Abstract
This prospective study investigates whether amino terminal proB-type natriuretic peptide (NT-proBNP) levels are elevated in neonates with congenital heart defect (CHD). NT-proBNP levels in the umbilical cord blood of 60 neonates with prenatally diagnosed CHD, in the cord blood of 200 control subjects, and in the plasma of the respective mothers were analyzed using an automated enzyme immunoassay. NT-proBNP concentrations in the cord blood of the CHD group were significantly elevated compared with controls [median (range): 158 pg/mL (378–3631 pg/mL) and 626 pg/mL (153–2518 pg/mL); p value <0.001]. The NT-proBNP concentrations of the newborns and their mothers did correlate neither in the CHD nor in the control group. In 54 patients with CHD, NT-proBNP levels were measured on the median 5th day of life. They showed a significant increase (median 1665 pg/mL and 19222 pg/mL; p < 0.001). NT-proBNP levels in the cord blood of neonates with CHD are significantly elevated and show a marked increase in the first week of life. Furthermore, this study confirms previously published reference intervals of NT-proBNP in the cord blood of healthy neonates as well as the finding that there is no exchange of NT-proBNP across the placenta.
Similar content being viewed by others
Main
Brain-type natriuretic peptide (BNP) is a natriuretic hormone secreted from the cardiac ventricular myocytes in response to an increase in ventricular wall stretch and filling pressures (1). Furthermore, hypoxia has been shown to be a direct stimulus for secretion of BNP in human cardiac myocytes (2). On secretion from the cardiac myocyte, pro-BNP, the inactive precursor, is cleaved into the biologically active BNP and the amino terminal fragment (NT-proBNP). Several studies have shown an excellent correlation between BNP and NT-proBNP plasma levels of adults, children, and in umbilical cord blood (3–5).
Concentrations of BNP and its biologically inactive fragment NT-proBNP are of high relevance in the diagnosis and treatment management of congestive heart failure in adults and children (3,6–11). Both peptides are useful markers in a variety of cardiac diseases in children and adolescents (11–18). It is well imaginable that also premature and newborn patients could benefit from the use of natriuretic peptide levels for diagnosis and treatment guidance of cardiac diseases. However, data on the usefulness of natriuretic peptides for diagnosis and management of congenital heart disease in this age group are limited. Even the published neonatal reference values show a variation depending on the kits used, the age at blood sampling, and the population studied (19–25). There are several studies published on the role of natriuretic peptides in the management of patent ductus arteriosus (26–30), persistent pulmonary hypertension of the newborn (31), as a marker of neonatal sepsis (32,33), and of left ventricular function (32,34). Previous investigations did neither show differences of NT-proBNP levels between arterial and venous cord blood nor a gender-related difference of NT-proBNP in neonates and children (22–24). Previously published NT-proBNP concentrations in the cord blood of healthy neonates and their mothers showed no correlation indicating that there is no placental exchange of NT-proBNP (22,23). NT-proBNP values in the cord blood of healthy neonates at time of delivery are elevated compared with older age groups and show a further increase after birth. They peak on the first to second day of life and constantly decrease beyond the second day of life (19–21,25). This elevation at birth is assumed to be a result of the dramatic circulatory changes during transition from fetal to neonatal life. With birth, the function of gas exchange is transferred from the placenta to the lungs. In healthy individuals, the venous and arterial circulation is separated, and from then on, cardiac output is equally divided between two ventricles. Congenital cardiac anomalies may affect the fetal circulation and normal adaptation after birth (35,36). So far, there are limited data published on NT-proBNP levels in the cord blood of neonates with congenital heart defect (37).
The purpose of this prospective study was to test the hypothesis that NT-proBNP is elevated in the cord blood of neonates with congenital heart defect compared with healthy neonates. Furthermore, this study investigates whether NT-proBNP decreases beyond the second day of life in neonates with congenital heart defect as it is reported in healthy neonates.
PATIENTS AND METHODS
Congenital heart defect group.
From May 2005 to January 2008, NT-pro BNP levels in the umbilical cord blood of 60 neonates with prenatally diagnosed congenital heart defect (CHD) at time of delivery and in the plasma of their mothers were measured using an automated enzyme immuno assay (Elecsys 2010, Roche Diagnostics). Fifty-three patients had ductus dependent lesions and 36 had a univentricular heart defect. The population characteristics of the patient group and the control group are shown in Table 1. The most common diagnosis was transposition of the great arteries (n = 10) in patients with biventricular heart defect, and hypoplastic left heart syndrome (n = 25) in patients with univentricular heart defect.
One patient with severe Ebstein anomaly died on the 5th day of life due to heart failure. Five patients did not need neonatal heart surgery (one patient with single ventricle, one with tricuspid atresia, two patients with tetralogy of Fallot, and one patient with congenitally corrected transposition of the great arteries). The remaining 54 patients with CHD underwent neonatal heart surgery. In this CHD subgroup, blood was drawn preoperatively on the median 5th day of life (range, 3–10 d) and NT-proBNP levels were additionally analyzed.
Control group.
To serve as controls, the NT-pro BNP levels in the umbilical cord blood of 200 healthy neonates (100 males and 100 females) at time of delivery and in the plasma of their healthy mothers were analyzed during a 3-month period from March 2008 to July 2008. In a physical examination by the neonatologist and a routinely performed ultrasound of the kidneys on the second to 5th day of life, all neonates in the control group were considered to be healthy. The history of all mothers was unremarkable; mothers with any abnormality, such as gestationally induced diabetes or arterial hypertension were excluded from the study.
Because of the policy of our perinatologists to induce labor in the 38th week of gestation in fetuses affected with congenital heart defect, the CHD group showed a significantly lower gestational age and birth weight than the term born control subjects. Signs of fetal distress (reflected by Apgar score and umbilical pH), mode of delivery, and maternal age did not differ in the two groups (Table 1).
The Upper Austrian ethic's committee approved this study and informed consent was obtained from the guardian of each subject enrolled in the study.
Statistical analysis.
Puncture of either the umbilical artery or the umbilical vein was technically not performed successfully in 14 subjects of the CHD group and in 45 subjects of the control group. These missing values of umbilical pH were not replaced; all other data were complete. All metric variables were tested for a normal distribution (test of normality: Kolmogorov-Smirnov with Lilliefors significance correction, type I error = 5%). For the comparisons of normally distributed variables between the cohorts and subgroups, the t test for independent samples was used. Not normally distributed metric data were analyzed with the Mann-Whitney U test. Within the cohorts not normally distributed, metric variables were compared by the dependent Wilcoxon test. Spearman's rank correlation coefficients were calculated for not normally distributed metric variables. All tests are two-tailed with a confidence level of 95% (p < 0.05). Data are reported as median with range.
RESULTS
Control group.
The NT-proBNP levels in the cord blood of healthy neonates at time of delivery were median (range) 626 pg/mL (153–2518 pg/mL). NT-proBNP levels did not differ between male and female neonates in the control group [median (range): 640 pg/mL (153–2518 pg/mL) and 607 pg/mL (214–2346 pg/mL); p value: 0.42]. The NT-proBNP concentrations for the newborns and their respective mothers did neither correlate in the control group [median (range): 66 pg/mL (5–368 pg/mL); r2: 0.11] nor in the CHD group [median (range): 71 pg/mL (10–2191 pg/mL); r2: −0.17].
Congenital heart defect group.
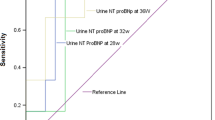
NT-proBNP levels were significantly higher in the cord blood of neonates affected with CHD [median (range): 1589 pg/mL (378–7626 pg/mL)] compared with healthy neonates [median (range): 626 pg/mL (153–2518 pg/mL); p value <0.001] (Fig. 1). Further subgroup analysis of the CHD group showed that NT-proBNP levels in the umbilical cord blood of patients with univentricular heart defects were significantly higher compared with patients with biventricular heart defects [median (range): 2001 pg/mL (378–7626 pg/mL) and 962.5 pg/mL (443–2585 pg/mL); p value <0.001].
In 54 patients with CHD, who underwent neonatal open-heart surgery, the preoperative NT-proBNP levels on the median 5th day of life (range, 3–10 d) were analyzed. NT-proBNP showed an increase compared with the cord blood levels in every single patient [median (range): 1665 pg/mL (378–7626 pg/mL) and 19222 pg/mL (1429–172800 pg/mL); p value <0.001] (Fig. 2). To exclude renal impairment, creatinine levels in the CHD group were measured on the median 5th day of life in all patients. Creatinine levels were within the normal range for age with median (range) 0.42 mg/dL (0.21–0.85 mg/dL).
One mother in the congenital heart defect group showed a markedly elevated NT-proBNP level of 2191 pg/mL. Further medical examination of this mother revealed pulmonary embolism due to factor V Leiden mutation.
DISCUSSION
The major new finding of this study is that NT-proBNP levels in the umbilical cord blood of neonates with congenital heart defect at time of delivery are significantly elevated compared with healthy neonates. Neither in the CHD group nor in the control group, the NT-proBNP umbilical cord blood levels correlated with the plasma concentrations of the respective mothers. This clearly indicates that the fetal heart on its own is able to secret NT-proBNP in response to altered loading conditions. This hormonal activation might be either the result of immoderate up-regulation of genes in the fetal heart affected with CHD (38) or it reflects the fact that the circulatory anomalies in the fetus and the hemodynamic changes during labor contribute to an overexposure to myocardial stretch in congenital heart defect patients, leading to excessive secretion of NT-proBNP (35,36). The highest cord blood levels were found in patients with univentricular heart defects, suggesting that maintenance of cardiac output by a single ventricle leads to volume overload of this ventricle and subsequently to an increased secretion of natriuretic hormones. Although we found a significant difference between the cord blood NT-proBNP levels of healthy neonates compared with neonates with CHD, a single NT-proBNP measurement in the cord blood cannot separate healthy neonates from neonates with CHD due to the marked overlap between both groups (Fig. 1).
Caused by perinatal circulatory changes and the immaturity of the kidneys, NT-proBNP concentrations in healthy neonates show a marked increase after birth and peak on the first to second day of life. Beyond the second day of life, maturation of the renal function leads to a steady decrease of NT-proBNP levels in healthy infants (19–21,25).
The second important finding of our study is that in patients with CHD NT-proBNP concentrations remain markedly elevated beyond the second day of life. It remains unknown, whether this elevation is due to the volume overload observed with prostaglandin induced open arterial ductus or it is genuinely present in neonates with complex CHD.
This study confirms in a significant number of subjects the previously reported finding (21–24) that NT-proBNP in the umbilical cord blood of healthy neonates is elevated compared with the reference interval for older age groups. This assumedly physiologic elevation in healthy neonates might be explained by the changes in ventricular volume load and ventricular stroke volume during transition from fetal to neonatal life. Using the same assay, Bar-Oz et al. (23) reported a mean umbilical cord blood concentration in healthy neonates of 600 pg/mL, and Bakker et al. (24) found a mean concentration of 80 pmol/L (670 pg/mL). Both reports are well comparable to our results of median 626 pg/mL in the healthy control group. In agreement with previous publications our study confirms that NT-proBNP levels in umbilical cord blood show no gender-related difference (21–24).
In conclusion, NT-proBNP levels in the umbilical cord blood of neonates with congenital heart defect are significantly elevated at time of delivery compared with healthy neonates. Additionally, NT-proBNP concentrations in neonates with CHD significantly increase beyond the second day of life.
Abbreviations
- BNP:
-
brain-type natriuretic peptide
- CHD:
-
congenital heart defect
- NT, proBNP:
-
amino terminal pro brain-type natriuretic peptide
- r2:
-
correlation coefficient
References
Kinnunen P, Vuolteenaho O, Ruskoaho H 1993 Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology 132: 1961–1970
Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM 2004 Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation 109: 2872–2877
Mueller T, Gegenhuber A, Poelz W, Haltmayer M 2005 Diagnostic accuracy of B type natriuretic peptide and amino terminal proBNP in the emergency diagnosis of heart failure. Heart 91: 606–612
Jefic D, Lee JW, Jefic D, Savoy-Moore RT, Rosman HS 2005 Utility of B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide in evaluation of respiratory failure in critically ill patients. Chest 128: 288–295
Hammerer-Lercher A, Neubauer E, Müller S, Pachinger O, Puschendorf B, Mair J 2001 Head-to-head comparison of N-terminal pro-brain natriuretic peptide, brain natriuretic peptide and N-terminal pro-atrial natriuretic peptide in diagnosing left ventricular dysfunction. Clin Chim Acta 310: 193–197
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, Mc Cullough PA 2002 Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347: 161–167
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 10: 933–989
Cohen S, Springer C, Avital A, Perles Z, Rein AJ, Argaman Z, Nir A 2005 Amino-terminal pro-brain-type natriuretic peptide: heart or lung disease in pediatric respiratory distress?. Pediatrics 115: 1347–1350
Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr 2004 Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation 109: 3176–3181
Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H 2006 B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 47: 742–748
Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, Rupprecht HJ, Bickel C, Tiret L, Cambien F, Gerstein H, Münzel T, Yusuf S 2006 Comparative impact of multiple biomarkers and N-Terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 114: 201–208
Law YM, Keller BB, Feingold BM, Boyle GJ 2005 Usefulness of plasma B-type natriuretic peptide to identify ventricular dysfunction in pediatric and adult patients with congenital heart disease. Am J Cardiol 95: 474–478
Lechner E, Gitter R, Mair R, Pinter M, Schreier-Lechner E, Vondrys D, Tulzer G 2008 Aminoterminal brain natriuretic peptide levels in children and adolescents after Fontan operation correlate with congestive heart failure. Pediatr Cardiol 29: 901–905
Berry JG, Askovich B, Shaddy RE, Hawkins JA, Cowley CG 2008 Prognostic value of B-type natriuretic peptide in surgical palliation of children with single-ventricle congenital heart disease. Pediatr Cardiol 29: 70–75
Koch AM, Zink S, Singer H, Dittrich S 2008 B-type natriuretic peptide levels in patients with functionally univentricular hearts after total cavopulmonary connection. Eur J Heart Fail 10: 60–62
Ozhan H, Albayrak S, Uzun H, Ordu S, Kaya A, Yazici M 2007 Correlation of plasma B-type natriuretic peptide with shunt severity in patients with atrial or ventricular septal defect. Pediatr Cardiol 28: 272–275
Ohuchi H, Takasugi H, Ohashi H, Okada Y, Yamada O, Ono Y, Yagihara T, Echigo S 2003 Stratification of pediatric heart failure on the basis of neurohormonal and cardiac autonomic nervous activities in patients with congenital heart disease. Circulation 108: 2368–2376
Cantinotti M, Clerico A, Murzi M, Vittorini S, Emdin M 2008 Clinical relevance of measurement of brain natriuretic peptide an N-terminal pro-brain natriuretic peptide in pediatric cardiology. Clin Chim Acta 390: 12–22
Koch A, Singer H 2003 Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart 89: 875–878
Mir TS, Laux R, Hellwege HH, Liedke B, Heinze C, von Buelow H, Laer S, Weil J 2003 Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: marked and rapid increase after birth. Pediatrics 112: 896–899
Schwachtgen L, Herrmann M, Georg T, Schwarz P, Marx N, Lindinger A 2005 Reference values of NT-proBNP serum concentrations in the umbilical cord blood and in healthy neonates and children. Z Kardiol 94: 399–404
Hammerer-Lercher A, Mair J, Tews G, Puschendorf B, Sommer R 2005 N-terminal pro-B-type natriuretic peptide concentrations are markedly higher in the umbilical cord blood of newborns than in their mothers. Clin Chem 51: 913–915
Bar-Oz B, Lev-Sabie A, Arad I, Salpeter L, Nir A 2005 N-terminal pro-B-type natriuretic peptide concentrations in mothers just before delivery, in cord blood and in newborns. Clin Chem 51: 926–927
Bakker J, Gies I, Slavenburg B, Bekers O, Delhaas T, van Dieijen-Visser M 2004 Reference values for N-terminal pro-B-type natriuretic peptide in umbilical cord blood. Clin Chem 50: 2465
Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, Koch A, Falkenberg J, Mir TS 2009 NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 30: 3–8
Flynn PA, da Graca RL, Auld PA, Nesin M, Kleinman CS 2005 The use of a bedside assay for plasma B-type natriuretic peptide as a biomarker in the management of patent ductus arteriosus in premature neonates. J Pediatr 147: 38–42
Holmström H, Hall C, Thaulow E 2001 Plasma levels of natriuretic peptides and hemodynamic assessment of patent ductus arteriosus in preterm infants. Acta Paediatr 90: 184–191
Choi BM, Lee KH, Eun BL, Yoo KH, Hong YS, Son CS, Lee JW 2005 Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics 115: e255–e261
Czernik C, Lemmer J, Metze B, Koehne PS, Mueller C, Obladen M 2008 B-type natriuretic peptide to predict ductus intervention in infants <28 weeks. Pediatr Res 64: 286–290
Farombi-Oghuvbu I, Matthews T, Mayne PD, Guerin H, Corcoran JD 2008 N-terminal pro-B-type natriuretic peptide: a measure of significant patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 93: F257–F260
Reynolds EW, Ellington JG, Vranicar M, Bada HS 2004 Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics 114: 1297–1304
Fried I, Bar-Oz B, Algur N, Fried E, Gavri S, Yatsiv I, Perles Z, Rein AJ, Zonis Z, Bass R, Nir A 2006 Comparison of N-terminal pro-B-type natriuretic peptide levels in critically ill children with sepsis versus acute left ventricular dysfunction. Pediatrics 118: e1165–e1168
El-Khuffash A, Molloy EJ 2007 Are B-type natriuretic peptide (BNP) and N-terminal-pro-BNP useful in neonates?. Arch Dis Child Fetal Neonatal Ed 92: F320–F324 Review
Fried I, Bar-Oz B, Perles Z, Rein AJ, Zonis Z, Nir A 2006 N-terminal pro-B-type natriuretic peptide levels in acute versus chronic left ventricular dysfunction. J Pediatr 149: 28–31
Friedman AH, Fahey JT 1993 The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol 17: 106–121
Rudolph AM 2009 The fetal circulation and congenital heart disease. Arch Dis Child Fetal Neonatol Ed Mar 25, Epub ahead of print
Kocylowski RD, dubiel M, Gudmundsson S, Sieg I, Fritzer E, Alkasi O, Breborowicz GH, von Kaisenberg CS 2009 Biochemical tissue-specific injury markers of the heart and brain in postpartum cord blood. Am J Obstet Gynecol 200: 273.e1–273.e25
Cameron VA, Aitken GD, Ellmers LJ, Kennedy MA, Espiner EA 1996 The sites of gene expression of atrial, brain and C-type natriuretic peptides in mouse fetal development: temporal changes in embryos and placenta. Endocrinology 137: 817–824
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lechner, E., Wiesinger-Eidenberger, G., Wagner, O. et al. Amino Terminal pro B-Type Natriuretic Peptide Levels Are Elevated in the Cord Blood of Neonates With Congenital Heart Defect. Pediatr Res 66, 466–469 (2009). https://doi.org/10.1203/PDR.0b013e3181b3aee4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b3aee4
This article is cited by
-
Fetal NT-proBNP levels and their course in severe anemia during intrauterine treatment
Archives of Gynecology and Obstetrics (2023)
-
Hemodynamic changes in neonates born to mothers with Graves’ disease
Endocrine (2021)
-
Factors affecting N-terminal pro-B-type natriuretic peptide levels in preterm infants and use in determination of haemodynamic significance of patent ductus arteriosus
European Journal of Pediatrics (2018)
-
N-terminal B-type natriuretic peptide urinary concentrations and retinopathy of prematurity
Pediatric Research (2017)
-
Amino-Terminal proB-Type Natriuretic Peptide Levels in the Umbilical Cord Blood of Neonates Differ According to the Type of Prenatally Diagnosed Congenital Heart Disease
Pediatric Cardiology (2015)