Abstract
The pathogenesis of idiopathic nephrotic syndrome (INS) remains unknown. Several findings suggest a role for the immune system. This study aimed to evaluate immune mediators in INS by measuring plasma and urinary levels of transforming growth factor β1 (TGF-β1), monocyte chemoattractant protein-1 (MCP-1/CCL2), regulated on activation normal T-cell expressed and secreted (RANTES/CCL5) and IL-8 (IL-8/CXCL8) in pediatric patients with INS and in age-matched healthy controls. Patients were divided according to their response to corticosteroids: steroid-sensitive (SS, n = 8), or steroid-resistant (SR, n = 24). Immune mediators were also compared in regard with disease activity (relapse and remission). Immune mediators were measured by ELISA. Plasma TGF-β1 levels in SR patients were approximately 2.8-fold higher than control values (p < 0.05). Urinary IL-8/CXCL8 was 2.9-fold higher in INS patients in relapse (proteinuria >100 mg/m2/24 h) when compared with patients in remission (p < 0.05), and levels had a positive correlation with individual proteinuria values (p < 0.05). Urinary IL-8/CXCL8 was significantly higher in relapsed SR than in SS patients in remission. No changes in MCP-1/CCL2 and RANTES/CCL5 levels were detected. Our findings suggest that IL-8/CXCL8 and TGF-β1 are involved in the pathogenesis of INS: IL-8/CXCL8 associated with local changes in glomerular permeability and TGF-β1 could be related to worse response to corticosteroids.
Similar content being viewed by others
Main
Idiopathic nephrotic syndrome (INS), characterized by heavy proteinuria, edema and hypoalbuminemia, is the most common glomerular disease in childhood (1). Common histologic variants are minimal change nephrotic syndrome (MCNS) and focal segmental glomerulosclerosis (FSGS) (2). The main predictive factor for disease evolution is not histologic diagnosis, but the response to treatment with corticosteroids (1,3). Indeed, children with steroid-resistant (SR) nephrotic syndrome have a greater chance to develop end-stage renal disease than those with steroid-sensitive (SS) nephrotic syndrome (1–3).
The pathogenesis of INS is not yet fully understood. Many studies have proposed a role for the immune system (2,4–6), and this hypothesis is supported by a favorable response to anti-inflammatory drugs (2), a relation between relapses and viral infections or allergic reactions (5), the recurrence of the disease in transplanted patients (1) and an association with immunologic disorders (2).
More recently, it has been proposed that alterations in the cytokine and chemokine profile of INS patients might contribute to proteinuria and glomerular damage (7,8). Cytokines are a group of proteins produced by several kinds of cells that function as soluble mediators with intercellular signaling functions (4). Chemokines constitute a large family of low-molecular-weight cytokines whose main action is the recruitment and activation of leukocyte subsets in various models of inflammation—the word “chemokine” is a contraction of the terms “chemoattractant” and “cytokine” (9,10).
A cytokine that is often associated with the progression of kidney disease is transforming growth factor-β (TGF-β) (11,12), which exhibits fibrogenic and proinflammatory properties in the kidney (12–14). One of the actions of TGF-β is to induce the accumulation of monocytes and stimulation of fibroblasts by increasing the expression of two C–C-chemokines: monocyte chemoattractant protein-1 (MCP-1/CCL2) and regulated on activation normal T-cell expressed and secreted (RANTES/CCL5) (15–17). Studies have found increased levels of MCP-1/CCL2 and RANTES/CCL5 in progressive glomerular diseases, allograft rejection and interstitial nephritis (18,19). Another mediator of interest when studying the INS pathogenesis is IL-8 (IL-8/CXCL8), a chemokine produced by endothelial cells and macrophages that attracts neutrophils and lymphocytes to the inflammation site (17,18), and may be involved in the pathogenesis of proteinuria in INS (7,20).
In this context, the present study aimed to evaluate circulating and urinary immune mediators in INS patients and to compare their levels according to steroid sensitiveness and disease activity at the time of blood and urine collection. We specifically measured plasma and urinary levels of MCP-1/CCL2 and IL-8/CXCL8, plasma TGF-β1 and urinary levels of RANTES/CCL5 in pediatric patients with INS, to disclose possible changes that might contribute to the pathogenesis of this syndrome.
SUBJECTS AND METHODS
Study design.
The present cross-sectional study used a convenience sample of children and adolescents with INS, followed-up at the Pediatric Nephrology Unit of our institution from 2005 to 2007. Diagnostic criteria for INS were based on the International Study of Kidney Disease in Children (21). Our Pediatric Nephrology Unit was established in 1980 and has followed-up approximately 300 children with nephrotic syndrome, according to a protocol that includes definition of disease etiology, assessment of clinical course and laboratory alterations, institution of treatment protocols and indication of renal biopsy based on clinical (corticosteroid unresponsiveness) and laboratory findings.
Patients with nephrotic syndrome.
Inclusion criteria included children and adolescents with well-established INS with still preserved renal function, followed-up from 2005 to 2007, whose parents gave their consent to participate in the study protocol. Children and adolescents with congenital or secondary forms of nephrotic syndrome and INS patients at stages 2–5 of chronic kidney disease were automatically excluded from the study.
Controls.
The control group consisted of sex and age-matched healthy subjects from our Pediatric Primary Care Center. Healthy status was determined through the subjects' medical history and either a parental report or self-report to rule out the presence of chronic or acute diseases.
Ethical aspects.
The Ethics Committee of the Federal University of Minas Gerais approved the study. Informed consent was obtained from parents of all included subjects. The research protocol did not interfere with any medical recommendations or prescriptions. Our Ethics Committee did not allow 24-h urine collection in healthy controls. Blood samples in control group were only drawn simultaneously to other routine blood exams. The follow-up of the INS patients and healthy controls was guaranteed even in cases of refusal to participate in the study.
Study protocol.
We allocated INS patients into two groups according to their response to treatment with corticosteroids. Steroid-sensitive (SS group), if complete remission of INS was obtained after an 8-wk course of corticosteroids, or SR group, if no remission or only partial remission occurred with steroids and if the patient needed to use other medications as an attempt to achieve disease control (3). We also subdivided SR and SS patients in two subgroups, according to disease activity (SR in relapse, SR in remission, SS in relapse and SS in remission) at the time of blood and urine collection for immune mediators measurements. In accordance to standard recommendations (2,3), our INS patients were considered in remission if their proteinuria levels were below or equal to 100 mg/m2/24 h, and in relapse if their proteinuria levels were above 100 mg/m2/24 h (2,3).
Clinical characteristics and casual measurements.
Clinical characteristics and casual measurements were obtained at the same time of blood and urine collection. The clinical variables analyzed were age, gender, height, weight, body mass index, and systolic and diastolic blood pressure. In INS patients, serum levels of urea, creatinine, albumin, cholesterol, triglycerides, and uric acid were assessed using the same blood sample obtained for the measurements of immune mediators. Urinary determinations of creatinine levels and 24-h protein excretion were also performed simultaneously to the measurements of urine chemokines levels. GFR was estimated using the Schwartz et al. (22) formula in INS patients and in healthy controls. Renal biopsy results and medications used at the time of blood sampling are also provided (Table 1).
Blood sampling.
After informed consent, all subjects (INS patients and controls) were submitted to blood collection for the measurement of immune mediators. Blood sampling occurred at only one occasion, simultaneously to other routine exams, as mentioned before. The samples were collected into sterile citrate tubes, which were immediately immersed in ice, and processed within 30 min after collection. Cells were sedimented by centrifugation at 700 g for 10 min at 4°C; then supernatant plasma was collected and respun for another 20 min at 1300 g to sediment platelets (23). Cell-free plasma was aliquoted into 0.5 mL samples and stored at −80°C until measurements.
Urine sampling.
According to the recommendations of our local Ethics Committee, 24-h urine samples were not allowed to be collected from healthy controls. For this reason, 24-h urine samples were obtained only from patients with INS, at the same day of blood collection. After homogenization, 10 mL of the collected urine were centrifuged at 4°C for 20 min at 1300 g. Cell-free urine was aliquoted into 0.5 mL tubes and stored at −80°C until measurements.
Cytokines and chemokines measurement.
Plasma and urinary levels of MCP-1/CCL2 and IL-8/CXCL8, plasma TGF-β1 and urinary RANTES/CCL5 were measured by specific enzyme-linked immunoassay (ELISA) kits (R&D Systems, Minneapolis, MN), following the manufacturer's instructions, as described elsewhere (24). Urine chemokine levels were standardized to urine creatinine measured in the same spot urine and expressed as pg/mg cr. All samples were assayed in duplicate in a single assay to avoid interassay variation. Our intra-assay variation for the ELISA measurements was below 3%. For measurement of TGF-β1, we used a Quantikine kit (R&D Systems, Minneapolis, MN), and the samples were activated before the assay. The detection limits were 6 pg/mL for TGF-β1, 8 pg/mL for MCP-1/CCL2, 2 pg/mL for RANTES/CCL5, and 6 pg/mL for IL-8/CXCL8.
Statistical analysis.
The values are expressed as medians or means and SD, when appropriate. Analysis of variance, followed by the Newman-Keuls test, was used for multiple comparisons of means. The Mann-Whitney test was used to compare medians between two groups and Kruskal-Wallis test for multiple medians comparisons. Spearman test was used to test correlations. The level of significance was set at p < 0.05.
RESULTS
Subject characteristics and casual measurements.
The control group (n = 12) included 5 boys and 7 girls ranging in age from 6.1 to 13.5 y. The mean values of weight, height, body mass index, systolic and diastolic pressures, and renal function parameters were within normal range (Table 1).
INS patients were divided according to steroid-responsiveness (SS or SR). The clinical and laboratorial features of each group at the time of blood and urine sampling are shown in Table 1. No differences were detected in age, sex distribution, weight, height, body mass index, nitrogen waste levels (urea and creatinine), uric acid, albumin, triglycerides, total cholesterol and GFR among INS groups and controls (p > 0.05, Table 1). Diastolic blood pressure was higher in the SR group than in SS or controls (p < 0.05). In addition, triglycerides, proteinuria and percentage of relapsing patients were significantly higher in the SR group when compared with SS (p < 0.05, Table 1).
As expected, almost all SR patients (22 among 24) were submitted to renal biopsies, which showed focal segmental sclerosis in 16 patients (73%) and diffuse mesangial proliferation in six (27%). In sharp contrast, only one SS patient was biopsied and histologic pattern evidenced MCNS (Table 1).
The majority of SR patients had previously received a course of cyclophosphamide (19 patients—79%) and/or cyclosporine (four patients—17%) according to our protocol recommendations as an attempt to induce disease remission. However, only one of them was still using cyclophosphamide associated with a low dose of corticosteroid at the time of blood and urine collection. As shown in Table 1, the others were receiving the combination of corticosteroids with angiotensin converting enzyme inhibitors (ACEi) (46%), or the isolated use of corticosteroids (25%) or ACEi (25%). None of the SS patients was treated with other medications rather than corticosteroids and only 3 among 8 patients were taking corticosteroids at the time of blood and urine collection due to present or recent disease relapse (Table 1).
As also evidenced in Table 1, the majority of our SR group (19 among 24 patients) still had an active disease at the time of collection, contrasting with the SS group in which only 2 out of 8 patients were in relapse.
Plasma and urinary levels of cytokines and chemokines according to steroid responsiveness.
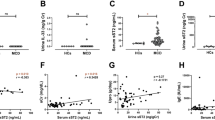
No significant differences were detected in plasma concentrations of IL-8/CXCL8 and MCP-1/CCL2 in SR, SS or controls (Table 2). No differences were observed in the comparison of TGF-β1 levels in SS patients and healthy controls. In contrast, plasma TGF-β1 levels were 2.8-fold higher in SR when compared with controls (p < 0.05; Table 2 and Fig. 1A). Additionally, plasma TGF-β1 levels were slightly but not significantly increased in SR patients when compared with SS group (Table 2 and Fig. 1A). We further divided the groups into four subgroups, according to disease activity (SR in relapse, SR in remission, SS in relapse and SS in remission). Although the highest TGF-beta levels occurred in relapsed SR patients, they only reached statistical significance when compared with control values (p < 0.05, Fig. 1B). Unfortunately, the Quantikine kit was not able to detect urinary TGF-beta 1 levels in the majority of urine samples from our INS patients. On the other hand, urinary concentrations of IL-8/CXCL8, MCP-1/CCL2 and RANTES/CCL5 were adequately measured and did not differ between SR and SS patients (Table 2).
A, Comparison between plasma TGF-β1 levels in steroid-resistant (SR, ▪), steroid-sensitive (SS, ▴) nephrotic patients and age-matched healthy controls (Controls, ♦). B, Comparison between plasma TGF-β1 levels in steroid-resistant patients in relapse (SR-relapse, ▪), steroid-resistant in remission (SR-remission, •), steroid-sensitive patients in relapse (SS-relapse, ▴), steroid-sensitive in remission (SS-remission, ▾) and in age-matched healthy controls (Controls, ♦). *p < 0.05 (Kruskal-Wallis test).
Plasma and urinary levels of cytokines and chemokines according to disease activity.
As shown in Table 3, plasma concentrations of TGF-β1, IL-8/CXCL8 and MCP-1/CCL2, and urinary levels of MCP-1/CCL2 and RANTES/CCL5 were not significantly different between patients during remission and in relapse. However, the urinary measurements showed a significant elevation of IL-8/CXCL8 levels in INS patients in relapse, reaching values 2.9-fold higher than patients during disease remission (p < 0.05; Table 3 and Fig. 2A). We also divided the disease activity groups into subgroups according to steroid responsiveness (SR in relapse, SR in remission, SS in relapse and SS in remission). Figure 2B shows a clear trend of higher urine urinary IL-8/CXCL8 levels in the subgroups in relapse when compared with remission ones, but the small number of relapsed SS patients and SR in remission did not allow conclusive statistical comparisons. On the other hand, urinary IL-8/CXCL8 levels of relapsed SR patients were significantly higher than those of SS patients in remission (p < 0.05; Fig. 2B). Additionally, urinary levels of IL-8/CXCL8 positively correlated to proteinuria (p < 0.05; Fig. 3).
A, Comparison between urinary IL-8 (IL-8/CXCL8) levels in nephrotic patients in relapse (▪) and remission (▴). B, Comparison between urinary IL-8 (IL-8/CXCL8) levels in relapsed steroid-resistant patients (Relapse-SR, ▪), steroid-resistant in remission (Remission-SR, •), relapsed steroid-sensitive patients (Relapse-SS, ▴), and steroid-sensitive in remission (Remission-SS, ▾). *p < 0.05 (Relapse vs Remission, Mann-Whitney test); **p < 0.05 (Relapse-SR vs Remission-SS, Kruskal-Wallis test).
DISCUSSION
The pathogenesis of INS still remains obscure. The immune system is thought to play a pivotal role, and there is a lot of evidence that supports this theory (2,4–6). In 1974, Shalhoub (25) developed a hypothesis that INS was an immune disorder, with increased levels of a lymphocyte-derived permeability factor. Since then, several groups have studied possible factors that could be responsible, at least in part, for the physiologic abnormalities of INS (4,26). In this study, we specifically focused on the evaluation of blood and urinary concentrations of TGF-β1, MCP-1/CCL2, RANTES/CCL5 and IL-8/CXCL8 in children with INS, since previous reports described alterations of these immune mediators in diverse renal diseases, including glomerulopathies (8,12,15,19,27).
Steroid resistance in children with INS is considered a stronger factor for a poor prognosis than histologic findings (28). In this context, it has been suggested that TGF-β might contribute to the progression of renal disease, leading to accumulation of extracellular matrix (ECM) and tissue fibrosis (12,15). High TGF-β expression and an elevation in its urinary concentration were previously detected in patients with FSGS, but not in those with MCNS (15,27,29). In our study, plasma TGF-β1 was found to be elevated in children with SR nephrotic syndrome when compared with healthy controls. It was also higher than the levels of SS patients, although the difference was not statistically significant. The absence of statistical difference between SR and SS could be due to the intrinsic limitations of the convenience sample. The small number of SS patients as well as the use of other medications in SR group such as ACEi and prednisone could interfere with the results. As previously reported, the treatment with ACEi reduced plasma TGF-β levels in experimental models of proteinuria and diabetic nephropathy (30,31). In addition, steroid administration also decreased renal expression of TGF-β in patients with severe proteinuria (15). Indeed, the use of these medications might have reduced plasma TGF-β1 concentration in our SR patients, thus attenuating the differences between INS groups in our study. Another important aspect was the collection of samples from patients at different time-points during disease evolution. This fact could also interfere with the measurements. Our findings were not conclusive enough to establish a role for TGF-β in INS. However, we suggest that increased plasma TGF-β1 in SR patients could be another pathogenic factor for the progression of renal disease. Further studies are obviously necessary to address this issue.
TGF-β1 is known to induce production of MCP-1/CCL2 and RANTES/CCL5, chemokines that have been associated to ECM deposition and proteinuria (16,17,19). We evaluated these chemokines in INS patients, but we did not find differences in their plasma and urinary levels. However, this result did not exclude a role for TGF-β1 in INS, since the increase in expression of MCP-1/CCL2 and RANTES/CCL5 is only one of the proposed mechanisms of TGF-β action in progressive renal diseases. This cytokine is also implicated in the accumulation of extracellular matrix via increased synthesis and decreased degradation of its components and via up-regulation of integrins on cell surface, thus facilitating the deposition of matrix in the interstitial space (12).
There has been a long debate as to whether or not MCNS and FSGS are part of the same disease spectrum, since MCNS can evolve into FSGS (32). There have been reports on some cases of INS in children who were initially corticosteroid responsive with MCNS histology, but progressed to FSGS over a 10-y period of repeated renal biopsies (32). It has also been suggested that long-standing proteinuria may contribute to the transformation of MCNS to FSGS (33). In this context, the involvement of a circulating permeability factor in the pathogenesis of proteinuria has been suggested by several findings. In patients with FSGS, proteinuria often recur after receiving a normal kidney transplant (34), although kidneys with FSGS were successfully transplanted in other subjects with total remission of the disease (35). In addition, there have been reports of successfully treated FSGS by plasmapheresis (36), and induction of proteinuria in a newborn of a mother with FSGS (37). A potential candidate for a permeability factor is IL-8/CXCL8. Most studies that assessed this chemokine in INS found increased serum levels in patients during relapse, and normal concentrations in remission (7,8,38). IL-8/CXCL8 was also found to have effects on the metabolism of the glomerular basement membrane, possibly increasing glomerular permeability (7). Infusion of this chemokine in rats induced proteinuria, an effect that could be reversed by the infusion of anti-IL-8/CXCL8 neutralizing antibodies (7). In our study, plasma levels of IL-8/CXCL8 were not significantly different between groups, although the urinary levels were increased in INS patients in relapse. Increased urinary IL-8/CXCL8 was also described in patients with lupus nephritis and IgA nephropathy (39). On the other hand, in the same study, the levels of this chemokine remained unchanged in nephritic patients compared with controls (39). Although, it should be mentioned that all nephritic patients were in remission (39). More recently, Cho et al. (40) reported for the first time high concentrations of IL-8/CXCL8 in the urine and plasma of pediatric patients with MCNS during relapse. Accordingly, in our study, the increase of this chemokine was independent of histology or response to corticosteroids, and was also related to urinary protein excretion. Moreover, urinary levels of IL-8/CXCL8 were positively correlated to proteinuria. This finding cannot be attributed merely to the increased permeability itself, because the same was not found for MCP-1/CCL2 or RANTES/CCL5, proteins with similar functions and molecular weights (9,10).
We are aware of the limitations associated with the cross-sectional design of our study. The main possible weakness was the use of a convenience sample, which makes homogeneity among the selected groups very difficult to obtain, since the levels of proteinuria, the presence and previous use of immunosuppressive drugs and the stage of disease activity at the time of collection may interfere with cytokine and chemokine measurements. Nevertheless, some aspects of the study may increase the strength of our findings, such as the utilization of strictly defined inclusion and exclusion criteria and a well-established protocol for the measurements of cytokines and chemokines with very low intra-assay variability (24).
In summary, we found increased plasma TGF-β1 in SR patients with preserved renal function when compared with healthy controls, suggesting that, at least in relapsed SR patients, some degree of fibrogenesis may be underway even at early stages of the disease. On the other hand, no changes in plasma TGF-beta levels were detected in the comparison of SS groups and controls. In addition, the cytokine levels in SR patients had a trend to be higher than in SS patients, but did not reach statistical difference probably due to the interference of disease treatment (corticosteroids and ACEi). Further studies are obviously necessary to confirm this possibility. More importantly, we also detected increased urinary IL8/CXCL8 in relapsed SR children when compared with SS patients in remission, with a positive correlation with urinary protein levels. This finding could be related to the effect of this chemokine on glomerular basal membrane and protein excretion, as described by other groups (7). Since plasma levels of IL8/CXCL8 were not different between groups, this result probably indicates an inflammatory process localized to renal tissue. Finally, our findings suggest that IL-8/CXCL8 and TGF-β1 could be involved in the pathogenesis of INS: IL-8/CXCL8 associated with glomerular inflammation and local changes in permeability and TGF-β1 probably correlated with worse response to corticosteroids.
Abbreviations
- ACEi:
-
angiotensin converting enzyme inhibitors
- FSGS:
-
focal segmental glomerulosclerosis
- IL-8/CXCL8:
-
Interleukin-8
- INS:
-
idiopathic nephrotic syndrome
- MCNS:
-
minimal change nephrotic syndrome
- MCP-1/CCL2:
-
monocyte chemoattractant protein-1
- RANTES/CCL5:
-
regulated on activation normal T-cell expressed and secreted
- SR:
-
steroid-resistant
- SS:
-
steroid-sensitive
- TGF-β1:
-
transforming growth factor-β1
References
Schachter AD 2004 The pediatric nephrotic syndrome spectrum: clinical homogeneity and molecular heterogeneity. Pediatr Transplant 8: 344–348
Eddy AA, Symons JM 2003 Nephrotic syndrome in childhood. Lancet 362: 629–639
Niaudet P 2004 Steroid-sensitive idiopathic nephrotic syndrome in children. Avner ED, Harmon WE, Niaudet P Pediatric Nephrology. 5th ed. Philadelphia, Lippincott Williams & Wilkins 543–556
van den Berg JG, Weening JJ 2004 Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci (Lond) 107: 125–136
Grimbert P, Audard V, Remy P, Lang P, Sahali D 2003 Recent approaches to the pathogenesis of minimal-change nephrotic syndrome. Nephrol Dial Transplant 18: 245–248
Camici M 2007 The nephrotic syndrome is an immunoinflammatory disorder. Med Hypotheses 68: 900–905
Garin EH 2000 Circulating mediators of proteinuria in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol 14: 872–878
Araya CE, Wasserfall CH, Brusko TM, Mu W, Segal MS, Johnson RJ, Garin EH 2006 A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol 21: 603–610
Mackay CR 2001 Chemokines: immunology's high impact factors. Nat Immunol 2: 95–101
Gerard C, Rollins BJ 2001 Chemokines and disease. Nat Immunol 2: 108–115
Harris RC, Neilson EG 2006 Toward a unified theory of renal progression. Annu Rev Med 57: 365–380
August P, Suthanthiran M 2003 Transforming growth factor beta and progression of renal disease. Kidney Int Suppl 64: S99–S104
Liu Y 2006 Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217
Reidy K, Kaskel FJ 2007 Pathophysiology of focal segmental glomerulosclerosis. Pediatr Nephrol 22: 350–354
Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P, Vlachojannis JG 2002 Transforming growth factor-beta(1) in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant 17: 2145–2152
Wang SN, Hirschberg R 1999 Tubular epithelial cell activation and interstitial fibrosis. The role of glomerular ultrafiltration of growth factors in the nephrotic syndrome and diabetic nephropathy. Nephrol Dial Transplant 14: 2072–2074
Qi W, Chen X, Polhill TS, Sumual S, Twigg S, Gilbert RE, Pollock CA 2006 TGF-beta1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am J Physiol Renal Physiol 290: F703–F709
Mukaida N, Harada A, Matsushima K 1998 Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev 9: 9–23
Segerer S 2003 The role of chemokines and chemokine receptors in progressive renal diseases. Am J Kidney Dis 41: S15–S18
Garin EH, West L, Zheng W 1997 Effect of interleukin-8 on glomerular sulfated compounds and albuminuria. Pediatr Nephrol 11: 274–279
1978 Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13: 159–165
Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A 1976 A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263
Reinhold D, Bank U, Buhling F, Junker U, Kekow J, Schleicher E, Ansorge S 1997 A detailed protocol for the measurement of TGF-beta1 in human blood samples. J Immunol Methods 209: 203–206
Sousa-Pereira SR, Teixeira AL, Silva LC, Souza AL, Antunes CM, Teixeira MM, Lambertucci JR 2006 Serum and cerebral spinal fluid levels of chemokines and Th2 cytokines in Schistosoma mansoni myeloradiculopathy. Parasite Immunol 28: 473–478
Shalhoub RJ 1974 Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 2: 556–560
Brenchley PE 2003 Vascular permeability factors in steroid-sensitive nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant 18: vi21–vi25
Yamamoto T, Noble NA, Cohen AH, Nast CC, Hishida A, Gold LI, Border WA 1996 Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int 49: 461–469
Schwartz MM, Evans J, Bain R, Korbet SM 1999 Focal segmental glomerulosclerosis: prognostic implication of the cellular lesion. J Am Soc Nephrol 10: 1900–1907
Strehlau J, Schachter AD, Pavlakis M, Singh A, Tejani A, Strom TB 2002 Activated intrarenal transcription of CTL-effectors and TGF-beta1 in children with focal segmental glomerulosclerosis. Kidney Int 61: 90–95
Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G 2002 Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins. A central pathway in progressive glomerulosclerosis. Am J Pathol 161: 2179–2193
Erman A, Veksler S, Gafter U, Boner G, Wittenberg C, van Dijk DJ 2004 Renin Angiotensin system blockade prevents the increase in plasma transforming growth fator beta 1 and reduces proteinuria and kidney hypertrophy in the streptozotocin-diabetic rat. J Renin Angiotensin Aldosterone Syst 5: 146–151
Ahmad H, Tejani A 2000 Predictive value of repeat renal biopsies in children with nephritic syndrome. Nephron 84: 342–346
Tanaka H, Waga S, Nakahata T, Onodera N, Monma N 2000 Focal segmental glomerulosclerosis: unremitting proteinuria of long duration as a possible etiology?. Tohoku J Exp Med 192: 157–163
Weber S, Tönshoff B 2005 Recurrence of focal-segmental glomerulosclerosis in children after renal transplantation: clinical and genetic aspects. Transplantation 80: S128–S134
Rea R, Smith C, Sandhu K, Kwan J, Tomson C 2001 Successful transplant of a kidney with focal segmental glomerulosclerosis. Nephrol Dial Transplant 16: 416–417
Yokoyama H, Wada T, Furuichi K 2003 Immunomodulation effects and clinical evidence of apheresis in renal diseases. Ther Apher Dial 7: 513–519
Kemper MJ, Wolf G, Muller-Wiefel DE 2001 Transmission of glomerular permeability factor from a mother to her child. N Engl J Med 344: 386–387
Neuhaus TJ, Wadhwa M, Callard R, Barratt TM 1995 Increased IL-2, IL-4 and interferon-gamma (IFN-gamma) in steroid-sensitive nephrotic syndrome. Clin Exp Immunol 100: 475–479
Wada T, Yokoyama H, Tomosugi N, Hisada Y, Ohta S, Naito T, Kobayashi K, Mukaida N, Matsushima K 1994 Detection of urinary interleukin-8 in glomerular diseases. Kidney Int 46: 455–460
Cho MH, Lee HS, Choe BH, Kwon SH, Chung KY, Koo JH, Ko CW 2003 Interleukin-8 and tumor necrosis factor-alpha are increased in minimal change disease but do not alter albumin permeability. Am J Nephrol 23: 260–266
Author information
Authors and Affiliations
Additional information
Supported by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) and PRONEX (Programa de Grupos de Excelência-FINEP, Brazil).
Rights and permissions
About this article
Cite this article
Souto, M., Teixeira, A., Russo, R. et al. Immune Mediators in Idiopathic Nephrotic Syndrome: Evidence for a Relation Between Interleukin 8 and Proteinuria. Pediatr Res 64, 637–642 (2008). https://doi.org/10.1203/PDR.0b013e318186ddb2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318186ddb2
This article is cited by
-
Histone deacetylase-2 expression and activity in children with nephrotic syndrome with different glucocorticoid response
Pediatric Nephrology (2018)
-
Posterior urethral valve in fetuses: evidence for the role of inflammatory molecules
Pediatric Nephrology (2017)
-
Montelukast as an add-on treatment in steroid dependant nephrotic syndrome, randomised-controlled trial
Journal of Nephrology (2016)
-
Effects of the mTOR inhibitor Rapamycin on Monocyte-Secreted Chemokines
BMC Immunology (2014)
-
The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies
Inflammation Research (2014)