Abstract
Our objectives were to establish antecedent factors and patterns of brain injury and their prognostic value in preterm infants with hypoxic-ischemic encephalopathy (HIE). Essential inclusion criteria were gestation (GA) ≤36 wk, Apgar scores <5/<7 at 1/5 min, major resuscitation at birth, and a brain MRI <6 postnatal wk. At least one additional criterion was required of the following: abnormal intrapartum CTG, sentinel event, meconium, cord pH <7.0, neonatal seizures, and multiorgan failure. Antenatal and perinatal data and ≥2 y neurodevelopmental outcome were documented. Fifty-five infants (GA 26–36+6; median, 35 wk) were eligible; all had 1–6 (median, 3) additional criteria. Placental abruption was the commonest identifiable antecedent event. Evidence of infection was not prominent. Main sites of injury were basal ganglia (BG, 75%), mostly severe, white matter (WM, 89%), mostly mild, brainstem (44%), and cortex (58%). Brainstem injury was associated with severe BG, WM, and cortical injury. Two-year outcome: death (32%), cerebral palsy (26%, mostly severe quadriplegia), mild impairment (10%), and normal (32%). Significant central gray matter and brainstem injury was found in many preterm infants with HIE. Neonatal MRI findings allowed accurate prediction of neurodevelopmental outcome. Early MRI is feasible and a valuable tool in this poorly reported group of infants.
Similar content being viewed by others
Main
Hypoxic-ischemic encephalopathy (HIE), and subsequent morbidity and mortality, is seldom reported in preterm infants. Criteria used in term infants to support a diagnosis of HIE occur for other reasons in preterm infants, where suboptimal Apgar scores, a need for respiratory support, and an inability to suck–feed are common. Clinical seizures are often subtle in preterms and defining encephalopathy may be difficult.
Few studies have evaluated brain injury patterns in preterm infants with signs of HIE (1,2). Barkovich and Sargent (1) described five profoundly asphyxiated preterm infants finding basal ganglia (BG), thalamic and brainstem injury, and relative sparing of peri-rolandic cortex. None had isolated periventricular leukomalacia (PVL). BG and brainstem necrosis are reported following major hypoxia-ischemia (HI) in utero at <37 wk gestation (3–6), but most studies of preterm ischemic brain injury suggest the commonest lesion is widespread WM gliosis (3,6). Placental abruption is an identified antecedent factor (7).
Regional differences in vulnerability to HI exist (8,9). Preterm WM is susceptible to HI, and inflammation due to the selective vulnerability of the preoligodendrocyte (9,10) and diffuse WM injury is common by term-equivalent age (11); severe WM abnormality after acute preterm perinatal HI might therefore be expected. The thalamus and posterior brainstem myelinate earlier (12) than the BG and anterior brainstem (13), so may be especially vulnerable. The preterm cortex may be less susceptible to calcium-induced excitotoxic injury than later in gestation because the proportion of a subtype of glutamate receptor allowing calcium influx into the cell is lower in the early third trimester (9). Single photon emission computed tomography studies show regional BG blood flow in preterms is almost twice that to the cortex and over 4-fold that to the subcortical WM (14), suggesting that the BG may be more susceptible to injury following acute HI than WM. This pattern is similar in preterm infants at term and term-born infants (15); however, positron emission tomography studies demonstrate a lower glucose transport capacity in the preterm brain (16). Injury after preterm HI might therefore be severer but similar in distribution to term HI injury.
The main aim of this study was to identify patterns of brain injury in preterm infants with clinical evidence of HIE. Additional aims were to assess antenatal and intrapartum antecedents, and the prognostic value of the early postnatal course, and neonatal brain MRI for 2-y neurodevelopmental outcome.
PATIENTS AND METHODS
Approval was obtained from the Hammersmith Hospital Trust Research Ethics Committee. MRI reports of all preterm infants born at or referred to our hospital from January 1990 to April 2005 were searched. Informed parental consent was obtained.
Essential inclusion criteria included: 1) gestational age (GA) ≤36 completed wk, 2) Apgar scores <5 at 1 and <7 at 5 min, 3) major resuscitation (intubation/cardiopulmonary resuscitation/adrenaline) at birth, 4) brain MRI within 6 postnatal wk. Additional inclusion criteria were abnormal intrapartum CTG, sentinel event, meconium staining, cord pH <7.0, neonatal seizures, and multiorgan failure.
Exclusion criteria were metabolic disorders, congenital malformations/infections, and genetic abnormalities.
Detailed antenatal and perinatal information and evidence of sepsis was documented (Supplemental Methods 1, Supplemental Digital Content 1, http://links.lww.com/PDR/A41).
Infants were grouped by time of scan: 1) scan <4 postnatal wk (HIE/early scan group) or 2) scan at 4–6 postnatal wk (HIE/late scan group). We included infants with a later first scan as some infants were considered too unstable for early MR imaging or early referral had not been considered. We excluded infants with sepsis and a later scan, as we considered this combination complicated scan interpretation of HI injury.
Magnetic resonance imaging.
A standard neonatal MR imaging protocol (17) was used at 1.0, 1.5, or 3 Tesla. Images were assessed by an experienced neuroradiologist (MAR); the pattern of signal intensity (SI) abnormalities was documented (Supplemental Methods 2, Supplemental Digital Content 2, http://links.lww.com/PDR/A42).
Neurodevelopmental outcome.
Outcome was assessed at 2 y corrected age (minimum). Developmental quotients (DQ) were determined using the Griffiths developmental scales (18). A structured neurologic exam (19) was performed. Cerebral palsy (CP) was defined (20). Outcome classification is as follows:
-
Normal: normal neurologic exam/DQ >85;
-
Mild: delay in motor milestones but no CP and/or DQ 75–85;
-
Moderate: athetoid/diplegic CP, DQ >75;
-
Severe: spastic/dystonic CP, DQ <50 if assessable, ± seizures;
-
Death: from severe neurologic problems
Statistical analysis.
The most abnormal scan was used for analysis. Categorical variables were compared using χ2 tests unless expected frequencies were <5, when Fisher's exact test was used. Numerical data were assessed for normality using the Shapiro-Wilk test. When underlying distributions were skewed, two medians were compared using the Mann-Whitney U test and multiple medians using the Kruskal-Wallis; with normally distributed data, means were compared by ANOVA testing. We used the software package Stats Direct (version 2.5.6).
RESULTS
Seventy infants met essential inclusion criteria; 15 were excluded: metabolic diagnosis (2), large cerebral abscess (1), inadequate clinical records (4), postnatal sepsis and MRI >4 postnatal wk (8). Of the 55 remaining infants, 47 had an MRI at ≤4 postnatal wk (HIE/early scan group) and 8 between 4 and 6 postnatal wk (HIE/late scan group). Nineteen infants had two or more scans.
Eleven infants had evidence of perinatal sepsis. Details were available for 10: GBS on high vaginal swab (2), GBS on nasogastric aspirate (1), positive blood culture (2, one GBS), predelivery maternal diarrhea with fever (1), maternal fever (4). Findings for these infants did not differ significantly from the rest of the cohort and all results were analyzed together.
Infant GA ranged from 26 to 36+6 wk (median, 35; 5 were <32 wk) and birth-weight 860–4460 g (5 were <10th centile, 9%). Of the 55 infants, 42 (76%) had two or more and 27 (49%) had three or more additional inclusion criteria (Table 1). The proportions of infants with available data having each additional criteria were abnormal CTG 83% (24/29), sentinel event 56% (31/55), meconium 60% (13/22), pH <7.0 71% (21/42), neonatal seizures 82% (38/45), and multiorgan failure 42% (21/50).
Information on delivery mode was available for 47 infants: emergency caesarean section, n = 26; spontaneous vaginal delivery, n = 21. One labor was induced. Mean umbilical cord pH was 6.99 (6.6–7.35), and Apgar scores were 2 (0–5) at 1 min and 4 (0–7) at 5 min. Seven infants (13%) were inborn. Infant characteristics are summarized (Table 1 and Fig. 1A).
Antecedent factors.
No pre-pregnancy or early antenatal factors were identified. Immediate predelivery placental abruption occurred in 15 infants (4 concealed, 11 overt). Other potential aetiologies for HIE were dystocia (n = 5), preeclampsia (n = 3), maternal trauma (n = 2), cord prolapse (n = 2), maternal seizures (n = 2), other antepartum hemorrhage (n = 3), and tight nuchal cord (n = 2). No potential etiology was identified for 20 infants, 7 of whom required emergency caesarean for fetal distress.
Patterns of injury.
Severe injury was predominantly in the central gray matter but mild WM abnormality was the commonest finding (Table 2 and Fig. 1B). Atrophy was only seen ≥19 postnatal days.
Basal ganglia and thalami.
HIE/early scan group: Thirty-four infants (72%) had BG lesions of which 21 (62%) were severe. Twenty-nine infants (62%) had thalamic lesions of which 19 (66%) were severe. BG and thalamic lesions co-occurred in 27 infants (57%) (Figs. 2A,B,3A,B—compare with normal images Fig. 2C, D). The commonest sites of injury were the lentiform nuclei (66%) and ventrolateral thalamic nuclei (53%). Only three of eight infants in the HIE/late scan group had thalamic injury and one BG injury.
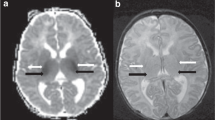
A 33-wk gestation infant imaged at 1 Tesla on day 9. (A) Axial T1- and (B) axial T2-weighted images through the BGT. The BGT are abnormal with diffuse high SI on T1-weighted images (A) and mixed high and low SI on T2-weighted images (B, short black arrows). There is diffuse homogeneous abnormal SI throughout the WM, low in (A) and high in (B, longer black arrows). The PLIC is abnormal being very wide with obvious low SI (A) and high SI in (B) (white arrows). Extracerebral hemorrhage is seen posteriorly and in the interhemispheric fissure. This infant died. Normal appearances at 34-wk gestation at 1 Tesla. (C) Axial T1-weighted and (D) axial T2-weighted images through the basal ganglia/thalami. Compared with images 2A, B, the PLIC is narrower and less defined on both T1- and T2-weighted images (white arrows). The globus pallidus (short arrow) is of relatively high SI on the T1-weighted images, normal for gestation.
Axial T1-weighted images. (A) A 36-wk gestation infant at 1 Tesla on day 16 showing mild/moderate focal central gray matter abnormality in the posterior and lateral lentiform nuclei and the thalamic ventrolateral nuclei (black arrows). The relatively high SI in the globus pallidus (white arrow) is normal. The PLIC has an abnormal appearance with uneven excessive high SI; outcome was severe but with eventual independent walking. (B) A 34-wk gestation infant at 1 Tesla on day 22 showing severe focal abnormality in the central gray matter (black arrows) and moderate diffuse low SI in the WM. There is some high SI within the PLIC but the appearance is abnormal for age. This infant also had brainstem injury (not shown) and died.
White matter (WM).
HIE/early scan group: Forty-two infants (89%) had some WM involvement, mild in 23 (55%), moderate in 10 (24%), and severe in 9 (21%); diffuse changes were the commonest finding (49%). HIE/late scan group: WM injury was seen in three infants (severe two, moderate one) (Figs. 2B,3B,4B,and 5B).
(A) Axial T1-weighted (with contrast) and (B) axial T2-weighted images showing marked focal abnormality (arrows) throughout the mesencephalon in a 33-wk gestation infant on day 9 at 1 Tesla (same infant as Fig. 2A, B). The WM shows a diffusely abnormal SI, best seen as high SI in (B).
The posterior limb of the internal capsule (PLIC).
At scan, 36 infants were ≥37 wk postmenstrual age (PMA); abnormal/equivocal PLIC myelination was seen in 16 of the HIE/early scan group and 2 of the HIE/late scan group (Figs. 2 and 3).
Brainstem.
Injury was seen in 20 (43%) of the HIE/early scan and 2 (25%) of the HIE/late scan group (Fig. 4).
Cortex.
HIE/early scan group: Loss of cortical markings was only seen in one infant whose first scan was on day 9 (Fig. 5). Cortical highlighting was seen in 22 infants (47%, median postnatal age, 12 d; range, 2–60 d); in 5, this was seen before day 5 (median, day 3; range, 2–4). The highlighting was mostly mild (widespread 7, focal 15) and never seen in isolation. Only three (38%) in the HIE/late scan group had overt cortical injury.
Hemorrhage.
HIE/early scan group: Parenchymal hemorrhage occurred in 12 infants, 10 in the WM, usually small punctate lesions (Fig. 6), and 1 each in the BG and thalamus. Four infants had intraventricular hemorrhage (IVH) (birth GA 30, 32, 33, 35 wk). None developed severe posthemorrhagic ventriculomegaly. Significant extracerebral hemorrhage (Figs. 2 and 4) was seen on five early scans and none on late scans.
Associations between the injured regions.
Thirty-six of 37 infants with basal ganglia and thalami (BGT) lesions (97%) also had WM involvement, diffuse in 24 (65%) and severe in 11 (31%). In all such cases, the BGT were the predominant sites of injury. Twenty of 22 (91%) with brainstem involvement had severe BGT injury and vice versa. All infants with brainstem injury had some WM involvement.
All 13 infants with abnormal PLICs had abnormal BGT and 9 (69%) had an abnormal brainstem. Four of six (67%) with equivocal PLICs had abnormal BGT and three had abnormal brainstems. Of 17 infants with normal PLICs, 10 (59%) had normal BGT, 6 (35%) had mild, and 1 (6%) moderate injury; all had a normal brainstem (Table 3).
Widespread cortical highlighting was seen in 10 infants; 9 (90%) had brainstem injury, 8 (80%) severe BGT injury, and 7 (70%) severe WM injury. Only 2 of 15 infants (13%) with focal cortical highlighting had severe WM injury although 7 (47%) had severe BGT injury.
Variation in injury pattern with gestation.
Infants with cortical injury were of higher birth GA (35.5 wk) than those without (34.1 wk, p = 0.02) and focal cortical highlighting was seen at a higher PMA (median, 37.3 wk) than widespread highlighting (36 wk, not statistically significant) (Table 2). No significant differences were found in GA at birth or PMA at scan with other injury patterns or severities. The median GA of infants with BG lesions was 36 wk (n = 7), with BGT lesions 34 wk (n = 28) and with thalamic lesions alone 33 wk (n = 4).
Outcome.
Outcome was available for 52/55 infants. Two had moved abroad and information was unobtainable, and one was untraceable (Table 4).
HIE/early scan: (n = 45) 15 (33%) died, 10 (22%) severe, 2 (4%) moderate, 3 (7%) mild, and 15 (33%) normal. HIE/late scan: (n = 7) none died, 3 (43%) severe, 1 (14%) mild, and 3 (43%) normal.
Injury patterns and outcome.
Severe BGT and brainstem injury was associated with a severe outcome or death in 19/20 infants (95%) (Table 5). One infant had a moderate outcome. Mild/moderate BGT injury, WM lesions, and no brainstem injury resulted in more variable outcomes; seven were normal, one mild, two severe, and three died. Isolated WM signal abnormalities were associated with a normal outcome in two infants and a mild outcome in one. Both infants with mild BGT lesions in the absence of WM or brainstem lesions had a normal outcome. Normal imaging was associated with a normal outcome in all six cases.
Nineteen infants had two or more scans; in six, the later scans gave extra information on evolving atrophy/cystic WM or BGT change. Injury severities were consistent between scans in all but one, the exception being a 33 wk infant with BGT injury classified as moderate at 21 d but severe at 60 d. The outcome was severe.
PLIC lesions and outcome.
(infants ≥37 wk PMA at scan, n = 36), Table 6.
Thirteen of 18 infants (72%) with normal PLICs had a normal motor outcome, 4 were mild (24%), and 1 had no available outcome data. One of five with equivocal PLICs was normal, one moderate, and three severe/died. All 13 with abnormal PLICs had severe outcomes (42%) or died (54%).
Ten infants were scanned before and after 37-wk PMA. In six, the severity of early BGT findings was concordant with the later PLIC data, i.e. normal BGT on early scan was associated with a normal PLIC on later scan and vice versa. Four of these infants had expected outcomes; however two, one with a normal BGT and PLIC but some WM change developed mild motor signs and the other with mild BGT lesions and an equivocal PLIC but also very severe WM injury died. In four infants, the imaging data were not completely concordant. In one infant, early scans showed normal BGT but the later scan an equivocal PLIC; this child had WM change and developed a mild diplegia with independent walking. The other three infants had mild BGT lesions on early scan but all had normal PLICs. Later scans showed normally appearing basal ganglia; two were normal at follow-up and one had mild motor problems. One of these infants was cooled in a pilot study (21).
Neonatal presentation and outcome.
GA, sex, birth weight, IUGR had no significant independent effect on outcome.
Apgar scores and cord pH.
One and 5-min Apgar scores did not predict outcome or lesion pattern; all but four infants had a 1-min score of ≤3; the 5-min score was lower in infants who died (median, 3.5) than in survivors (median, 4). The 10-min Apgar score was significantly lower in infants who died (median, 5), compared with survivors, (median, 7; p = 0.048) and to those with a normal outcome (median, 7; p = 0.041). Thirteen of 18 infants (72%) with a 10-min Apgar <7 developed CP/died compared with 7/17 (41%) with a score ≥7 (p = 0.006). Cord pH data (n = 42 infants) were not helpful in predicting outcome.
Artificial ventilation.
Data on the need for ongoing artificial ventilation (AV) was available for 43 infants. Nine were ventilated at the time of their MRI. Those who died or had a severe outcome required AV significantly longer than those with a mild/normal outcome (p = 0.005; died: median, 3 d; range, 2 h to 30 d; severe outcome: median, 4 d; range, 0–35 d; mild and normal outcomes: median, 1 d; range, 0–6 d). This difference remained significant when infants with respiratory distress syndrome (n = 12) were excluded (p = 0.037). Five infants required AV >2 wk; two had a severe outcome and three died neonatally.
Sixteen infants died, seven neonatally including three who had AV up to the time of death (median age, 17 d); one infant had planned withdrawal of care from AV. The remaining nine died at a median age of 8 (range 2–60) mo.
Nineteen infants required AV ≤24 h; 10 (53%) had a normal outcome, 3 (16%) mild, 2 (11%) severe, and 4 (21%) died, all later at 6, 8, 30, and 60 mo. Five infants only ventilated briefly at birth had a normal outcome.
Neonatal seizures.
Information was available for 45 infants; 38 had clinical seizures all starting ≤24 h. Five of six infants without seizures had a normal and one a moderate outcome. Twenty-four of 37 infants with seizures (65%) had a severe outcome or died; this association occurred in 86% of infants exposed to perinatal infection.
DISCUSSION
This is the first large neuroimaging study assessing injury patterns in preterm infants with clinical criteria consistent with HIE and the first to assess outcome beyond infancy. There was a high incidence of severe BG (38%) and brainstem (40%) injury; this may reflect either an enhanced vulnerability to HI at these sites or a greater severity of insult.
Our data suggest that brainstem injury is more likely with severe insults; all but three infants with severe BGT injury also had brainstem injury. Thalamic involvement co-occurred with BG injury and both sites seemed affected to equal degrees at the time we scanned them, despite earlier thalamic myelination.
As expected in a preterm population most infants (82%) had some WM injury; however, this was usually mild and diffuse. Severe WM injury occurred considerably less frequently than severe central gray matter or brainstem injury. All but two infants with severe WM injury also had severe BGT injury. It is likely that WM abnormalities occurred secondarily to severe BGT injury as a result of severing thalamocortical connections (8,22). Isolated WM involvement was uncommon and generally mild. The milder WM signal abnormalities may be a separate phenomenon related to preterm ex utero exposure and represent diffuse excessive high signal intensity (DEHSI) though our cohort is predominantly a late preterm group not thought so susceptible to DEHSI. No infant had classic cystic PVL suggesting that HIE in preterm infants is not a common prequel for this.
The cortex was relatively spared as reported by Barkovich and Sargent (1). The median GA of infants with cortical injury was significantly higher than that of infants without, possibly explained by the caudo-cranial pattern of myelination, and by an increase in cortical glutamate receptors as term is approached (9). Cortical highlighting becomes less obvious with time and was only seen in two infants after 4 wk. Cortical highlighting in term infants is usually seen after 5 postnatal days but was seen in nine infants in this study earlier than this—it is possible but unlikely that antenatal injury explained this; six of these infants had events precipitating emergency delivery, in four cases large placental abruptions. None had evidence of preceding fetal concerns.
No other injury patterns were significantly associated with GA, although the paucity of infants of <32 wk GA in our cohort might at least partly account for this.
Placental abruption was the single commonest identifiable etiology (7). Uterine rupture and cord prolapse were uncommon in contrast to the term population (23). Placental abruption may be a more common sentinel event in preterms, accounting both for HIE and initiation of preterm birth. Events initiating abruption in our study were unknown (7) but an association with histologic chorioamnionitis is reported (24,25). Chorioamnionitis is a risk factor for preterm labor, so subclinical infection may be the initiating process. Cytokines and inflammatory mediators might prime the fetal brain, especially WM, to be susceptible to HI. However, the 11 infants with definite evidence of infection did not have placental abruption or severe WM injury. Many died, with severe BGT and brainstem injury, suggesting gray matter susceptibility. Unfortunately, placental histology was seldom available. There was a lack of apparent effect of perinatal sepsis on injury patterns except that, paradoxically, severe WM injury was less common. Proportionately, but not significantly, more infants with perinatal sepsis died (45%) than infants without (33%) perhaps explained by the dual-pathology in the former—these infants did not have worse scans but were of younger gestation.
Severe IVH and hemorrhagic parenchymal infarction were not common as expected in late preterm infants though small IVH might have been underestimated from later scans. Parenchymal hemorrhages were mostly small punctate WM lesions not seen on later scans. Although these had the MR characteristics of hemorrhage, they may represent nonhemorrhagic injury, in particular small infarcts (26). They did not cause major damage and were not more common in the HIE/infection group.
It was not possible to determine accurately the timing of insults, although in all but one case the imaging findings were consistent with a perinatal timing. No infant had visible atrophy before 19 d suggesting that injury did not predate delivery.
Outcome is poorer than expected for term HIE; only one-third of infants had normal outcomes at 2 y, a third died, and nearly a quarter developed quadriplegic CP. We cannot exclude that only more severe infants were referred to us as milder HIE may not be suspected or thought so significant in preterm infants.
The pattern and severity of lesions on neonatal MRI was of good prognostic value, regardless of scan timing within the 6 postnatal wk. Later scans might show more atrophy or cystic change but the injury severity classification was the same in all but one infant. Severe BGT and brainstem lesions were associated with severe outcomes or death. Normal brain imaging and mild isolated BGT or WM lesions were associated with normal or mild outcomes. Infants referred later, therefore with a later first MRI, had milder BGT injury. The reasons for later referral are unclear but the infants were more preterm and their early symptoms may not have been so obvious; severely affected very preterm infants may have died.
Abnormal PLIC myelination in term infants predicts abnormal neurodevelopmental outcome with high sensitivity and specificity (27). Before 37 wk PMA, assessment of PLIC myelination is not reliable though it often looked abnormal (Fig. 2). All infants scanned ≥37 wk PMA with abnormal PLICs had a severe outcome or died while none with normal PLICs did. The predictive value of PLIC appearance probably relates to co-occurrence of BGT lesions. There was concordance between early BGT and later PLIC findings in infants who died or had severe outcomes but some discordance in those with milder outcomes. This observation supports rescanning at term in those with more subtle findings.
Only 10-min Apgar scores <7 were significantly associated with CP or death. Cord pH values did not predict outcome. All infants requiring AV for >2 wk died or had severe outcomes. Although many with severe outcomes did not require prolonged ventilation, none with a good outcome was ventilated >6 d. Clinical seizures were associated with quadriplegic CP/death in 65% and more strongly predictive of a poor outcome with evidence of infection; the absence of clinical seizures was associated with a normal outcome. Other intrapartum or neonatal factors were nonpredictive.
In conclusion, our data show that preterm infants with a presentation consistent with HIE have a high incidence of severe BGT and brainstem involvement associated with significant mortality and neurologic morbidity. WM lesions were common though often mild and cystic PVL was not found. Cortical injury was commoner in older gestation infants. Placental abruption was a clear risk factor. Importantly, early self-ventilation did not preclude severe injury; however, no infant ventilated for >6 d had a normal outcome.
We suggest that, in preterm infants with HIE and especially with seizures, early MR imaging allows accurate prediction of neurologic outcome and therefore may contribute significantly to the management and clinical decision making in this vulnerable preterm group. Early therapies, notably hypothermia, are being introduced in the term population and it will be necessary to evaluate them in preterms starting we suggest with the more mature preterm infants who are most vulnerable to HIE.
Abbreviations
- BG:
-
basal ganglia
- BGT:
-
basal ganglia and thalami
- DQ:
-
developmental quotient
- HIE:
-
hypoxic-ischemic encephalopathy
- HI:
-
hypoxia-ischemia
- PLIC:
-
posterior limb of the internal capsule
- PVL:
-
periventricular leukomalacia
- WM:
-
white matter
References
Barkovich AJ, Sargent SK 1995 Profound asphyxia in the premature infant: imaging findings. AJNR Am J Neuroradiol 16: 1837–1846
Keeney SE, Adcock EW, McArdle CB 1991 Prospective observations of 100 high-risk neonates by high-field (1.5 Tesla) magnetic resonance imaging of the central nervous system. II. Lesions associated with hypoxic-uschemic encephalopathy. Pediatrics 87: 431–438
Salhab WA, Perlman JM 2005 Severe fetal acidaemia and subsequent neonatal encephalopathy in the larger premature infant. Pediatr Neurol 32: 25–29
Cohen M, Roessmann U 1994 In utero brain damage: relationship of gestational age to pathological consequences. Dev Med Child Neurol 36: 263–268
Yokochi K 1997 Thalamic lesions revealed by MR associated with periventricular leukomalacia and clinical profiles of subjects. Acta Paediatr 86: 493–496
Marin-Padilla M 1997 Developmental neuropathology and impact of perinatal brain damage. White matter lesions of the neocortex. J Neuropathol Exp Neurol 56: 219–235
Squier M, Keeling JW 1991 The incidence of prenatal brain injury. Neuropathol Appl Neurobiol 17: 29–38
Johnston MV 2001 Excitotoxicity in neonatal hypoxia. Ment Retard Dev Disabil Res Rev 7: 229–234
Talos DM, Follett PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, Jensen FE 2006 Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in the forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol 497: 61–77
Back SA, Rivkees S 2004 Emerging concepts in periventricular white matter injury. Semin Perinatol 28: 405–414
Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea N, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA 2003 Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112: 1–7
Counsell SJ, Maalouf EF, Fletcher AM, Duggan P, Battin M, Lewis H, Herlihy AH, Edwards AD, Bydder GM, Rutherford MA 2002 MR Imaging assessment of myelination in the very preterm brain. AJNR Am J Neuroradiol 23: 872–881
Hasegawa M, Houdou S, Mito T, Takashima S, Asanuma K, Ohno T 1992 Development of myelination in the human fetal and infant cerebrum: a myelin basic protein immunohistochemical study. Brain Dev 14: 1–6
Borch K, Greisen G 1998 Blood flow distribution in the normal human preterm brain. Pediatr Res 43: 28–33
Miranda MJ, Olofsson K, Sidaros K 2006 Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res 60: 359–363
Powers WJ, Rosenbaum JL, Dence CS, Markham J, Videen TO 1998 Cerebral glucose transport and metabolism in preterm human infants. J Cereb Blood Flow Metab 18: 632–638
Rutherford MA 2002 MRI of the Neonatal Brain. WB Saunders, London, pp 3–14
Griffiths R, Huntley M 1996 The Griffiths Mental Development Scales—Revised manual: from birth to 2 years. ARICD, High Wycombe
Mercuri E, Guzzetta A, Laroche S, Ricci D, Cowan FM, Dubowitz LM 2003 Neurological examination of preterm infants at term age: comparison with term infants. J Pediatr 142: 647–655
Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D 2005 Proposed definition and classification of cerebral palsy. Dev Med Child Neurol 47: 571–576
Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD 2000 Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics 106: 684–694
Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, Hajnal J, Edwards D, Cowan F 2004 Diffusion weighted MR imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics 114: 1004–1014
Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, Cowan FM 2008 Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121: 906–914
Rana A, Sawhney H, Gopalan S, Panigrahi D, Nijhawan R 1999 Abruptio placentae and chorioamnionitis-microbiological and histologic correlation. Acta Obstet Gynecol Scand 78: 363–366
Major CA, de Veciana M, Lewis DF, Morgan MA 1995 Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications?. Am J Obstet Gynecol 172: 672–676
Cornette LG, Tanner S, Ramenghi L, Miall L, Childs A, Arthur RJ, Martinez D, Levene MI 2002 Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Arch Dis Child Fetal Neonatal Ed 86: F171–F177
Rutherford MA, Pennock JM, Counsell S, Mercuri E, Cowan FM, Dubowitz LM, Edwards AD 1998 Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischaemic encephalopathy. Pediatrics 102: 323–328
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Philips Medical System, The Health Foundation, The Academy of Medical Sciences, and Medical Research Council.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.pedresearch.org).
Rights and permissions
About this article
Cite this article
Logitharajah, P., Rutherford, M. & Cowan, F. Hypoxic-Ischemic Encephalopathy in Preterm Infants: Antecedent Factors, Brain Imaging, and Outcome. Pediatr Res 66, 222–229 (2009). https://doi.org/10.1203/PDR.0b013e3181a9ef34
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a9ef34
This article is cited by
-
Brainstem hypoxic–ischemic lesions on MRI in infants treated with therapeutic cooling: effects on the length of stay and mortality
Journal of Perinatology (2021)
-
Structural Changes in the Cortico-Ponto-Cerebellar Axis at Birth are Associated with Abnormal Neurological Outcomes in Childhood
Clinical Neuroradiology (2021)
-
COX5A over-expression protects cortical neurons from hypoxic ischemic injury in neonatal rats associated with TPI up-regulation
BMC Neuroscience (2020)
-
A Review of Functional Near-Infrared Spectroscopy Studies of Motor and Cognitive Function in Preterm Infants
Neuroscience Bulletin (2020)
-
Upregulation of miR-376c-3p alleviates oxygen–glucose deprivation-induced cell injury by targeting ING5
Cellular & Molecular Biology Letters (2019)