Abstract
Ethnic disparity in preterm delivery between African Americans and European Americans has existed for decades, and is likely the consequence of multiple factors, including socioeconomic status, environment, and genetics. This review summarizes existing information on genetic variation and its association with preterm birth in African Americans. Candidate gene-based association studies, in which investigators have evaluated particular genes selected primarily because of their potential roles in the process of normal and pathologic parturition, provide evidence that genetic contributions from both mother and fetus account for some of the disparity in preterm births. To date, most attention has been focused on genetic variation in pro- and anti-inflammatory cytokine genes and their respective receptors. These genes, particularly the pro-inflammatory cytokine genes and their receptors, are linked to matrix metabolism because these cytokines increase expression of matrix degrading metalloproteinases. However, the role that genetic variants that are different between populations play in preterm birth (e.g. the SERPINH1 - G56 SNP) cannot yet be quantified. Future studies based on genome wide association or admixture mapping may reveal other genes that contribute to disparity in prematurity.
Similar content being viewed by others
Main
Preterm birth, defined as delivery before 37 wk of gestation, accounts for about 70% of all neonatal morbidity and mortality (1). In 2005, the incidence of preterm delivery among African Americans and European Americans in the United States was 18.4 and 11.7%, respectively (2). This ethnic disparity in preterm delivery between African Americans and European Americans has existed for decades.
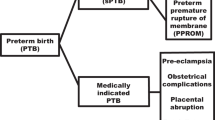
Approximately, one third of all preterm births are indicated with the remaining two third being spontaneous. Preterm premature rupture of membranes (PPROM), which accounts for one third of all spontaneous preterm births (3,4), is more likely to precede spontaneous preterm delivery in African Americans than in European Americans (5).
RISK FACTORS FOR PRETERM BIRTH AND ETHNIC DISPARITIES
There are multiple potential risk factors for prematurity including education level, income, physical environment, and marital status. These factors impact birth outcomes in ethnic subgroups differently (6–11).
The association between cigarette smoking or illicit drug use and preterm delivery is well established. Notably, African American women do not engage in high-risk behaviors, such as cigarette smoking, alcohol and illicit drug use, at a higher rate than European Americans (5,12–14).
Deficiency of iron, folic acid, and vitamin D during pregnancy may increase the risk for a preterm delivery and differences in nutritional status are likely contributors to ethnic/racial disparities in prematurity. African American women are more likely than European Americans to be anemic during pregnancy (15). African American women are also more likely to have lower serum and red blood cell folate concentrations (16). More African American women are vitamin D deficient compared with European American women (17). Poor weight gain during pregnancy has been associated with prematurity and low birth weight (18). African American women are more likely than European Americans to gain inadequate weight during pregnancy (5). Conversely, obesity is independently associated with preterm birth and more African American women have a prepregnancy weight of 200 pounds or more compared with European Americans (19).
Disparities in birth outcomes between African Americans and European Americans in the United States have been attributed, in part to delayed, and inadequate, utilization of prenatal care. However, studies conducted in military settings, where both groups have approximately equal healthcare access, have shown higher preterm birth rate among African Americans (20,21). In other studies, African American women who initiated prenatal care in the first trimester still had higher rates of infant mortality than European American women with late prenatal care (22).
The studies summarized above provide evidence that socioeconomic indicators, nutrition and the environment significantly impact birth outcomes among different ethnic groups, and can contribute, at least in part, to the disparity in preterm birth rates between African Americans and European Americans.
EPIDEMIOLOGIC EVIDENCE CONSISTENT WITH GENETIC CONTRIBUTIONS TO PRETERM BIRTH
Epidemiologic studies that have included diverse populations are consistent with the notion that genetic factors contribute to disparate rates of prematurity. Adams et al. (23) reported a higher rate of a second preterm delivery after a first pregnancy that ended in a spontaneous preterm delivery in African Americans than in European Americans. Both maternal and paternal ethnicities are significant contributors (24). Palomar et al. (25) reported an increase in risk of preterm delivery at less than 35 wk of gestation if the mother is European American and the father is African American (odds ratio [OR] = 1.28, 95% confidence interval [CI] 1.13–1.46). Using a United States natality cohort of over 2.8 million singleton births, Simhan et al. (26) also reported similar findings. On the basis of the timing of preterm births, and the overrepresentation of preterm births in African American mothers, Kistka et al. (27) concluded that genetic factors have a greater etiologic role in prematurity than previously thought.
CANDIDATE GENES AND INCREASED RISK FOR PREMATURE DELIVERY IN AFRICAN AMERICANS
Evidence that genetic factors contribute to preterm birth derives mainly from the association of prematurity with single gene defects (28,29). These rare mutations (e.g. those causing Ehlers-Danlos syndrome) make only minor contributions to preterm birth, and do not account for ethnic disparities.
Evidence for genetic contributions to the disparity in preterm birth among ethnic groups comes from candidate gene-based association studies, in which investigators have evaluated particular genes selected primarily because of their potential roles in the process of normal and pathologic parturition. These genes fall into two major groupings including those involved 1) in the host response to infection/inflammation, and 2) those involved in the synthesis and degradation of the extracellular matrix. Candidate genes potentially involved in decidual hemorrhage and activation of uterine contractility have not been investigated in any detail. To date, most attention has been focused on genetic variation in pro- and anti-inflammatory cytokine genes and their respective receptors. These genes, particularly the pro-inflammatory cytokine genes and their receptors, are linked to matrix metabolism because these cytokines increase expression of matrix degrading metalloproteinases.
There are a number of obstacles to pursuing genetic studies on preterm birth. Common conditions like preterm birth are generally influenced by multiple genes. Moreover, the genetics of complex traits do not obey classical Mendelian laws as the impact of these genes is greatly influenced by environmental factors. Additionally, there must be consideration of both the maternal and fetal genetic background, which may independently confer risk of prematurity, or may have synergistic interactions. Recent advances in genome technology and in the corresponding knowledge about the human genome and genetic variation across populations have greatly changed how we can look for genes contributing to complex diseases. A better understanding of relationships among genetic markers, facilitated by the International Haplotype Map (www.hapmap.org) project, allows genome-wide association studies to be carried out in which large numbers of genetic markers are screened in large samples of patients and unaffected controls. Once population stratification has been controlled (i.e., exclusion of significant differences in ethnic/racial compositions of the control and case groups, which could produce spurious finding because of varying allele frequencies among different racial/ethnic populations that are not associated or linked to the condition under investigation), association between a trait and a genetic marker indicates very close linkage.
Unfortunately, genome-wide association studies of preterm birth have yet to be reported, and the existing literature is based almost exclusively on analysis of candidate genes using the classical case-control study design. Most of these studies have not conformed to recommended guidelines for the conduct of genetic epidemiologic studies (30,31). The majority of the published association studies on preterm birth are based on very small sample sizes, sometimes less than 100, as noted in the subsequent text. Consequently, the statistical power to reject the null hypothesis (no association) is low, challenging the importance of “negative” association results. Replication studies, a necessity when initial reports are based on small sample sizes, have been infrequent. In addition, when positive results have been reported the statistical significance of the associations has been marginal, and usually well below thresholds recommended by critical appraisers. When multiple alleles have been examined, a correction for multiple statistical tests has not routinely been considered. Moreover, the potential confounding effects of population stratification, a particular concern when highly heterogeneous populations like African Americans are being studied, has rarely been addressed in a formal manner. Indeed, in some instances disparate populations, like African Americans and European Americans have been combined in a single analysis. The contribution of genetic variants that are often coupled, or in the parlance of population genetics, in linkage disequilibrium (LD), with the alleles under investigation has only recently been incorporated into studies of prematurity. For example, chromatin immunoprecipitation studies suggest that the tumor necrosis factor alpha (TNFA) −308A allele, thought to increase transcriptional activity of the TNFA promoter, is in LD with a lymphotoxin-α (LTA) haplotype that is associated with increased LTA transcription (32). Thus, the reported association between the TNFA −308A allele and prematurity may actually reflect the genetic contribution of the LTA alleles and the level of LTA rather than a causal influence of the TNFA gene variant. Few studies have addressed gene-environment (including nutrition) interactions or gene-gene interactions, and those that have are based on very limited sample sizes. Finally, the contribution of epigenetic factors (e.g., DNA methylation), acting on the background of DNA sequence variation, as a contributor to risk of preterm delivery has only recently been described (33). Recognizing these significant shortcomings, we have summarized existing information on genetic variation and its association with preterm birth in African Americans (Table 1).
Genes Involved in Response to Infection
Toll-like receptor 4 and caspase recruitment domain-containing protein 15.
Lipopolysaccharide, a component of the cell wall of Gram-negative bacteria, which is thought to play a key role in eliciting an inflammatory response, is recognized by proteins of the innate immune system, including Toll-like receptor 4 (TLR4) (34) and caspase recruitment domain-containing protein 15 (CARD15) (also referred to as NOD2) (35). Ferrand et al. (36) reported similar frequencies of the CARD15 insertion mutation and the hyporesponsive TLR4 allele (896G allele) in African Americans and European Americans. In a case-control study, they found no significant difference in the TLR4 896G allele frequencies among cases and controls, and all carriers of the G allele were heterozygous. The CARD15 2936insC mutation was only detected in controls.
Genes Involved in Inflammation
Tumor necrosis factor-α.
TNF-α is a pro-inflammatory cytokine that promotes production of collagen-degrading matrix metalloproteinases (MMPs) while suppressing the biosynthesis of tissue inhibitors of metalloproteinases (37). The MMPs are involved in degradation of fetal membrane collagens and loss of tensile strength. TNF-α also can antagonize the action of progesterone, which may promote uterine contractile activity (38). The TNFA gene is the most studied candidate gene for prematurity.
Roberts et al., (39) in 1999, reported a significant association between allelic variants of the polymorphism at position −308 in the promoter of the TNFA gene and preterm birth after PPROM in a case-control study of African American women. There were significantly more carriers of the rare −308A allele (homozygous and heterozygous) among women who had preterm delivery after PPROM (58%, n = 15/26) than among the controls (30%, n = 33/110) (p = 0.008; OR = 3.18, 95% CI 1.33–7.83). In women who had idiopathic preterm birth, the rare allele carrier rate among cases and controls was not significantly different.
Macones et al. (40) found maternal carriers of the TNFA −308A allele (heterozygous or homozygous) to have a significantly increased risk of spontaneous preterm birth (OR = 2.7, 95% CI 1.7–4.5). The relationship between the TNFA −308A allele and preterm birth was modified by ethnicity. The OR (95% CI) was 2.5 (1.4–4.5) for African Americans, and 1.6 (0.5–5.2) for European Americans. The authors also reported an interaction between carriage of the rare allele and bacterial vaginosis with preterm birth. Mothers with bacterial vaginosis who carried the rare allele had an increased risk of preterm birth (OR = 6.1, 95% CI 1.9–21.0).
Moore et al. (41) described a significant association between the TNFA −308A allele and preterm birth. The study sample was 45% black, 44% European, and 11% other races. Forty-eight percent of cases carried the TNFA −308A allele compared with 29% of controls (p = 0.026; OR = 2.2, 95% CI 1.0–5.0).
In contrast, Menon et al. (42) found no significant association between TNFA −308 alleles and preterm birth in either African Americans or European Americans. However, these authors found that amniotic fluid TNF-α concentrations were associated with preterm birth in African Americans, but not European Americans (43). Among African Americans, the mean TNF-α concentration in cases (1287 pg/mL) was significantly higher than that in controls (67.3 pg/mL) (p < 0.001). There was also a significant difference in the molar ratios of TNF-α/TNF-α receptors between cases and controls among African Americans. Menon et al. described a significant genotype association for the TNF-α receptors 1 and 2 (TNFR1 and TNFR2) gene polymorphisms and preterm birth including a TNFR1 single nucleotide polymorphism (SNP) at +4203 (p = 0.04), and a significant allele and/or genotype association (p values 0.01–0.02) for the TNFR2 SNPs at +19027 and +35150 in African Americans.
Speer et al. (44) studied a population that comprised of 21.5% African Americans, 75.5% European Americans, and 3.0% of mixed/other races and found no significant association between the maternal TNFA −308A allele and preterm birth.
Engel et al. (45) examined polymorphisms in the TNFA and LTA genes as part of a common haplotype configuration and did not find any differences in haplotype frequency distribution between spontaneous preterm birth cases and control subjects.
In a study in western Kenya, Aidoo et al. (46) reported that fetal carriage of the −308A allele was significantly associated with preterm birth. The relative risks (95% CI) were 7.3 (2.85–18.9) and 6.7 (2.0–23.0) for homozygotic and heterozygotic genotypes, respectively.
Menon et al. (42) reported a significant association between fetal carriage of the TNFA −308 genotype and preterm birth in European Americans, but not in African Americans.
Interleukin-1β.
Infusion of interleukin-1β (IL-1β) into the amniotic cavity of pregnant rhesus monkeys resulted in the production of TNF-α and prostaglandins with subsequent preterm uterine contractions (47).
Genc et al. (48) reported that among children of African descent, absence of homozygous carriage of IL1B (+3953*2) was associated with spontaneous preterm delivery (p = 0.033). The carriage rate among children delivered at term was 40.71% (n = 11/27) compared with 7.1% (n = 1/14) among preterm babies. Mothers of African descent also showed a similar trend in carriage rate 55.6% (n = 15/27) for term delivery, and 28.6% (n = 4/14) for preterm delivery but the difference was not statistically significant. There was also no significant association between fetal and maternal carriage of IL-1 receptor antagonist allele 2 (IL1RN*2) and preterm delivery.
Murtha et al. (49) found maternal carriage of at least one copy of the IL1RN*2 to be associated with increased risk of preterm birth. The racial breakdown of the study population was 45.5% European American, 42.5% African American, and 12% were of other races. After adjusting for maternal age and race, the OR (95% CI) for the association between IL1RN*2 and preterm birth was 3.2 (1.68–6.14) for the whole sample and 4.66 (1.85–11.74) for European Americans. A similar pattern of increased IL1RN*2 allele frequency was observed among African American subjects (cases: 17.4% vs. controls: 9.7%), but the difference was not statistically significant.
Hao et al. (50) reported a significant association between IL-1 receptor 2 (IL1R2) gene haplotypes and preterm birth in African Americans (p = 0.002).
Engel et al. (45) found no differences in IL1B haplotype frequencies between preterm birth cases and controls, among both African Americans and European Americans. They also found no differences in IL1A haplotype frequencies among African Americans. Moore et al. (41) reported no significant difference in the carriage of the IL1B (+3953) allelic variants between cases and controls in a study in which blacks comprised of 45% of population.
Interleukin-2.
Engel et al. (45) concluded that an IL2 −385 SNP did not influence the risk of spontaneous preterm birth in African Americans or European Americans.
Interleukin-4.
IL-4 induces differentiation of T lymphocytes along the TH2 pathway, blocking production of interferon-γ, thereby leading to reduction in pro-inflammatory cytokine synthesis (51). IL-4, which increases during normal pregnancy, also activates B lymphocytes leading to B-cell proliferation and antibody synthesis (52). A −590 C>T SNP in the promoter of the IL4 gene increases gene transcription and elevates IL-4 levels (53,54). This polymorphism has been associated with elevated serum immunoglobulin levels and increased severity of asthma and atopic dermatitis (53,55).
Interleukin-6.
IL-6 is a pro-inflammatory agent that induces T lymphocytes, C-reactive protein synthesis and B-cell differentiation to activate the acute phase inflammatory response. Intra-amniotic inflammation and microbial invasion of the intrauterine compartment, elevates IL-6 concentrations in amniotic fluid, cervical fluid, and cord plasma (56–59). A polymorphism at −174 (G>C) in the promoter region of the IL6 gene, which results in decreased cytokine production, has been associated with decreased risk of preterm birth (60). Significant differences between European Americans and African Americans, in both genotype and allele frequencies in the IL6 gene and its receptor gene (IL6R), have been reported (60,61).
Simhan et al. (60) reported that the homozygous IL6 (−174C/C) genotype was significantly less frequent among women with spontaneous preterm birth. Thirty (19.2%) of the control subjects and 2 (6.3%) of the cases were homozygous for the IL6 (−174C/C) genotype (OR = 0.17, 95% CI 0.04–0.74). No African American woman carried the C/C genotype.
Velez et al. (61) examined the association of polymorphisms in the IL6 gene and its receptor gene (IL6R) with preterm birth, and reported a significant association between the SNP at +37672 for IL6R from maternal samples (allele frequency p = 0.04; genotype p = 0.05) among African Americans. No significant association was found between the polymorphism at position −174 of IL6 and preterm birth in either African Americans or European Americans.
Velez et al. (62) also reported a significant association between maternal IL6R SNP (rs4553185) and preterm birth among African Americans (allele p = 0.00449, and genotype p = 0.01). In African Americans, analysis of maternal samples showed an association of two SNPs, rs1800795, and rs2069840, which have alleles that form a haplotype, with preterm birth. Relative to the common C-C haplotype, the A-G haplotype showed a significant protective effect (OR = 0.32, 95% CI 0.12–0.73; p = 0.004). In analyses of IL-6 amniotic fluid concentrations the strongest associations were observed in IL6R haplotypes formed by alleles of the rs4601580-rs4845618-rs7549338 SNPs, from African American maternal samples (p = 0.0023), and rs4601580-rs4845618 SNPs, from European American fetal samples (p = 0.0016).
Speer et al. (44) found no significant difference in IL6 (−174) genotype frequencies between spontaneous preterm birth cases and controls, in either maternal or fetal samples. However, absence of the low-producer IL6 C/C genotype was noted in African Americans.
Menon et al. (59) compared amniotic fluid concentrations of IL-6 in preterm and term subjects and found significant differences only in European Americans (p = 0.003). The median IL-6 concentration in preterm birth cases was significantly higher in European Americans compared with African Americans (p = 0.03).
Interleukin-10.
IL-10 is an endogenous cytokine that promotes development of the TH2 type of immune response. IL-10 may be produced by TH1, TH2, and non-T cells (63). IL-10 is also produced by maternal decidua cells and, to a lesser extent, fetal membranes (64). In most pregnancies, IL-10 is present in amniotic fluid in detectable levels, at all gestational ages (65). Amniotic fluid IL-10 concentrations have been reported to be significantly higher in women with preterm labor and associated intrauterine infection (65). IL-10 has also been shown to be an autocrine inhibitor of MMP-9 production, in human cytotrophoblasts (66).
A study of 80 mother and infant pairs that was comprised of 21.5% African Americans, 75.5% European Americans, and 3% other ethnicities, found no association between IL10 polymorphisms and preterm birth in either maternal or infant sample (44). The authors, however, reported a significantly higher frequency of the IL10 A-T-A haplotype (low IL-10 producer) among African American women (p = 0.004).
Interferon-γ.
In a study in which 21.5% of the participants were African Americans, Speer et al. (44) reported the fetal interferon-γ gene (IFNG) polymorphism (+874T) to be associated with spontaneous preterm birth, conditioned on maternal IFNG genotype.
Genes Involved in Matrix Metabolism
The fetal membranes derive tensile strength from interstitial collagen, and membrane rupture has been attributed, in part, to collagen degradation, which is mediated by MMPs. Fetal membrane MMP activity increases at the time of parturition and elevated levels have been associated with PPROM (67). MMP-1 is one of the collagenases involved in the first step of interstitial collagen catabolism. Fibrillar collagen cleaved by MMP-1 is further broken down by other MMPs, including the gelatinases MMP-2 and MMP-9. Other enzymes associated with the degradation process are collagenases MMP-8 and MMP-13 (68).
Matrix metalloproteinase-1.
Fujimoto et al. (69) examined the relationship between the −1607 matrix metalloproteinase-1 (MMP1) promoter polymorphism (rs1799750) and PPROM in African Americans and reported significant differences in neonatal allele and genotype frequencies between cases and controls. The authors found the frequency of 1G/2G heterozygotes and 2G/2G homozygotes to be significantly higher in cases (88%, n = 66/75) than in controls (76.2%, n = 179/235) (p = 0.028; OR 2.29, 95% CI 1.09–4.82). Alternatively said, the 1G/1G genotype protects against PPROM, as does a −935C allele of a T>C promoter SNP described by Wang et al. (33).
Matrix metalloproteinase-8.
In a case-control study of 168 cases and 216 controls, in African Americans, Wang et al. (70) reported that neonatal (i.e., fetal) carriage of the three minor allele haplotype (−799T, −381G, +17G) in the MMP8 promoter region was significantly associated with PPROM (p < 0.0001; OR = 4.63, 95% CI 2.01–11.94). The individual SNPs did not, however, show any significant association with PPROM. The minor allele haplotype increased MMP8 promoter activity in cytotrophoblasts. In this study, population stratification was excluded through an analysis of genetic ancestry estimated with ancestry informative markers. Percent African ancestry among cases was 0.847 ± 0.141, and 0.827 ± 0.149 in controls (p = 0.487).
Matrix metalloproteinase-9.
A case-control study of African American neonates showed higher carriage rate of the 14 CA-repeat allele in the MMP9 promoter in offspring of women who had PPROM (63.5%, n = 47/74) compared with those delivered at term (36.2%, n = 78/207) (OR = 3.06, 95% CI 1.77–5.27; p < 0.0001) (71). The authors found that the low number 14 CA-repeat allele confers greater promoter activity in amnion epithelial cells. They also noted that previous studies have reported that African Americans have a lower 14 CA-repeat allele frequency compared with European Americans, suggesting that the higher PPROM incidence in African Americans cannot be attributed to the 14 CA-repeat allele frequency differences between the ethnic groups.
Heat shock protein 47 (SERPINH1).
The SERPINH1 gene encodes a heat shock protein (Hsp47), localized to the endoplasmic reticulum, that serves as a chaperone stabilizing the collagen triple helix. Reduced amounts of nascent SERPINH1 transcripts and mRNA have been found in amnion samples carrying the minor SERPINH1 −656T allele (72). Hsp47 is essential for collagen synthesis. Increasing levels of Hsp47 increases type I collagen production. The reduced collagen content in PPROM fetal membranes may be due partly to reduced synthesis or increased degradation. Wang et al. (72) concluded that the SERPINH1 −656T allele is the first example of an ancestry-informative marker associated with preterm birth in African Americans. The T allele is enriched in persons of African descent (73).
Wang et al. found the minor SERPINH1 −656T allele was significantly more frequent in African American neonates born from pregnancies complicated by PPROM compared with controls (p < 0.0009; OR = 3.22, 95% CI 1.50–7.22). Cases and controls showed no significant differences in ancestry. A replication case-control study conducted on a separate sample gave similar results.
Wang et al. (74) later identified a relatively infrequent 12-bp deletion in the 5′-flanking region of the SERPINH1 gene that increases promoter activity and protects against PPROM in African Americans. The 12-bp deletion is adjacent to and in LD with the minor SERPINH1 −656T allele. The allele frequency of the 12-bp deletion was 9/403 (2.3%) among controls and 0/291 (0%) among PPROM cases (p = 0.0124).
Other Genes
Significant association between factor V haplotypes and preterm birth has been reported in African Americans (50). Erichsen et al. (75) examined the association between haplotypes in the sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2, and preterm birth. The sample consisted of 366 African Americans (129 cases and 237 controls) and 477 European Americans (142 cases and 335 controls). African Americans showed no consistent pattern of association between the five common haplotypes in spontaneous preterm birth. However, significant associations were found for European Americans.
OPPORTUNITIES TO IDENTIFY OTHER GENES CONTRIBUTING TO DISPARITIES IN PRETERM BIRTH
Admixture mapping, an alternative to traditional linkage analysis, can be used to localize disease-causing genes that differ in frequency across populations. It has particular value when used in populations that have undergone recent admixture such as African Americans, and when applied to conditions with distinct prevalence differences between populations, like preterm birth. Admixture mapping gains its power from the fact that the LD between linked loci of previously isolated populations can be detected even after 20 generations. Thus, near a disease gene there will be enrichment of ancestry-informative genetic markers representing the population with the greater disease risk. The intermixing of Western European, Indigenous American, and West African populations started over 500 y ago during the European Colonial period. Because of the slow decay of admixture LD, it has been estimated (and observed) that admixture LD exists at large genetic recombination distances up to 10–20 cm in African Americans. This means studies to be conducted with many fewer genetic markers and fewer subjects than needed for genome scans using genetic association in unadmixed populations where genetic recombination distances are as small. Panels of markers for admixture mapping have been identified by several groups (76–78) and admixture LD has been used to study associations with resting metabolic rate and obesity, insulin-related phenotypes, and skin pigmentation among other traits (77). Moreover, informative markers have been established for other populations including Indigenous Americans, allowing for admixture calculations that can encompass Western European, African, and Indigenous American. This methodology has yet to be applied to the identification of genes contributing to ethnic disparities in prematurity, and consequently represents a major research opportunity.
CONCLUSIONS
Ethnic disparities in preterm birth may result from multiple factors. Although existing studies can be criticized based on study design, there is accumulating evidence that genetic contributions from both mother and fetus account for some of the disparity in preterm birth between African Americans and European Americans. Ethnic differences in allele and/or haplotype frequencies have been reported for the TNFA, and IL6 genes, and genes encoding their receptors, but the inconsistent finding on their association with preterm birth suggests that independently, these genes are not strong determinants of preterm birth. It is possible that any effects that these genes have on prematurity results from interaction with other genes or with environmental factors. The SERPINH1 −656T allele, which is enriched in African populations and African Americans, is an example of an ancestry-informative marker associated with preterm birth in African Americans. Future studies based on genome-wide association or admixture mapping may reveal other genes that contribute to disparity in prematurity.
Abbreviations
- LD:
-
linkage disequilibrium
- MMP:
-
matrix metalloproteinase
- PPROM:
-
preterm premature rupture of membranes
- SNP:
-
single nucleotide polymorphism
References
Giarratano G 2006 Genetic influences on preterm birth. MCN Am J Matern Child Nurs 31: 169–175
Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System 2007 Births: final data for 2005. Natl Vital Stat Rep 56: 1–103
Goldenberg RL, Culhane JF, Iams JD, Romero R 2008 Epidemiology and causes of preterm birth. Lancet 371: 75–84
Srinivas SK, Macones GA 2005 Preterm premature rupture of the fetal membranes: current concepts. Minerva Ginecol 57: 389–396
Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML 2005 Births: final data for 2003. Natl Vital Stat Rep 54: 1–116
Savitz DA, Kaufman JS, Dole N, Siega-Riz AM, Thorp JM Jr, Kaczor DT 2004 Poverty, education, race, and pregnancy outcome. Ethn Dis 14: 322–329
McGrady GA, Sung JF, Rowley DL, Hogue CJ 1992 Preterm delivery and low birth weight among first-born infants of black and white college graduates. Am J Epidemiol 136: 266–276
Pickett KE, Collins JW Jr, Masi CM, Wilkinson RG 2005 The effects of racial density and income incongruity on pregnancy outcomes. Soc Sci Med 60: 2229–2238
Vinikoor LC, Kaufman JS, MacLehose RF, Laraia BA 2008 Effects of racial density and income incongruity on pregnancy outcomes in less segregated communities. Soc Sci Med 66: 255–259
O'Campo P, Xue X, Wang MC, Caughy M 1997 Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. Am J Public Health 87: 1113–1118
Roberts EM 1997 Neighborhood social environments and the distribution of low birthweight in Chicago. Am J Public Health 87: 597–603
Chasnoff IJ, Landress HJ, Barrett ME 1990 The prevalence of illicit-drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas county, Florida. N Engl J Med 322: 1202–1206
Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE 1993 A review of smoking in pregnancy: effects on pregnancy outcomes and cessation efforts. Annu Rev Public Health 14: 379–411
Berg CJ, Wilcox LS, d'Almada PJ 2001 The prevalence of socioeconomic and behavioral characteristics and their impact on very low birth weight in black and white infants in Georgia. Matern Child Health J 5: 75–84
Hemachandra AH, Klebanoff MA, Furth SL 2006 Racial disparities in the association between birth weight in the term infant and blood pressure at age 7 years: results from the Collaborative perinatal project. J Am Soc Nephrol 17: 2576–2581
Centers for Disease Control and Prevention (CDC) 2002 Folate status in women of childbearing age, by race/ethnicity–United States, 1999–2000. MMWR Morb Mortal Wkly Rep 51: 808–810
Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA 2002 Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76: 187–192
Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM 2003 Low maternal weight, failure to thrive in pregnancy, and adverse pregnancy outcomes. Am J Obstet Gynecol 189: 1726–1730
Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA 2005 Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health 95: 1545–1551
Kugler JP, Connell FA, Henley CE 1990 Lack of difference in neonatal mortality between blacks and whites served by the same medical care system. J Fam Pract 30: 281–287
Alexander GR, Baruffi G, Mor JM, Kieffer EC, Hulsey TC 1993 Multiethnic variations in the pregnancy outcomes of military dependents. Am J Public Health 83: 1721–1725
Mathews TJ, MacDorman MF, Menacker F 2002 Infant mortality statistics from the period 2000 linked birth/infant death data set. Natl Vital Stat Rep 50: 1–28
Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA 2000 Rates of and factors associated with recurrence of preterm delivery. JAMA 283: 1591–1596
Tan H, Wen SW, Walker M, Demissie K 2004 Parental race, birth weight, gestational age, and fetal growth among twin infants in the United States. Early Hum Dev 80: 153–160
Palomar L, DeFranco EA, Lee KA, Allsworth JE, Muglia LJ 2007 Paternal race is a risk factor for preterm birth. Am J Obstet Gynecol 197: 152.e1–152.e7
Simhan HN, Krohn MA 2008 Paternal race and preterm birth. Am J Obstet Gynecol 198: 644.e1–644.e6
Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, Cole FS, DeBaun MR, Muglia LJ 2007 Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol 196: e1–e6
Parry S, Strauss JF III 1998 Premature rupture of the fetal membranes. N Engl J Med 338: 663–670
Varner MW, Esplin MS 2005 Current understanding of genetic factors in preterm birth. BJOG 112: 28–31
Cooper DN, Nussbaum RL, Krawczak M 2002 Proposed guidelines for papers describing DNA polymorphism-disease associations. Hum Genet 110: 207–208
Pennell CE, Jacobsson B, Williams SM, Buus RM, Muglia LJ, Dolan SM, Morken NH, Ozcelik H, Lye SJ, PREBIC Genetics Working Group Relton C 2007 Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol 196: 107–118
Knight JC, Keating BJ, Rockett KA, Kwiatkowski DP 2003 In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nat Genet 33: 469–475
Wang H, Ogawa M, Wood JR, Bartolomei MS, Sammel MD, Kusanovic JP, Walsh SW, Romero R, Strauss JF III 2008 Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet 17: 1087–1096
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr 1997 A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature 388: 394–397
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH 2001 A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603–606
Ferrand PE, Fujimoto T, Chennathukuzhi V, Parry S, Macones GA, Sammel M, Kuivaniemi H, Romero R, Strauss JF III 2002 The CARD15 2936insC mutation and TLR4 896 A>G polymorphism in African Americans and risk of preterm premature rupture of membranes (PPROM). Mol Hum Reprod 8: 1031–1034
So T, Ito A, Sato T, Mori Y, Hirakawa S 1992 Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol Reprod 46: 772–778
Brown AG, Leite RS, Strauss JF III 2004 Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann N Y Acad Sci 1034: 36–49
Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA, Strauss JF III, Parry S 1999 Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol 180: 1297–1302
Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF III 2004 A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol 190: 1504–1508
Moore S, Ide M, Randhawa M, Walker JJ, Reid JG, Simpson NA 2004 An investigation into the association among preterm birth, cytokine gene polymorphisms and periodontal disease. BJOG 111: 125–132
Menon R, Velez DR, Thorsen P, Vogel I, Jacobsson B, Williams SM, Fortunato SJ 2006 Ethnic differences in key candidate genes for spontaneous preterm birth: TNF-alpha and its receptors. Hum Hered 62: 107–118
Menon R, Thorsen P, Vogel I, Jacobsson B, Morgan N, Jiang L, Li C, Williams SM, Fortunato SJ 2008 Racial disparity in amniotic fluid concentrations of tumor necrosis factor (TNF)-alpha and soluble TNF receptors in spontaneous preterm birth. Am J Obstet Gynecol 198: 533.e1–533.e10
Speer EM, Gentile DA, Zeevi A, Pillage G, Huo D, Skoner DP 2006 Role of single nucleotide polymorphisms of cytokine genes in spontaneous preterm delivery. Hum Immunol 67: 915–923
Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF 2005 Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology 16: 469–477
Aidoo M, McElroy PD, Kolczak MS, Terlouw DJ, ter Kuile FO, Nahlen B, Lal AA, Udhayakumar V 2001 Tumor necrosis factor-alpha promoter variant 2 (TNF2) is associated with pre-term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet Epidemiol 21: 201–211
Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ 1996 Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig 3: 121–126
Genc MR, Gerber S, Nesin M, Witkin SS 2002 Polymorphism in the interleukin-1 gene complex and spontaneous preterm delivery. Am J Obstet Gynecol 187: 157–163
Murtha AP, Nieves A, Hauser ER, Swamy GK, Yonish BA, Sinclair TR, Heine RP 2006 Association of maternal IL-1 receptor antagonist intron 2 gene polymorphism and preterm birth. Am J Obstet Gynecol 195: 1249–1253
Hao K, Wang X, Niu T, Xu X, Li A, Chang W, Wang L, Li G, Laird N, Xu X 2004 A candidate gene association study on preterm delivery: application of high-throughput genotyping technology and advanced statistical methods. Hum Mol Genet 13: 683–691
Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE 1999 The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 17: 701–738
Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T 1999 Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol 117: 550–555
Rosenwasser LJ, Klemm DJ, Dresback JK, Inamura H, Mascali JJ, Klinnert M, Borish L 1995 Promoter polymorphisms in the chromosome 5 gene cluster in asthma and atopy. Clin Exp Allergy 2: 74–78
Wierenga EA, Messer G 2000 Regulation of interleukin 4 gene transcription: alterations in atopic disease?. Am J Respir Crit Care Med 162: S81–S85
Kawashima T, Noguchi E, Arinami T, Yamakawa-Kobayashi K, Nakagawa H, Otsuka F, Hamaguchi H 1998 Linkage and association of an interleukin 4 gene polymorphism with atopic dermatitis in Japanese families. J Med Genet 35: 502–504
Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C 1998 Interleukin-6 concentrations in cervical secretions in the prediction of intrauterine infection in preterm premature rupture of the membranes. Gynecol Obstet Invest 46: 91–95
Romero R, Avila C, Santhanam U, Sehgal PB 1990 Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 85: 1392–1400
El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN 2000 Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol 95: 1056–1064
Menon R, Camargo MC, Thorsen P, Lombardi SJ, Fortunato SJ 2008 Amniotic fluid interleukin-6 increase is an indicator of spontaneous preterm birth in white but not black Americans. Am J Obstet Gynecol 198: 77.e1–77.e7
Simhan HN, Krohn MA, Roberts JM, Zeevi A, Caritis SN 2003 Interleukin-6 promoter -174 polymorphism and spontaneous preterm birth. Am J Obstet Gynecol 189: 915–918
Velez DR, Menon R, Thorsen P, Jiang L, Simhan H, Morgan N, Fortunato SJ, Williams SM 2007 Ethnic differences in interleukin 6 (IL-6) and IL6 receptor genes in spontaneous preterm birth and effects on amniotic fluid protein levels. Ann Hum Genet 71: 586–600
Velez DR, Fortunato S, Williams SM, Menon R 2008 Interleukin-6 (IL-6) and receptor (IL6-R) gene haplotypes associate with amniotic fluid protein concentrations in preterm birth. Hum Mol Genet 17: 1619–1630
Lalani I, Bhol K, Ahmed AR 1997 Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol 79: 469–483
Trautman MS, Collmer D, Edwin SS, White W, Mitchell MD, Dudley DJ 1997 Expression of interleukin-10 in human gestational tissues. J Soc Gynecol Investig 4: 247–253
Greig PC, Herbert WN, Robinette BL, Teot LA 1995 Amniotic fluid interleukin-10 concentrations increase through pregnancy and are elevated in patients with preterm labor associated with intrauterine infection. Am J Obstet Gynecol 173: 1223–1227
Roth I, Fisher SJ 1999 IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol 205: 194–204
Vadillo Ortega F, Hernandez Miranda A, Bermejo Martinez L, Beltran Montoya J, Gonzalez Avila G 1995 Transition from the latent to the active enzymatic form as a model of regulation of extracellular matrix degradation in the chorioamnion during human labor. Ginecol Obstet Mex 63: 166–172
Vadillo-Ortega F, Estrada-Gutierrez G 2005 Role of matrix metalloproteinases in preterm labour. BJOG 112: 19–22
Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF III 2002 A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem 277: 6296–6302
Wang H, Parry S, Macones G, Sammel MD, Ferrand PE, Kuivaniemi H, Tromp G, Halder I, Shriver MD, Romero R, Strauss JF III 2004 Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet 13: 2659–2669
Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, Strauss JF III 2002 A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod 8: 494–501
Wang H, Parry S, Macones G, Sammel MD, Kuivaniemi H, Tromp G, Argyropoulos G, Halder I, Shriver MD, Romero R, Strauss JF III 2006 A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc Natl Acad Sci U S A 103: 13463–13467
Rocnik EF, van der Veer E, Cao H, Hegele RA, Pickering JG 2002 Functional linkage between the endoplasmic reticulum protein Hsp47 and procollagen expression in human vascular smooth muscle cells. J Biol Chem 277: 38571–38578
Wang H, Sammel MD, Tromp G, Gotsch F, Halder I, Shriver MD, Romero R, Strauss JF III 2008 A 12-bp deletion in the 5′-flanking region of the SERPINH1 gene affects promoter activity and protects against preterm premature rupture of membranes in African Americans. Hum Mutat 29: 332–337
Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, Levine M, Siega-Riz AM, Olshan AF, Chanock SJ 2006 Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol 163: 245–254
Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, Kessing BD, Malasky MJ, Scafe C, Le E, De Jager PL, Mignault AA, Yi Z, De The G, Essex M, Sankale JL, Moore JH, Poku K, Phair JP, Goedert JJ, Vlahov D, Williams SM, Tishkoff SA, Winkler CA, De La Vega FM, Woodage T, Sninsky JJ, Hafler DA, Altshuler D, Gilbert DA, O'Brien SJ, Reich D 2004 A high-density admixture map for disease gene discovery in African Americans. Am J Hum Genet 74: 1001–1013
Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, Pfaff C, Jones C, Massac A, Cameron N, Baron A, Jackson T, Argyropoulos G, Jin L, Hoggart CJ, McKeigue PM, Kittles RA 2003 Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet 112: 387–399
Shriver MD, Kennedy GC, Parra EJ, Lawson HA, Sonpar V, Huang J, Akey JM, Jones KW 2004 The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Hum Genomics 1: 274–286
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Institutes of Health (P60 MD002256 and R01 HD034612) and the March of Dimes.
Rights and permissions
About this article
Cite this article
Anum, E., Springel, E., Shriver, M. et al. Genetic Contributions to Disparities in Preterm Birth. Pediatr Res 65, 1–9 (2009). https://doi.org/10.1203/PDR.0b013e31818912e7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31818912e7
This article is cited by
-
Acknowledging and Addressing Allostatic Load in Pregnancy Care
Journal of Racial and Ethnic Health Disparities (2021)
-
Racial and social predictors of longitudinal cervical measures: the Cervical Ultrasound Study
Journal of Perinatology (2017)
-
Spontaneous preterm birth and single nucleotide gene polymorphisms: a recent update
BMC Genomics (2016)
-
Progesterone Receptor (PGR) gene polymorphism is associated with susceptibility to preterm birth
BMC Medical Genetics (2015)
-
Delivery of a very low birth weight infant and increased maternal risk of cancer and death: a population study with 16 years of follow-up
Cancer Causes & Control (2015)