then determined whether the observed beneficial effects of probiotics and transferred probiotics-primed DC on host protection against enteric bacterial pathogen are associated with an increased intestinal antibody response. Our results showed that mice with pre-La alone, pre-La+Cr, and t-LaDC+Cr had higher total IgA levels compared with the other groups (Fig. 3A). Furthermore, in the four groups with Cr infection, the mice pre-La or t-LaDC had significantly higher levels (>2 times, p < 0.05) of anti-Cr specific IgA than the mice with Cr infection alone (Fig. 3B). These results, therefore, provide evidence to suggest that pre-La or t-LaDC may enhance protective immunity of host by stimulating an increased amount of intestinal IgA production.
IgA level and cytokines were measured by ELISA. The data shown are the values of IgA (ng/g feces) in Cr-alone (black bar), t-NrDC+Cr (white bar), t-LaDC+Cr (black bar), pre-La+Cr (white bar), pre-La alone (black bar), and control (white bar) collected at 2-wk post-Cr infection. The mice pre-La or t-LaDC produced significantly higher level of total IgA (A) and anti-Cr IgA (B) than Cr-alone. MLN cells were cultured with Cr antigen (50 μg/mL) and the culture supernatants were collected 48 h later. Cytokines IFN-γ (C), IL-10 (D), and IL-12p70 (E) in culture supernatants of different groups (2-wk post-Cr infection) were determined by ELISA. The data show that pre-La and t-LaDC induced higher IFN-γ, IL-10, and IL-12 production. The results are demonstrated as the mean ± SEM. Statistical significant differences compared with the Cr-alone (*p < 0.05, n = 10 mice per group).
To examine the influence of colonizing La on cytokine response in the intestinal mucosa, we examined antigen-specific cytokine production of MLN by collecting lymphocytes from MLN and restimulating in vitro with bacterial antigen. Our results revealed that pre-La and t-LaDC resulted in an enhanced Cr antigen-specific IFN-r (Fig. 3C), IL-10 (Fig. 3D), and IL-12p70 (Fig. 3E) production in mice. This data indicates the impact of probiotic treatment and DC that were activated by probiotics on immune modulation of mucosal cytokine responses during enteric bacterial infection.
Probiotics La treatment results in up-regulation of DCs costimulatory molecules.
Modulation of DC activation status may result in functional alterations in DCs. To determine the action of the La on DCs, young mice (2-wk old) started receiving weekly probiotic inoculation. Mice were killed at 4 wk after probiotic inoculation (6-wk old). MLN and spleen were collected, and cells were stained to evaluate the frequency and activation of DCs. Our results showed that pre-La resulted in a significant expansion of CD11c+cells with an up-regulation of CD11c, CD80, CD86, CD40, and MHC-II (Fig. 4A and B). The enhancement in DC activation markers may suggest functional maturation of these cells. The above results suggested that pre-La might induce DC expansion and activation, contributing to an enhanced protective immune response of the host. Pre-La also resulted in significant higher CCR6 expression on DC from MLN, but not CCR7. As well, isolated DC from spleen of La-primed mice showed a trend toward expression than control, both on CCR6 and CCR7 (Fig. 4C). The above finding suggested that some population of isolated DC from MLN and spleen might migrate to the intestine after adoptive transfer.
La differentially up-regulate CD11c, CD80, CD86, CD40, and MHC-II expression on DC. (A) DC isolated from MLN of the BALBc/ByJ mice (6-wk old), and CD11c, CD40, and MHC-II expression were up-regulated in the mice pre-La since 2-wkold. (B) Histograms show flow cytometry results for CD11c, CD80, CD86, CD40, and MHC-II expression on MLN-derived DC (upper row) and DC isolated from spleen (lower row) of the BALBc/ByJ mice (6-wkold), which pre-La since 2-wk old (thick black line) in comparison with unstimulated DC of control mice (filled histograms with gray color). Curves are normalized to the values for unstimulated cells. The data are representative of two independent experiments, n = 10 mice per group. (C) Histograms show CCR7 and CCR6 expression on isolated DC from MLN and spleen of pre-La mice since 2 wk of age (thick black line) in comparison with unstimulated DC of control mice (filled histograms with gray color). The expression of mRNA (CCR7, CCR6) is also compared by RT-PCR. The data shown are mice pre-La since 2 wk of age (black bar), and unstimulated DC of control mice (gray bar).
Adoptive transfer with La-primed DC enhanced CD11c+DC infiltration in colonic tissue and MLN.
We next sought to explore the bases for such effects by examining how transferred DCs may alter colonic immune cells. Colonic tissue and MLN were removed from the mice t-LaDC or t-NrDC on day 10 after cells were transferred. We found that the counts of DCs in MLN of mice t-LaDC (Fig. 5A and C) were higher than those in mice t-NrDC (Fig. 5B and D). We also found that the counts of DCs in the colonic tissue of mice t-LaDC (Fig. 6A and C) were higher than those in mice t-NrDC (Fig. 6B and D). We isolated cells from LP of colon, and analyzed cell counts of LPMC:6.5 ± 1.1 × 106 cells/mouse in mice t-LaDC and 6.2 ± 0.9 × 106 cells/mouse in mice t-NrDC (n = 10). Moreover, higher cell counts of CD11c+DC from LPMC were detected in mice t-LaDC (5.6 ± 0.7 × 105 cells/mouse), compared with those in mice t-NrDC (3.2 ± 0.6 × 105 cells/mouse). T-cell subpopulations and activation, as well as DC activation and maturation were analyzed by FACS. There was no significant difference on the expression of CD4, CD8, and CD45RB, but t-LaDC up-regulated the expression of CD44 and CD69. The recipient mice of t-LaDC had higher expression on CD11c, CD80, CD86, and MHC-II (I-Ad), indicating enhancement of DC activation and maturation (Fig. 6E).
t-LaDC enhanced CD11c+DC infiltration in MLN, magnification ×400. DCs were purified from La-inoculated (4 wk after inoculation per week) or normal mice, and adoptively transferred to normal BALB/c mice. The recipient mice were killed 10 d later. The MLN tissue sections were stained with anti-CD11c FITC (green). We found the counts of CD11+DCs in the mice t-LaDC (A, C) are higher than those in the mice t-NrDC (B, D).
t-LaDC enhanced CD11c+DC infiltration in colonic tissue. Under the same experiment protocol, the colonic tissue sections were stained with anti-CD4 FITC (green) and anti-CD11c-biotin/streptavidin-Cy3 (red). We found the counts of CD11c+DCs in the mice t-LaDC (A, C) are higher than those in the mice t-NrDC (B, D). Magnification ×400. (E) Histograms show flow cytometry result (on isolated cells from LP) from the mice t-LaDC (thick black line) in comparison with the mice t-NrDC (filled histograms with gray color), n = 10. (F) The expression of chemokines and chemokine receptors (CCR7, CCR6, CXCR4, and SLC) are compared by RT-PCR. The data shown are mice pre-La (black bar), or without La (gray bar), n = 10.
DC infiltration in colonic tissue was assessed in mice preinoculated with or without La treatment for 4 wk followed by Cr challenge later. These mice were killed 1 wk post-Cr challenge and colonic tissues were homogenized for RNA isolation. The expression of chemokines recruiting DCs (such as CCR7, CCR6, CXCR4, and SLC) was up-regulated in the mice pre-La (Fig. 6F), indicating more DCs infiltrating the tissues during infection when pretreated with probiotics. These results suggest that inoculation of La may drive the mucosal immune response of the host, stimulating the proliferation or infiltration of CD11c+DC in digestive system, such as LP of colon and MLN.
DISCUSSION
DCs are important APCs in the generation of immune responses. The functions of DC in the gut may be modulated by microorganisms including the normal flora, those administered orally such as probiotics and pathogens. A recent report demonstrated that Lactobacillus group could regulate DC surface expression and cytokine production (16). High doses of probiotics treatments resulted in increased surface expression of co-stimulatory molecules CD80 (B7-1), CD86 (B7-2), CD40, and MHC-II I-Ad (17). In line with these observations, the results of our study revealed that pre-La-induced maturation and activation of DC, as evidenced by significant up-regulation of costimulatory molecules (Fig. 4), suggesting that La can act as an efficient immune modulator and stimulate productive T-cell response against enteric bacterial pathogens. In the current study, we further show that t-LaDC into normal BALB/c mice then infected with Cr produce a significant enhanced host protective mechanism against enteric bacterial infection, resulting in a decrease in the bacterial load, an increase in mucosal IgA response, and the attenuation of bacteria colitis. These observations are similar to the results from mice pre-La and infected with Cr. The results suggest that DC priming by probiotics play the key role in the immune modulation of Cr colitis.
Lactobacillus species have been shown to activate monocytes and macrophages, which are important in antigen processing, presentation, and activation of the antigen-specific immune response. The functional nature of APCs, in particular DCs, can have important impacts on Th cell polarization. IL-12 together with IFN-γ typically shifts immunity toward the type-1 response characterized by enhanced IFN-γ secretion. The type-1 response, which supports the development of cell-mediated and cytotoxic immunity, is required for host protection against Cr infection. The results from the current study revealed that pre-La or t-LaDC results in elevated Cr-specific IL-12 and IFN-γ response of MLN cells, suggesting that La may stimulate the induction and development of Th1 response, contributing to a reduced Cr infection and consequently, an attenuated bacteria-mediated colitis.
Interestingly, we also observed that La inoculation or t-LaDC lead to an increase in IL-10 response. It is possible that probiotics will induce both regulatory and Th1-polarizing cytokine production of MLN cells. This hypothesis is supported by previous reports showing that B. bifidum, B. infantis, and Lc. lactis reduced production of Th2 cytokines and were potent inducers of IL-10 production in peripheral blood mononuclear cells (PBMC) (18). Moreover, it has been shown that PBMC and cord blood-derived monocytes produce IL-6, TNF-α, IL-12, and IL-10 in response to commensal Gram-positive bacteria (19). It was also shown that DCs matured by B. bifidum exposure are able to stimulate CD4+T cells to produce significantly increased amount of IL-10 compared with maturation factors-stimulated DCs (20). Our results provide evidence to indicate that La may activate DC and prime T cells for the development of Th1 and/or regulatory T cells (Th3 or Tr1). However, future study will be necessary to elucidate the precise mechanisms by which probiotics alter DC function, contributing to the induction and development of T cell responses.
The inoculation of probiotics has been shown to increase the antibody response to gut pathogens. We show in the current study that probiotics inoculation results in an increase in both total and Cr-specific IgA response. This is in line with previous reports. Significantly, higher intestinal antibacterial IgA responses were recorded in probiotics-fed mice. Mice that were fed Bifidobacteria for 12 d showed significantly higher levels of fecal total IgA compared with controls (21). Importantly, in the current study, we observed an enhanced mucosal IgA response in mice t-LaDC, providing evidence to suggest that probiotics may enhance local IgA production in intestinal mucosa through effects on DC, contributing to an increase in host protective immunity against enteric bacterial infection. Intestinal IgA, besides providing protection against bacterial, parasitic and viral mucosal pathogens, plays a key role in selection and maintenance of a spatially diversified gut bacterial community (22).
We demonstrated that t-LaDC+Cr has similar effects on fecal bacteria output, mucosal IgA level response, cytokine production, and colonic histopathology compared with mice pre-La+Cr. These results suggest that DC play the key role in probiotics inhibiting Cr colitis. In addition, in the current study, we found the counts of DC in colonic tissue and MLN of the mice t-LaDC are higher than those in the mice t-NrDC. Higher CD11c+DC in LP, and higher activation and maturation of DC as well as T cell activation in LP of the mice t-LaDC are seen. These results suggest that La may stimulate the expansion and/or recruitment of DC in digestive system (MLN and colonic tissue), contributing to an enhanced antigen-presentation at the intestinal mucosa, promoting mucosal T cell immune response to pathogenic bacteria in the intestinal tract.
Our results also show that pre-La significantly altered the dynamics of Cr infection in the colon. Cr resides primarily in an extracellular location of the epithelial surface and in the LP or edematous submucosa (16). The mice pre-La had lower levels of Cr colonization, inhibited proliferation, and in addition, may facilitate bacterial clearance. The results of our experiment with colonic bacterial flora are also consistent with the finding that the pathogenic bacteria count was reduced in probiotic-colonized rats (23). In addition, our study demonstrates that t-LaDC results in enhanced host defense as evidenced by decreased bacterial loads and attenuated colonic histopathology in murine infectious colitis.
In summary, our results provide evidence to suggest that probiotic bacteria may have a direct effect on the functional capacity of DC, modulating mucosal T cell responses to enteric bacterial pathogens. We show that t-LaDC results in an enhanced mucosal IgA production, reduced bacterial loads, an increased mucosal IL-12, IL-10, and IFN-γ response, and attenuation of bacterial colitis. A better understanding of immunoregulation in the intestinal mucosa by probiotics may provide important information for establishing more effective preventive and therapeutic approaches to the treatment of both immune-mediated and infectious diseases.
Abbreviations
- Cr :
-
Citrobacter rodentium
- DCs:
-
dendritic cells
- La NCFM:
-
Lactobacillus acidophilus
- LP:
-
lamina propria
- MLN:
-
mesenteric lymph nodes
- pre-La,:
-
preinoculated with Lat-LaDC
- t-LaDC,:
-
adoptively transferred with La-primed DCs
- t-NrDC:
-
adoptively transferred with normal mice DCs
References
Nader de Macias ME, Apella MC, Romero NC, Gonzalez SN, Oliver G 1992 Inhibition of Shigella sonnei by Lactobacillus casei and L. acidophilus. J Appl Bacteriol 73: 407–411
Hudault S, Lievin V, Bernet-Camard MF, Servin AL 1997 Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol 63: 513–518
Shu Q, Gill HS 2002 Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol 34: 59–64
Bomba A, Nemcova R, Kastel R, Herich R, Pataky J, Cizek M 1996 Interactions of Lactobacillus spp. and enteropathogenic Escherichia coli under in vitro and in vivo conditions. Vet Med (Praha) 41: 155–158
Varcoe JJ, Krejcarek G, Busta F, Brady L 2003 Prophylactic feeding of Lactobacillus acidophilus NCFM to mice attenuates overt colonic hyperplasia. J Food Prot 66: 457–465
Chen CC, Louie S, Shi HN, Walker WA 2005 Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res 58: 1185–1191
Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, O'Kusky JR, Buchan AM, Jacobson K 2008 Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol 294: G295–G306
Wilson M, Seymour R, Henderson B 1998 Bacterial perturbation of cytokine networks. Infect Immun 66: 2401–2409
Sanders ME, Klaenhammer TR 2001 Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionally as a probiotic. J Dairy Sci 84: 319–331
Frankel G, Phillips AD, Novakova M, Field H, Candy DC, Schauer DB, Douce G, Dougan G 1996 Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect Immun 64: 5315–5325
MacDonald TT, Frankel G, Dougan G, Goncalves NS, Simmons C 2003 Host defences to Citrobacter rodentium. Int J Med Microbiol 293: 87–93
Banchereau J, Steinman RM 1998 Dendritic cells and the control of immunity. Nature 392: 245–252
Kronin V, Hochrein H, Shortman K, Kelso A 2000 Regulation of T cell cytokine production by dendritic cells. Immunol Cell Biol 78: 214–223
Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K 2001 Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology 121: 1428–1436
Drakes ML, Blanchard TG, Czinn SJ 2005 Colon lamina propria dendritic cells induce a proinflammatory cytokine response in lamina propria T cells in the SCID mouse model of colitis. J Leukoc Biol 78: 1291–1300
Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT 1999 Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun 67: 3031–3039
Drakes M, Blanchard T, Czinn S 2004 Bacterial probiotic modulation of dendritic cells. Infect Immun 72: 3299–3309
Niers LE, Timmerman HM, Rijkers GT 2005 Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type-2 cytokines. Clin Exp Allergy 35: 1481–1489
Karlsson H, Hessle C, Rudin A 2002 Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun 70: 6688–6696
Niers LE, Hoekstra MO, Timmerman HM, van Uden NO, de Graaf PM, Smits HH, Kimpen JL, Rijkers GT 2007 Selection of probiotic bacteria for prevention of allergic diseases:immunomodulation of neonatal dendritic cells. Clin Exp Immunol 149: 344–352
Fukushima Y, Kawata Y, Mizumachi K, Kurisaki J, Mitsuoka T 1999 Effect of bifidobacteria feeding on fecal flora and production of immunoglobulins in lactating mouse. Int J Food Microbiol 46: 193–197
Suzuki K, Ha SA, Tsuji M, Fagarasan S 2007 Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Semin Immunol 19: 127–135
Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G 2004 Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Dig Dis Sci 49: 320–327
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by Chang Gung Memorial Hospital Research Project Grant 34010 and National Science Council of Taiwan Grant NSC 95-2314-B-182A-108.
Rights and permissions
About this article
Cite this article
Chen, CC., Chiu, CH., Lin, TY. et al. Effect of Probiotics Lactobacillus acidophilus on Citrobacter rodentium Colitis: The Role of Dendritic Cells. Pediatr Res 65, 169–175 (2009). https://doi.org/10.1203/PDR.0b013e31818d5a06
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31818d5a06
This article is cited by
-
Lactobacillus delbrueckii subsp. lactis (strain CIDCA 133) stimulates murine macrophages infected with Citrobacter rodentium
World Journal of Microbiology and Biotechnology (2017)
-
Recombinant Lactobacillus plantarum expressing HA2 antigen elicits protective immunity against H9N2 avian influenza virus in chickens
Applied Microbiology and Biotechnology (2017)
-
Cross-protective efficacy of dendritic cells targeting conserved influenza virus antigen expressed by Lactobacillus plantarum
Scientific Reports (2016)
-
The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models
Nature Reviews Microbiology (2010)