Abstract
When a sheep loses its tail, it cannot regenerate it in the manner of lizards. On the other hand, it is possible to clone mammals from somatic cells, showing that a complete developmental program is intact in a wounded sheep's tail the same way it is in a lizard. Thus, there is a requirement for more than only the presence of the entire genetic code in somatic cells for regenerative abilities. Thoughts like this have motivated us to assemble more than just a factographic synopsis on tissue regeneration. As a model, we review skin wound healing in chronological order, and when possible, we use that overview as a framework to point out possible mechanisms of how damaged tissues can restore their original structure. This article postulates the existence of tissue structural memory as a complex distributed homeostatic mechanism. We support such an idea by referring to an extremely fragmented literature base, trying to synthesize a broad picture of important principles of how tissues and organs may store information about their own structure for the purposes of regeneration. Selected developmental, surgical, and tissue engineering aspects are presented and discussed in the light of recent findings in the field.When a sheep loses its tail, it cannot regenerate it in the manner of lizards. On the other hand, it is possible to clone mammals from somatic cells, showing that a complete developmental program is intact in a wounded sheep's tail the same way it is in a lizard. Thus, there is a requirement for more than only the presence of the entire genetic code in somatic cells for regenerative abilities. Thoughts like this have motivated us to assemble more than just a factographic synopsis on tissue regeneration. As a model, we review skin wound healing in chronological order, and when possible, we use that overview as a framework to point out possible mechanisms of how damaged tissues can restore their original structure. This article postulates the existence of tissue structural memory as a complex distributed homeostatic mechanism. We support such an idea by referring to an extremely fragmented literature base, trying to synthesize a broad picture of important principles of how tissues and organs may store information about their own structure for the purposes of regeneration. Selected developmental, surgical, and tissue engineering aspects are presented and discussed in the light of recent findings in the field.
Similar content being viewed by others
Main
Quality of healing depends on the capability to recall the structure of lost parts.
After the body is formed via morphogenesis and organogenesis, it is not easy to repair, replace, or regenerate most of its structures if they become damaged. Indeed, most injuries to our bodies are healed incompletely, with a loss of structure or function to a variable extent. For example, deep wounds in adult skin heal by granulation tissue formation and reepithelialization, which repairs the integumental defect but does not regenerate the original skin architecture (e.g., hair follicles and other appendages, dermal lines, folds). This is rather puzzling, because it is known that the skin contains all necessary components needed for a complete regeneration, including both mesenchymal and epidermal stem cells (1). On the other hand, nature also provides multiple examples of the body's ability to heal or even completely replace much more complex structures than the skin, such as a lizard tail or a deer's antlers (2).
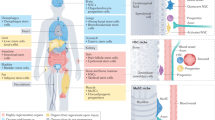
Comparing all these processes, it appears that they have a principle in common: a certain kind of “memory.” Cells, tissues, and organs somehow need to know what to do—and when and how to do it—to regenerate lost or damaged body parts. This ability of “remembering” the body's own structure becomes evident when regenerating structures that no longer exist in the injured body (such as a lost lizard's tail). Furthermore, there must be a mechanism that maintains body shape over the years (or even centuries) of life, except for the changes related to aging. We speculate that the extent and quality of this structural memory of tissues will determine the quality and completeness of the regenerative processes. Whether it is a perfect neoformation of an entire tail or just healing through bad scarring on a diabetic's leg, the tissues and cells always perform their reparative tasks using a predefined set of biologic functions, and under the influence of microenvironmental conditions. Below we review current knowledge on skin repair, and where appropriate, we make broader factual connections from the aspect of postulated structural memory that is encoded within tissues and organs (Fig. 1).
model of integration of different elements that maintain the body plan. Multiple biologic mechanisms are involved in the storage or production of the information necessary for preservation of the body's architecture. Although these individual mechanisms obey the known principles of physiologic regulation and can be categorized into groups of increasing complexity, their mutual interactions do not appear as a plain hierarchical relationship. Rather, the simpler (lower) levels form a foundation for, and are contained within, the more complex (upper) levels, and complex feedback loops exist as well. Thus, for illustration purposes, individual mechanisms are assigned different colors in a flame diagram depicting their recursive containment within each other and their mutual dependence. The functional complexity starts on the bottom with the DNA in the nuclei of individual cells, and concludes on the top with complex regulatory systems that synchronize the entire organism. This diagram was assembled as an aid or a “checklist” for analytical considerations in the fields of wound healing and tissue engineering. Extremely complex interactions between all structure-maintaining systems are simplified in the following analogy with a candle flame: All regulatory (instructional, structure-memorizing) levels are parts of the same “flame.” As such, they are mutually more or less interconnected, and changes in any level may influence the entire system. The genomic DNA sequence, when complete and correct, is a fundamental piece (a “wick” in the picture). Without it, the “flame” dies (e.g., fatal mutations, chromosomal loss, etc.). However, a candlewick on its own never makes a flame, while a flame cannot be without a wick and it can only be ignited with another flame. And so it is with DNA and life in tissue engineering: We do not understand the basis of life to create living structures, but we can use preexisting small functional pieces—live cells, tissues and gene constructs—to repair damaged body parts. The more that the basic (lower) regulatory levels are maintained in our engineered graft, and the more that the upper regulatory levels are compatible with it, then the better our tissue constructs will be. And similarly as we block the top of a burning flame with a candlesnuffer, in analogy it is possible to remove the upper levels of regulation in living systems. By doing this, we lose the top levels of body integration, but the lower regulatory levels persist with a reduced functionality (e.g., organs, tissues or cells dissected from live animals can temporarily survive in vitro in culture, slowly losing their original biologic properties because of being deprived of nerve impulses, hormonal peaks, blood stream, etc.). No diagram and no analogy is perfect, but we believe that this scheme may at least serve as a good starting point for young colleagues. It represents a broad concept of principles involved in organ regeneration, and their mutual interconnections during decades-long, day-by-day maintenance of the body's fine structure in health and disease.
THE CONCEPT OF STRUCTURAL MEMORY IN TISSUES
Anatomical and functional organization of the body's structural memory.
Partitioning the body into individual organ systems to be comprehensive in our understanding of anatomy, physiology, and diseases, is always somewhat artificial. And while some systems were quite easily identified centuries ago (cardiovascular, nervous, respiratory, digestive), other systems were difficult to discover—and until now, their definitions remain speculative, based on functional unity rather than anatomical demarcation (e.g., the endocrine system or immune system, whose elements can be scattered in multiple unrelated locations such as bones, intestine, spleen, etc.). When it comes to the definition of mechanisms that maintain the body's shape and restore its structure after injury or damage, we encounter even larger difficulties. First, it is clear that certain healing potentials exist literally everywhere within the organism, thus ranking tissue healing among ubiquitous processes such as metabolism or ion homeostasis and making any anatomical demarcation impossible. Second, the involvement of immunocytes and stem cells migrating into the wounded area from remote locations clearly demonstrates a coordinated action of the body, which is further underlined by the eventual involvement of the most complex regulators, such as nervous or endocrine stimuli in certain healing locations. Third, our understanding of how the body is formed during embryonic development is still insufficient, and so is our understanding of how structures once created are physiologically maintained during the lifespan, implying that our current insight into tissue repair may be rudimentary at best. And finally, the explosion of data on complex tissue- and organ-specific healing processes makes it impossible to review all the current knowledge within a journal article like this one. Therefore, we use a substantial level of abstraction and conceptual simplification, together with references from the literature for details, to delineate a generally applicable sketch of how tissues remember and preserve their own structure.
Explaining the term “memory” used in this article.
The mere repopulation of a damaged site by neighboring cells may be viewed as a basic healing mechanism and may eventually lead to perfect regeneration in some locations. However, healing may also involve sophisticated processes such as neural stimuli (e.g., in muscles) or complex differentiation events that recapitulate parts of embryonic development (e.g., lizard tails). Thus, we use the term structural “memory” in the broadest, all-inclusive sense. In other words, for purposes of this review, the structural memory of the body is understood as a set of multiple factors that includes biomolecules, subcellular and extracellular structures, and signaling networks, together with all related biologic mechanisms, that instruct cells to maintain or recreate complex structures of tissues and organs. This set may include a broad range of elements: from the chemistry of DNA sequences, through cell surface determinants (antigens, receptors, channels, etc.), latent growth factors silently deposited within the extracellular matrix (ECM), mechanical pressure and temperature changes, up to neural regulation and behavioral changes.
One important aspect of this definition is that we do not find any evidence of centralized storage of the body's structural information, so there is no analogy with computer memory or our mental cognition. Rather than this, tissue structural memory is distributed throughout all body structures and encoded directly within them, starting with DNA as the primary instructions for building all higher-order, more or less self-remembering systems. Immunologic memory is an example of generally accepted mechanisms of cell/tissue-encoded memory. Another important aspect is that there is very little real knowledge of how the body maintains its structural organization. The majority of facts remain to be discovered and this article cannot aim higher than to be a fair attempt at extrapolating to a bigger picture based on the current fragmented experimental data.
Some of the elements of structural memory may not exhibit any apparent roles under physiological conditions in vivo, and perhaps may be dispensable under in vitro culture conditions. For example, certain cell surface antigens are never exposed to the immune system, or certain growth factors are never released from the ECM unless the tissue is wounded—and unwounded tissues are maintained perfectly fine in their absence. Thus, genes of unknown functions or genes showing no phenotypical defect after knocking them out may fall under this category. The elements of structural memory also may be separated from each other by distance and multiple barriers (such as the multipotential bone marrow stem cells that reside far from lungs, or the presence of fibrinogen that usually does not occur within intact organs). However, under specific conditions—either natural, accidental, or medicinally induced—some or all of these memory elements may be brought together, start mutual interactions and thus acquire a new sense that directs somatic cells to regrow lost body parts or reactivates the original imprinting present during development.
Figure 1 shows the key components of the structural memory combined in a hierarchical scheme, being represented as elements of one integral, interconnected, multilevel body maintenance system. Inherently, the “upper levels” usually contain and use all “lower levels” as their functional components. For example, immunity (level IX) is based on genes, cell signaling, matrix interactions, functional vasculature, etc., although it does not depend on—but can be controlled by—the level X elements. Figure 1 is used as a framework for our review. For brevity, we explain the natural chronological order of wound healing processes, while referring to corresponding memory elements from Figure 1. For simplicity, skin healing is used as a major model system throughout the article, with reference to selected interesting details from other organs when appropriate. We will also make connection between what we learned from studying wound repair to its current and potential applications in the field of tissue bioengineering.
SIMILARITIES BETWEEN HEALING AND EMBRYONIC DEVELOPMENT
Scarless epithelial closures as an archetype of wound healing.
Inside a developing embryo, multiple organs and structures are formed by mutual fusion of primitive anlagens (such as pharyngeal arches, neural lips, palatal shelves, endocardial cushions, etc.). Embryonic tissues usually respond to new contacts—when established in the right place and at the right time—by removal of the epithelium from the contact area. Then, confluence of the underlying layers concludes the tissue union. Many of these processes are essentially akin to the embryo wounding itself (by removing the epithelium), and then healing the defect generated. Interestingly, all these essential morphogenetic processes are accomplished without notable traces or scars, similar to healing of wounds during the fetal period (3–5). The neural tube and the palate are among the best-studied epithelial closures occurring during embryonic development. Although the primary cellular mechanism and timing of epithelial disappearance from the surface of apposing palatal shelves remains controversial (6–9), it is known that these mucosal cells deposit transforming growth factor beta 3 (TGF-β3) into the ECM before they disappear (10). Although this growth factor pool may be used by the depositing cells themselves (10,11), it is possible that it also serves as a message for subsequent developmental processes governing the epithelial closure. Intriguingly, TGF-β/BMP is the same signaling pathway associated with scar formation and scarless wound healing (12–14), and TGF-β3 was demonstrated to mediate antiscarring effects. Important implications of TGF-β signaling (see Ref. 15 for a detailed review of all ligands and receptors) for healing of injured epithelial surfaces of the body such as skin, mucosa, or endothelium, are demonstrated by the decades-long promises in developing antiscarring agents (16).
Practical utilization of developmental knowledge.
The epithelial closures mentioned above serve as a limited example of how developmental biology helps tissue engineers understand the processes involved in organ formation. In general, the continuing elucidation of additional developmental mechanisms involved in tissue morphogenesis comes with the expectation that they could be repeated or mimicked i) to prevent postnatal manifestation of prenatal defects (17), or ii) to be used in engineering of tissues and organs. It is not even partially possible to mention here all the parallels between embryogenesis and organ regeneration. We have to refer here to a rapidly growing body of knowledge in the standalone field of developmental biology. For the readers' interest, the organs with the most understood morphogenesis with potential implications for tissue engineering or regeneration include the heart and cardiovascular system (18–25), teeth (26), craniofacial bones and tissues (27–36), the neural-crest-derived structures (37,38), bone marrow (39), skeleton (40), and skin (41–43).
THE INITIAL PHASE OF HEALING
Players in regeneration: cells, matrix, and soluble mediators.
When tissues are injured or damaged, organic debris has to be removed from the site of injury, and new components must be delivered and/or recreated, there. Everything must happen in an orchestrated manner and involves mutual interactions of cells, ECM and soluble factors (44). Not surprisingly, the same basic components are used in tissue engineering (usually referred to as cells or stem cells, scaffolds, and inducing compounds). Any imbalances between these key players or other elements of structural memory may result in improper repair, such as insufficient healing (chronic ulcers, recurrent hernia, unhealed fractures), overhealing (hypertrophic scars, keloids, fibrosis), or other aberrations (ectopic joints, teratomas). Whereas tissue engineering procedures are artificial and still highly experimental, natural wound healing generally involves three regular stages that overlap in space and time (45,46): i) hemostasis and (optional) inflammation, ii) a proliferative phase of granulation and tissue (neo)formation, and iii) tissue remodeling including scar maturation. Initially, hemostasis and platelet aggregation are followed by wound infiltration with lymphocytes, phagocytes, and cells involved in later proliferative phases. In the tissue formation phase, processes named granulation, angiogenesis and epithelialization begin to fill and cover the wounded area, accompanied by wound contraction (47,48). During tissue remodeling, there is increased repatterning and turnover (synthesis, deposition, remodeling, and degradation) of the ECM molecules (49,50), and loss of surplus cell types by apoptosis. Cell–cell and cell–matrix interactions, together with a correct balance of soluble molecular mediators, are vital for appropriate wound healing in all three phases.
First reaction—leukocytes and inflammation.
When the injury is large and deep enough to disrupt mesenchymal layers, vasoconstriction, blood clotting and aseptic inflammation are the body's first responses (Fig. 1, all levels involved). Following such a deep injury, platelets and other blood cells become entrapped and aggregate into a blood clot. Different cell types have different temporal involvements in wound closure, but in general, the platelets, polymorphonuclear neutrophils (51), as well as the endothelial cells lining the capillaries at the wound site, release chemotactic factors. In later phases of healing, PMNs must transmigrate across the endothelial cells of the capillaries and other nearby blood vessels. For this to occur, the mesenchymal endothelial cells must become activated by proinflammatory cytokines such as IL-1β, TNF-α, and IFN-γ. Proinflammatory cytokines prime the endothelial cells for leukocyte adhesion and diapedesis by inducing the expression of P- and E-selectins, as well as the intracellular adhesion molecule 1 and 2. These adhesins interact with the corresponding integrins present at the cell surface of PMNs, including CD11a/CD18 (LFA-1), CD11b/CD18 (MAC-1), CD11c/CD18 (gp150, 95), and CD11d/CD18 (49). Multiple proinflammatory cytokines also recruit and activate fibroblasts and epithelial cells. Another important player in wound healing are macrophages, which replace the PMNs after the first couple of days. Migration of blood monocytes (i.e., future macrophages) into the wound is regulated by the interaction of α4β1 integrin and endothelial vascular cell adhesion molecule 1. At the wound site, monocytes respond to growth factors, bind the ECM, and mature to become macrophages producing additional signaling molecules—TGF-β, TGF-α, basic fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor. These growth factors stimulate the production of adequate or excessive amounts of the ECM, as well as induce increasing cell proliferation and reepithelialization to replace damaged cells. Macrophage immunity aids wound healing, and in experiments targeted at the reduction of scarring, which inhibited macrophage invasion into the wound, the efforts were not successful and there was a resulting delay in healing (46,52).
Immunologic memory—the extent of inflammation affects healing outcomes.
In general, the amount of inflammation determines the quality of tissue repair, and inflammation is required for scar formation (49,53). The immunology and physiology of inflammation (Fig. 1, levels I–IX) is so complex that it is beyond the scope of this review. From the aspect of body structure maintenance, specific immune responses that involve creation of memory cells is not the only memorizing action characteristic of the immune system. Also, multiple mechanisms of nonspecific immunity, including inflammation, can be viewed as a part of the body's structural memory. Although specific immunity is more plastic and highly adjustable to novel antigens, nonspecific immunity provides a quite rigid set of defense tools against generally expected and commonly occurring agents (bacteria, foreign particles, dead tissue, etc.). Despite this functional difference, both types of immunity, in principle, carry the information needed to distinguish self from foreign and healthy from sick, thus serving as complementary body-structure-maintaining subsystems, and both play distinct roles in wound healing. Using the aspect of memory mechanisms in healing, we can distinguish two important impacts of immunologic defense: 1) Recognition of self versus foreign antigens, which is encoded in the immunologic memory (both specific and nonspecific), is crucial in determining which structures are to be destroyed within the wound and which will be saved; and 2) The extent of immunologic destruction affects the amount and types of other memory units (differentiated cells, acellular matrices/scaffolds … ) left in the wound to serve as a starting material for healing.
In the ideal situation, only the debris and devitalized tissue are removed from the wound, and a maximum of viable cells and ECM is saved and nurtured. Such a situation may occur in very small injuries, but in general, it is unlikely in most real wounds. Therefore, cell signaling and the production of various bioactive proteins during the inflammatory phase are actively investigated with the hope of uncovering therapeutic options for treating wounds associated with the up- or downregulation of inflammation (such as chronic ulceration, wounds in elderly and diabetic patients, and possibly certain types of fibrosis). Both the polymorphonuclear neutrophils and macrophages produce high levels of destructive enzymes and oxygen radicals that may cause tissue damage (54). In contrast to postnatal wound healing, healing in the early fetal skin exhibits scarless regeneration of the dermal architecture. Among the numerous differences between fetal and postnatal skin is the major hallmark of fetal repair, which is the lack of a typical inflammatory response (46,49).
WRECKED TISSUE REMNANTS, AND BUILDING NEW SCAFFOLDS
Scaffold memory and growth factors storage—the extracellular matrix.
It is known that if an epithelial surface or a small part of the parenchyma is lost, many organs may regenerate quite well, providing that the mesenchymal structures remain preserved (Fig. 1, level VI). For example, small skin abrasions or liver damage may demonstrate scarless healing. However, if the mesenchyme is damaged or lost, the tissue or organ may become mutilated. This is particularly evident in tissues with extremely complex architecture, such as the kidney, which represents a great challenge for artificial matrix microfabrication (55). It is apparent that the ECM serves as a destination area for cells, with the ability to instruct the attracted cells to perform certain functions and/or differentiation processes. In certain locations, ECM itself may play extremely specific roles that require unique physical properties, such as transparency in the eye (56). Furthermore, the intact mesenchymal stroma defines the shape of the healing structure, thus representing a kind of “tissue memory” (Fig. 1, level VI) that works even in certain cases of total cell loss (e.g., dermabrasion).
Accordingly, from the bioengineering point of view, a scaffold is prepared as the first structure during construction of artificial tissues or organs. So how is its function achieved? The function of providing the shape and size of a regenerating organ is clear—ECM serves as a mechanical support for cells in the same fashion as the shape of a cultivation flask demarcates the cell growth area. In addition to this mechanical role, it is known that molecules stored in the ECM can direct cell proliferation, migration, and differentiation (57–59). For example, growth factors released to and/or stored within the ECM (PDGF, TGF-β) and fibronectin encourage the proliferation and migration of cells to the wound bed, and production of ECM molecules by fibroblasts. Cells bound to the ECM often respond differently to growth factors and other stimuli when compared with unattached cells. In the absence of signals arising from attachment to the ECM, many cells will commit apoptosis (60). Recent studies have shown that double P- and E-selectin deficient mice and intracellular adhesion molecule 1 deficient mice all demonstrate dramatic delays in wound closure that is primarily associated with a lack of infiltration of neutrophils and macrophages into the wound site (49). Another recent study demonstrates that mechanical strain may significantly alter the storage behavior of the ECM, leading to different rates of growth factor release with direct effects on adjacent cells (61). Thus, fabrication of matrices capable of a simultaneous release of multiple growth factors in desired concentrations (62) under various mechanical conditions represents a major challenge. Future development of tissue engineering may include utilization of microdevices or advanced intelligent biomaterials for precisely controlled protein release. Design of temporary biodegradable matrices, such as fibrin for the delivery of cells, and research on 3D matrix properties are other active frontiers of current research (63–68).
Provisional ECM during wound healing.
The provisional ECM, present in healing wounds, is different in composition from the ECM found in normal tissue. It includes fibronectin, a mechanically weak type III collagen, elastin, glycosaminoglycans, glycoproteins, and proteoglycans. Impaired production of normal collagen in Ehlers-Danlos syndrome causes delayed wound healing (69). For years, it was believed that decorin deficiencies (a TGF-β binding factor) lead to weak connective tissues. However, it is interesting to note that a recent study demonstrates the opposite: improved cellularity and tensile strength is seen in collagen matrices engineered with decorin-deficient fibroblasts, and a similar effect can be achieved by stimulation of normal fibroblasts with TGF-β1 (70). This brings new promise for matrix engineering. The provisional ECM has an increased amount of hyaluronan and fibronectin, which creates an extremely hydrated matrix that provides for additional cell migration. There is also a steady increase in the amount of collagenase and other enzymes that are responsible for collagen degradation. Specifically designed compositions of the ECM are critical in the bioengineering of multiple tissues and organs, including bone and cartilage (71–74), cleft palate repair (4,75), brain injury repair (76), heart valves (77,78), and the skin, which is the most advanced living artificial organ at the present time (42). The elastic component of the ECM, characterized by molecules of elastin that crosslink with one another, confers a resilience to recoil after stretching. Thus, it is extremely important in the bioengineering of heart valves and large blood vessels. The fibronectin component of the ECM that binds with numerous molecules and interacts with collagen, fibrin, heparin, and specific membrane receptors on responsive cells, is consistent with its role as a part of the instructional memory operating during wound healing. The cell-binding domain, made up of amino acids Arg-Gly-Asp (RGD), functions in helping cells adhere to and migrate within the ECM (54). The RGD component has been a recent focus of study for scientists investigating the targeting of cancer cells that upregulate RGD (79), with possible implications for healing of cancer lesions via ECM manipulation.
DEVELOPMENTAL PROGRAMS IN CELLS
Memory within the wound neighborhood.
The neighborhood of a wounded tissue may serve as an additional type of “tissue memory” or a “material pool” needed for repair (Fig. 1, levels I–VIII). In the simplest situation, fully differentiated cells or alternatively, progenitor cells, just simply migrate into the wound from surrounding unwounded areas (epithelial cells, macrophages, endothelial cells, fibroblats, parenchymal cells, etc.). The regenerative potential of neighboring cells may go far beyond this repopulating capability. For example, differentiated corneal keratocytes not only heal eye scratches extremely efficiently, but they also retain progenitor cell properties when injected into a developing embryo, with the ability to migrate together with the neural crest and differentiate to multiple tissues (80). However, in the majority of epithelial cell types, the developmental program has already been executed once and its outcomes are permanently embossed in the differentiated state of these cells, which are now ready to simply repopulate the damaged site. This situation is analogous to the recovery of a burnt forest: The soil is there, the shape of hills is preserved (“remembered”), and the only requirement is to add some seedlings there, nurture them, and then wait. In clinical practice, reepithelialization of skin abrasions or liver regeneration are typical examples of this healing mechanism that requires a certain degree of mesenchymal stromal integrity. In tissue engineering, this mechanism is exercised when more or less differentiated cells are seeded into scaffold matrices of future implants in vitro (81) or delivered straight to the target tissue in vivo (82).
Developmental memory recalled from local or circulating stem cells.
However, if the natural stroma is totally lost, or if an artificial scaffold cannot be manufactured, or if the structural or functional interconnections of cells are too complex (e.g., neural tissues or muscles), simple cell replacement cannot warrant full regeneration. The only hope is tissue engineering with the use of stem cells including embryonic stem cells (ESCs; Fig. 1, level IV). Stem cells possess the developmental plasticity (83) enabling them to recapitulate certain developmental pathways. In naturally healing tissues, there are three major sources of new materials: i) differentiated cells and stem cells remaining in the wounded area, ii) the same cell types plus blood and lymphatic vessels in the wound neighborhood, and iii) cells coming from remote locations, usually via the bloodstream, such as fibrocytes and circulating stem cells. Whereas tissue engineering can wisely use all of these natural resources, its main mission is to find alternative solutions in cases where any of the natural healing mechanisms are missing or insufficient. This may turn out to be an extremely complex task, since the stem cells used in experiments, including ESCs, often show unexpected affinities to adult tissues together with surprising differentiation capabilities—e.g., adipose or pancreatic stem cells can form neurons or glial and other cell types (84,85), and amniocytes can form kidney structures (86). Some of the natural and artificial cell repopulation processes will be discussed in the paragraphs below.
Age, mitotic memory, and acquired mutations.
The developmental potential, i.e., a differentiated state versus multi- or totipotency, is not the only memory carried by cells used in tissue engineering. Nonstem cells also remember their relative senescence (i.e., their “mitotic age” causing the Hayflick limit in cell culture; this essential vitality indicator has a loose correlation with the overall age of the organism) through telomere-independent as well as telomere-shortening-dependent mechanisms (Fig. 1, level I) (87,88). Thus, senescence assays may become an important tool in tissue engineering to avoid premature ageing and loss of engineered implants. Moreover, mutation and chromosomal analysis of cells used for organ construction is an adequate prerequisite to prevent implant failure or malignant transformation. This is particularly important when ESCs are used. Even well-developing early embryos are known to carry de novo originated chromosomal aberrations, and frequent remarkable genetic mosaicism has been well documented. Thus, single-cell ESC genetic diagnosis becomes a frontier in assisted reproduction and bioengineering (89–91), fulfilling one of the most important roles—providing a source of genetically healthy cells.
ORGAN LEVEL: INTEGRATION OF ALL MEMORY MECHANISMS
Granulation.
Possibly one of the best examples of the fact that wound healing is a predefined, uniformly programmed process, is granulation. Centuries ago it was noticed that healing wounds, regardless of their location, acquire a very similar and quite uniform appearance characterized by soft, moist gelatinous tissue with a bumpy surface comprised of small red nodules (Lat. granulationes) formed by new capillary loops (46). Granulation tissue always consists of i) revascularization components: new blood vessels and endothelial cells, ii) immune components consisting of inflammatory cells—mostly macrophages and neutrophils, but also other leukocytes in lesser amounts, iii) structural cells, that is fibroblasts and myofibroblasts, and iv) enzymes and components of a provisional ECM produced by fibroblasts. At the end of the granulation period, ECM production levels in the wound no longer exceed the amount of its degradation; granulation ceases and the excess fibroblasts commit apoptosis, which converts the granulation tissue from a cell rich environment into a one that consists mostly of collagen (92,93).
Fibroplasia—The beginning of tissue neoformation.
After the inflammatory period, cells within the wound begin to organize a new, primitive tissue (Fig. 1, levels V–VI). Tissue formation and remodeling begins about 2–3 days after a wound occurs, overlapping with the inflammatory phase. This requires selective apoptosis of certain cell types such as the PMNs and macrophages, as well as selective proliferation of mesenchymal cells such as endothelial cells and fibroblasts (93). Fibroblasts are a part of the mesenchymal stroma, and their migration into a wound is referred to as fibroplasia. By the end of the first week, fibroblasts are the predominant cells within the wound site. Factors such as fibronectin, PDGF, FGF, TGF-α and -β, and C5a, facilitate fibroblast migration and proliferation. TGF-β has been suggested as having a dual role: as a down-regulator of inflammation, as well as being the key growth factor for inducing fibrosis. To get to the wound site, fibroblasts migrate utilizing the fibrin/fibronectin wound clot, adhering to fibronectin. After migration and proliferation, fibroblasts begin to lay down ground substance and collagen. The collagen laid down early in wound healing, type III, is extremely important for the maintenance of wound strength. Fibroblasts also secrete growth factors that attract epithelial cells to the wound site.
Fibrocytes—Fibroblast-like progenitor cells.
The peripheral blood circulation contains a distinct population of bone marrow-derived fibroblast-like progenitor cells, referred to as fibrocytes, that retain the plasticity to differentiate along the mesenchymal lineages. Fibrocytes stimulated with profibrotic cytokines and growth factors in vitro produce large quantities of ECM components and further differentiate into cells identical to contractile myofibroblasts and fibroblasts observed to emerge at healing (94). Fibrocytes compose approximately 0.1–0.5% of circulating nonerythrocytic mononuclear cells. Fibrocytes express the MHC class I and II and costimulatory molecules CD80 and CD86, which allows them to exhibit antigen-presenting activity and activate both the CD4+ and CD8+ T lymphocytes. Therefore, it is hypothesized that undifferentiated circulating fibrocytes may possess monocyte-like characteristics, and that human fibrocytes may be present in the peripheral blood as mononuclear CD14+ precursors. As nonstimulated immature mesenchymal cells, fibrocytes do not produce large amounts of collagenous proteins or other ECM components until they differentiate into mature myofibroblasts or fibroblasts. Instead, fibrocytes are a vital source of matrix metalloproteinase 9, an ECM-degrading enzyme that facilitates wound remodeling (94). Fibrocytes can differentiate into several mesenchymal lineages: fibroblasts, myofibroblasts, and adipocytes (95), thus having a potential to functionally populate bioengineered scaffolds.
Fibroblasts and their role in wounds.
Once fibrocytes have completed their maturation from CD14+ mononuclear cells, differentiation into cells ultrastructurally and phenotypically similar to myofibroblasts and fibroblasts is promoted by stimulation with TGF-β. Fibroblasts are mesenchymal cells with a major role in building and remodelling the ECM. About a week after wounding, contraction of the wound takes place. Fibroblasts differentiate into myofibroblasts, which contain actin similar in structure to the actin found in smooth muscle, and function in wound contraction (96). Myofibroblasts attach to one another and the ECM at the wound edges, and pull on the ECM when they contract, thus reducing the wound size. This may be critical in healing of vitally important organs, such as the heart after myocardial infarction (97). The contraction period ends when myofibroblasts commit apoptosis (93,98). Contraction of a wound can last for several weeks and may continue, even after the wound is completely reepithelialized. It can lead to disfigurement and loss of function if excessively prolonged. Tensile strength is enhanced primarily by the degradation of type III collagen and the reorganization and production of type I collagen found in scar tissue. Tensile strength is also increased by the enzyme lysyl-oxidase, which covalently cross-links the collagen molecules. Over several months or more, the tensile strength of scar tissue can only achieve a maximum of about 80% of normal tissue strength (54). Recent findings indicate the involvement of TGF-β3 and PAI-1 in scar contraction (3), and specific fibroblast contractile and migratory properties may underlie scarless wound healing in the fetal period (99).
Fibroblast identity, regulation, and utilization in tissue engineering.
As a part of the body's architectural memory, the original mesenchymal fibroblasts that normally constitute tissues and organs have very specific gene expression patterns, defining their anatomical position within an axial coordinate system (Fig. 1, level II) (100). On the other hand, fibroblasts immigrate to wounded tissues, similar to immune cells involved in regeneration, carry the developmental memory of a cell type designed to execute a predefined set of functions during wound healing, regardless of the wound location. Unfortunately, there is poor understanding of whether these immigrated fibroblasts permanently carry a bone marrow positional imprinting or whether they will be reprogrammed to match the destination tissues. How this reprogramming could be regulated and how cells from remote locations can reconstruct the architecture of damaged organs remains to be shown in future research. Current data suggest that fibroblasts are subject to a broad and quite nonspecific external regulation and modification that can be used not only to enhance healing (101), but also may result in pathology (102–104). For example, invasive tumors can take over the regulation and start to use fibroblasts for the production of cell-invasion-favorable ECM, a situation referred to as normal wound healing gone awry (105). Recently, identical stereotyped transcriptional signatures of fibroblasts have been described in wounds and tumors (48). This is a major step forward, and a complete understanding of fibroblast regulation may become a powerful tool for tissue engineers, especially after it has been demonstrated that genetic engineering can revert fibroblasts back to the embryonic stem cell level with all the ESC-related developmental memory (106). Additional current studies support a hypothesis that cells within tissues sense environmental changes and adjust their reactions to the usual versus injurious stimuli (107). Differences in the wound healing of burns versus mechanical wounds, together with intriguing findings that fibroblast can memorize former exposure to specific biologic and mechanical stimuli, including wound coverage and outside pressure (103,108–111), demonstrate that fibroblasts are effective biosensors that can carry acquired memories over a certain period of time. Therefore, not only the source and age of fibroblasts, but also the methods of handling, culture media used, mechanical irritation such as tension, stretching or centrifugal forces, or 2D- versus 3D-culture, may substantially alter the experimental or therapeutic outcomes (66). Interestingly, similar properties have been discovered in mesenchymal stem cells (112). Subsequently, due to a need to repeat the same experiment with just subtle changes in fibroblast treatment and/or handling, the fibroblast plasticity and memory may make many bioengineering studies incredibly long and cumbersome. Precise standardization of all bioengineering procedures and their detailed description in publications emerges as a must in the current tissue engineering field.
Epithelization.
Epithelial plasticity plays an important role in development, wound healing, and cancer (113–115). Depending on the wound size, epithelialization occurs within hours to days after the injury of skin or mucosal membranes (Fig. 1, level VII). Epithelial cells migrate from the neighborhood to the surface of the granulation tissue. The cells undergo morphologic changes that enable them to detach from the neighboring cells and basement membrane without undergoing apoptosis, thus facilitating migration. This morphologic change includes degradation of desmosomes and hemidesmosomes. Epithelial cells also function in phagocytosis of the scab tissue and foreign matter, and are able to dissolve or dissect the clot, debris, and the ECM utilizing plasminogen activators, collagenases, and matrix metalloproteinases to help degrade the granulation tissue below. The epithelial cells continue to migrate until cells from either side meet in the middle. At this point, contact inhibition stimulates the epithelial cells to revert to their normal morphology and resume cell–cell and cell–matrix interaction. The basal cells continue to divide and proliferate until the normal epithelial structure is attained (46).
Full-thickness healing of epithelial structures.
Deep wounds in the adult skin severely impair the regenerative capability of hair follicles and skin glands to the extent that they may be completely lost, despite a presence of fully competent stem cells. The latest findings support the idea that these stem cells do not just get a proper developmental signal during natural wound healing, but they may be primed to recapitulate the skin morphogenesis under artificially induced Wnt signaling stimuli (1). This is in agreement with the latest study demonstrating that such regeneration may occur, although rarely, even without artificial stimulation, triggered via the same signaling pathway (116). Altogether, these exciting findings may accelerate development of new treatments for wounds, hair loss and other degenerative skin disorders. From the aspect of tissue memory, keratinocytes seem to be less sensitive to external stimuli than fibroblasts, and a successful fully functional replacement of urethral epithelium with epidermal cells (117) demonstrates that maintaining the body's original architecture is not always necessary for achieving good therapeutic results. Moreover, although certain changes in gene expression may occur in cells taken from individuals with metabolic disorders, recent findings suggest that keratinocytes from diabetic patients may be equally good for wound coverage as normal keratinocytes. This allows for the use of immunologically less problematic autografts for reepithelialization of chronic ulcers in diabetic patients (118). However, it is the denuded matrix of the wound surface that carries the memory of what epithelial type should reside on it, and in wound healing, the matrix determines whether normal epithelial cells would or would not be prone to populate the wound area. Therefore, considerable effort is made in engineering instructive signals into substrates that would enhance epithelialization, using the methods ranging from soaking matrices with growth factors, through chemical matrix surface activation, up to the use of whole organisms to make changes in wounded tissues, such as medicinal maggot application (119,120). Cell memory and sensitivity to mechanical stimuli has been recently used in promising studies of acute and chronic wound epithelialization with the use of ultrasound and shock waves, and are now entering phase III clinical evaluation (121). From a scientific point of view, intravital observation of wound healing processes is currently becoming a seminal source of information on natural cell behavior that could also be used in tissue engineering and assessment of biologic functionality of bioengineered tissues (122–124). The effects of temporary wound coverage, antibacterial protection, and other external conditions on the healing process are also an integral part of current research (125–128).
Vasculature, blood supply, and oxygen during wound healing.
Tissue healing increases metabolic turnover in tissues and is highly dependent on nutrition (129), energetic supplies, oxygen (130), and prompt waste removal. For example, fibroblasts, the main cells that deposit granulation tissue, depend on oxygen to proliferate and lay down the new ECM. Too little oxygen may inhibit their growth and deposition of ECM components, and can lead to excessive fibrotic scarring. Thus, healing processes would fail without effective blood and lymph circulation (46,131).
Blood vessels are often interrupted in wounds. Thus, new vessels must grow into the wounded tissue from the neighborhood (Fig. 1, level VIII), and not surprisingly, most active healing processes occur in the front line of revascularization. Endothelial cells are attracted to the wound area by fibronectin found in the fibrin scab and by growth factors released by other cells. Endothelial growth and proliferation is also stimulated by hypoxia and the presence of lactic acid in the wound. In a low-oxygen environment, macrophages and platelets produce angiogenic factors which attract endothelial cells chemotactically, such as macrophage-derived angiogenic factor (93). The IL-8, FGF, and vascular endothelial growth factor secreted by macrophages are also important for angiogenesis. New capillaries bud from existing capillaries and progress forward, binding the fibrin within the wound site. To migrate, endothelial cells need collagenases and plasminogen activator to degrade the clot and part of the ECM. Zinc-dependent metalloproteinases digest basement membranes and ECM to allow for cell proliferation and angiogenesis (54). When macrophages and other growth-factor-producing cells are no longer in a hypoxic, lactic-acid-rich environment, they stop producing angiogenic factors; when tissue is adequately perfused, migration and proliferation of endothelial cells is reduced. Eventually, blood vessels that are no longer needed die by apoptosis (132). All these events demonstrate that “memory” for proper healing does not always need to be something explicitly physical (like an ECM scaffold), but it also can be encoded indirectly, e.g., as the reactivity of cells to external stimuli.
Practical problems with vasculature in healing, regeneration, and bioengineering.
A widespread problem encountered in everyday life of the elderly is decreased natural angiogenesis in chronically photodamaged skin, while acute UV irradiation increases angiogenesis (133). Resolving this puzzle seems to be one of the keys to understanding and the treatment of skin aging and photodamage, as well as to development of the best practices for suntanning. In clinical practice, unavoidable vascular damage is often created during actinic, cytostatic or surgical therapy of tumors, and thus, development of gentle and specific tumoricidal therapies like targeted photodynamic approaches may help spare the normal tissue of oncology patients (134). Although in some other oncological strategies there has been a considerable effort made toward inhibition and/or destruction of blood vessels to devitalize tumor tissues (135), the opposite effort is usually exercised in tissue engineering and wound healing research (136,137) to keep regenerating or engineered tissues alive and functional. Three major areas of vessel engineering can be distinguished: i) reconstruction of large vessels using transplants and prostheses, ii) creation or induction of vessels in bioengineered constructs (i.e., imitation of embryonic vasculogenesis), and iii) enhancement of vessel invasion into the engineered material from the neighboring tissues (angiogenesis). Promising approaches, such as novel chemical compounds (138), modified protein matrices (139), genetically engineered stem cells (140), innovative application systems (141), and recombinant biomolecules (142), have demonstrated the potential to enhance angiogenesis and protect against ischemia in exciting experiments with wounded organs, including the skin and heart. However, attempts to engineer blood vessels may face severe difficulties due to a local tissue memory that imprints organ-specific phenotypes to endothelial cells (143). This phenotypic change will be extremely difficult to reproduce in engineered organs (Fig. 1, level VII), because the plasticity, reprogrammability, natural developmental timing and physiologic consequences of endothelial functional heterogenity are not fully understood. Recent findings support another intriguing possibility related to phenotypic endothelial variations: Endothelial cells may be involved in scarless wound healing via regulation of leukocyte transmigration (144). Overall, vascular problems currently represent a major source of problems in organ engineering. Certain wound types, such as chronic ulcers (145,146), are difficult to revascularize, and alternative oxygen delivery techniques via diffusion are a viable temporary solution (147). Because of the complex spatiotemporal dynamics of the vasculature, computer modeling is gaining increasing involvement in planning and assessing vascular replacements and other operations (135,148).
CONCLUSIONS
Although we have tried to capture a simplified image of the current knowledge of regenerative mechanisms in their broad complexity and mutual interconnections within a living organism, this article does not cover the maintenance of the body integrity at the highest levels such as neurohumoral regulation circuits, psychological influences on tissue physiology, or the concepts of life and its maintenance per se (149–151). We have concentrated on mechanisms that are known in molecular detail and have a direct influence on current biotechnologies. Whether it is wound healing or bioengineering, cells always need guidance to create proper structures. It is a goal of bioengineers to construct the best possible tissue scaffolds and to generate or mimic as many natural instructive signals as possible. Giving a complete list of these signals would be a task comparable to explaining the entire body of developmental biology. Thus, this article has rather attempted to provide an overview of the topic. By summarizing known principles of body structure maintenance in a systematic manner, we aim to make it easier to think about the body plan in a categorical and structured fashion. Hierarchization of the key principles of tissue memory is important for making decisions on the dispensability vs. indispensability of the individual components used in the fabrication of artificial organs, and may help with troubleshooting when our construct does not function in an expected manner. Furthermore, it is a general expectation that computational biology will bring a substantial insight into systems biology and will help to understand the self-preserving dynamics of body organization. We hope that our article will contribute to establishing a simplified, easily understandable framework effective in communicating the biologic events of wound healing not only among biomedical researchers, but also between biologists and computer scientists.
Abbreviations
- ECM:
-
extracellular matrix
- ESC:
-
embryonic stem cell
- FGF:
-
fibroblast growth factor
- PMN:
-
polymorphonuclear neutrophils
References
Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon R, Isik F 2006 Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 7: 4
Gilbert SF 2003 Developmental Biology. Sinauer Associates, Inc, Sunderland, pp 1–838
Li WY, Huang EY, Dudas M, Kaartinen V, Warburton D, Tuan TL 2006 Transforming growth factor-beta3 affects plasminogen activator inhibitor-1 expression in fetal mice and modulates fibroblast-mediated collagen gel contraction. Wound Repair Regen 14: 516–525
Lorenz HP, Longaker MT 2003 In utero surgery for cleft lip/palate: minimizing the “Ripple Effect” of scarring. J Craniofac Surg 14: 504–511
Yannas IV, Kwan MD, Longaker MT 2007 Early fetal healing as a model for adult organ regeneration. Tissue Eng 13: 1789–1798
Dudas M, Li WY, Kim J, Yang A, Kaartinen V 2007 Palatal fusion—where do the midline cells go? A review on cleft palate, a major human birth defect. Acta Histochem 109: 1–14
Takigawa T, Shiota K 2004 Terminal differentiation of palatal medial edge epithelial cells in vitro is not necessarily dependent on palatal shelf contact and midline epithelial seam formation. Int J Dev Biol 48: 307–317
Brown NL, Sandy JR 2007 Tails of the unexpected: palatal medial edge epithelium is no more specialized than other embryonic epithelium. Orthod Craniofac Res 10: 22–35
Ahmed S, Liu CC, Nawshad A 2007 Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3. Dev Biol 309: 193–207
Dudas M, Nagy A, Laping NJ, Moustakas A, Kaartinen V 2004 Tgf-beta3-induced palatal fusion is mediated by Alk-5/Smad pathway. Dev Biol 266: 96–108
Dudas M, Kim J, Li WY, Nagy A, Larsson J, Karlsson S, Chai Y, Kaartinen V 2006 Epithelial and ectomesenchymal role of the type I TGF-beta receptor ALK5 during facial morphogenesis and palatal fusion. Dev Biol 296: 298–314
Kryger ZB, Sisco M, Roy NK, Lu L, Rosenberg D, Mustoe TA 2007 Temporal expression of the transforming growth factor-Beta pathway in the rabbit ear model of wound healing and scarring. J Am Coll Surg 205: 78–88
Cabrera RC, Siebert JW, Eidelman Y, Gold LI, Longaker MT, Garg HG 1995 The in vivo effect of hyaluronan associated protein-collagen complex on wound repair. Biochem Mol Biol Int 37: 151–158
Prime SS, Pring M, Davies M, Paterson IC 2004 TGF-beta signal transduction in oro-facial health and non-malignant disease (part I). Crit Rev Oral Biol Med 15: 324–336
Dudas M, Kaartinen V 2005 Tgf-beta superfamily and mouse craniofacial development: interplay of morphogenetic proteins and receptor signaling controls normal formation of the face. Curr Top Dev Biol 66: 65–133
Ferguson MW, O'Kane S 2004 Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 359: 839–850
Drahovsky P, Dankovcik J, Bodnarova A, Drahovska I, Sabovcik R 1999 [Congenital diaphragmatic hernia manifesting after the neonatal period]. Rozhl Chir 78: 123–126
Xu H, Baldini A 2007 Genetic pathways to mammalian heart development: recent progress from manipulation of the mouse genome. Semin Cell Dev Biol 18: 77–83
van Wijk B, Moorman AF, van den Hoff MJ 2007 Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res 74: 244–255
Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K 2006 Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development 133: 3473–3484
McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP 2005 Extracellular matrix gene expression in the developing mouse aorta. In: Wassarman P, Miner J (eds) Advances in Developmental Biology 15, Extracellular Matrix in Development and Disease. Elsevier, Amsterdam, pp 81–128
Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS 2005 Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci USA 102: 8916–8921
Choi M, Stottmann RW, Yang YP, Meyers EN, Klingensmith J 2007 The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ Res 100: 220–228
Ma L, Lu MF, Schwartz RJ, Martin JF 2005 Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132: 5601–5611
Mercado-Pimentel ME, Runyan RB 2007 Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185: 146–156
De Coster PJ, Mortier G, Marks LA, Martens LC 2007 Cranial suture biology and dental development: genetic and clinical perspectives. J Oral Pathol Med 36: 447–455
Jiang R, Bush JO, Lidral AC 2006 Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn 235: 1152–1166
Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC 2006 Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nat Genet 38: 1335–1340
Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF 2005 Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132: 1453–1461
Warner DR, Horn KH, Mudd L, Webb CL, Greene RM, Pisano MM 2007 PRDM16/MEL1: a novel Smad binding protein expressed in murine embryonic orofacial tissue. Biochim Biophys Acta 1773: 814–820
Rice DP 2005 Craniofacial anomalies: from development to molecular pathogenesis. Curr Mol Med 5: 699–722
Depew MJ, Simpson CA 2006 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn 235: 1256–1291
Havens BA, Rodgers B, Mina M 2006 Tissue-specific expression of Fgfr2b and Fgfr2c isoforms, Fgf10 and Fgf9 in the developing chick mandible. Arch Oral Biol 51: 134–145
Mukhopadhyay P, Greene RM, Pisano MM 2006 Expression profiling of transforming growth factor beta superfamily genes in developing orofacial tissue. Birth Defects Res A Clin Mol Teratol 76: 528–543
Nie X, Luukko K, Kettunen P 2006 BMP signalling in craniofacial development. Int J Dev Biol 50: 511–521
Oka K, Oka S, Sasaki T, Ito Y, Bringas P Jr, Nonaka K, Chai Y 2007 The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development. Dev Biol 303: 391–404
Mancini ML, Verdi JM, Conley BA, Nicola T, Spicer DB, Oxburgh LH, Vary CP 2007 Endoglin is required for myogenic differentiation potential of neural crest stem cells. Dev Biol 308: 520–533
Sommer L 2006 Growth factors regulating neural crest cell fate decisions. Adv Exp Med Biol 589: 197–205
Hogan BM, Layton JE, Pyati UJ, Nutt SL, Hayman JW, Varma S, Heath JK, Kimelman D, Lieschke GJ 2006 Specification of the primitive myeloid precursor pool requires signaling through Alk8 in Zebrafish. Curr Biol 16: 506–511
Wan M, Cao X 2005 BMP signaling in skeletal development. Biochem Biophys Res Commun 328: 651–657
Chuong CM, Cotsarelis G, Stenn K 2007 Defining hair follicles in the age of stem cell bioengineering. J Invest Dermatol 127: 2098–2100
Gibran NS, Boyce S, Greenhalgh DG 2007 Cutaneous wound healing. J Burn Care Res 28: 577–579
Macneil S 2007 Progress and opportunities for tissue-engineered skin. Nature 445: 874–880
Li J, Chen J, Kirsner R 2007 Pathophysiology of acute wound healing. Clin Dermatol 25: 9–18
Stadelmann WK, Digenis AG, Tobin GR 1998 Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176: 26S–38S
Clark RA 1996 The molecular and cellular biology of wound repair. Plenum Press, New York, pp 1–620
Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE 2004 Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell 15: 5670–5677
Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO 2004 Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol 2: E7
Eming SA, Krieg T, Davidson JM 2007 Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127: 514–525
Iba Y, Shibata A, Kato M, Masukawa T 2004 Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol 4: 1873–1880
Schymeinsky J, Then C, Sindrilaru A, Gerstl R, Jakus Z, Tybulewicz VL, Scharffetter-Kochanek K, Walzog B 2007 Syk-mediated translocation of PI3Kdelta to the leading edge controls lamellipodium formation and migration of leukocytes. PLoS ONE 2: e1132
Gosain A, Muthu K, Gamelli RL, DiPietro LA 2007 Norepinephrine suppresses wound macrophage phagocytic efficiency through alpha- and beta-adrenoreceptor dependent pathways. Surgery 142: 170–179
Martin P, Leibovich SJ 2005 Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15: 599–607
Schultz SS 2005 Adult stem cell application in spinal cord injury. Curr Drug Targets 6: 63–73
Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP 2007 Microfabrication of three-dimensional engineered scaffolds. Tissue Eng 13: 1837–1844
Orwin E, Shah A, Voorhees A, Ravi V 2007 Bioreactor design for cornea tissue engineering: material-cell interactions. Acta Biomater 3: 1041–1049
Macri L, Silverstein D, Clark RA 2007 Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev 59: 1366–1381
Berrier AL, Yamada KM 2007 Cell-matrix adhesion. J Cell Physiol 213: 565–573
Libby P, Lee RT 2000 Matrix matters. Circulation 102: 1874–1876
Ruoslahti E, Reed JC 1994 Anchorage dependence, integrins, and apoptosis. Cell 77: 477–478
Petrigliano FA, English CS, Barba D, Esmende S, Wu BM, McAllister DR 2007 The effects of local bFGF release and uniaxial strain on cellular adaptation and gene expression in a 3D environment: implications for ligament tissue engineering. Tissue Eng 13: 2721–2731
Lee M, Chen TT, Iruela-Arispe ML, Wu BM, Dunn JC 2007 Modulation of protein delivery from modular polymer scaffolds. Biomaterials 28: 1862–1870
Sanclimens G, Shen H, Giralt E, Albericio F, Saltzman MW, Royo M 2005 Synthesis and screening of a small library of proline-based biodendrimers for use as delivery agents. Biopolymers 80: 800–814
Karp JM, Langer R 2007 Development and therapeutic applications of advanced biomaterials. Curr Opin Biotechnol 18: 454–459
Ryu W, Huang Z, Prinz FB, Goodman SB, Fasching R 2007 Biodegradable micro-osmotic pump for long-term and controlled release of basic fibroblast growth factor. J Control Release 124: 98–105
Rhee S, Grinnell F 2007 Fibroblast mechanics in 3D collagen matrices. Adv Drug Deliv Rev 59: 1299–1305
Ito K, Yamada Y, Naiki T, Ueda M 2006 Simultaneous implant placement and bone regeneration around dental implants using tissue-engineered bone with fibrin glue, mesenchymal stem cells and platelet-rich plasma. Clin Oral Implants Res 17: 579–586
Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B 2006 Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng 12: 2385–2396
Yen JL, Lin SP, Chen MR, Niu DM 2006 Clinical features of Ehlers-Danlos syndrome. J Formos Med Assoc 105: 475–480
Ferdous Z, Wei VM, Iozzo RV, Hook M, Grande-Allen KJ 2007 Decorin-TGF beta interaction regulates matrix organization and mechanical characteristics of 3-D collagen matrices. J Biol Chem 282: 35887–35898
Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, Kurata H, Williams GR, Polak J, Fisk NM 2008 Intrauterine transplantation of human fetal mesenchymal stem cells from first trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood 111: 1717–1725. Doi:10.1182/blood-2007-08-105809
Qiao B, Padilla SR, Benya PD 2005 Transforming growth factor (TGF)-beta-activated kinase 1 mimics and mediates TGF-beta-induced stimulation of type II collagen synthesis in chondrocytes independent of Col2a1 transcription and Smad3 signaling. J Biol Chem 280: 17562–17571
Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL 2007 Regulation of immature cartilage growth by IGF-I, TGF-beta1, BMP-7, and PDGF-AB: role of metabolic balance between fixed charge and collagen network. Biomech Model Mechanobiol, in press. Doi:10.1007/s10237-007-0096-8
Parker WL, Finnson KW, Soe-Lin H, Knaus P, Philip A 2007 Expression and function of TbetaRII-B, a variant of the type II TGF-beta receptor, in human chondrocytes. Osteoarthritis Cartilage 15: 442–453
Moreau JL, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP 2007 Tissue engineering solutions for cleft palates. J Oral Maxillofac Surg 65: 2503–2511
Israelsson C, Lewen A, Kylberg A, Usoskin D, Althini S, Lindeberg J, Deng CX, Fukuda T, Wang Y, Kaartinen V, Mishina Y, Hillered L, Ebendal T 2006 Genetically modified bone morphogenetic protein signalling alters traumatic brain injury-induced gene expression responses in the adult mouse. J Neurosci Res 84: 47–57
Breuer CK, Mettler BA, Anthony T, Sales VL, Schoen FJ, Mayer JE 2004 Application of tissue-engineering principles toward the development of a semilunar heart valve substitute. Tissue Eng 10: 1725–1736
Levi DS, Raff E, Stepan L, Liu J, Williams RJ, Moore JW, Carman G 2005 Use of a covered stent modification to produce a transcatheter valve: laboratory and animal testing. ASAIO J 51: 719–724
Hu Z, Luo F, Pan Y, Hou C, Ren L, Chen J, Wang J, Zhang Y 2007 Arg-Gly-Asp (RGD) peptide conjugated poly(lactic acid)-poly(ethylene oxide) micelle for targeted drug delivery. J Biomed Mater Res A, in press. Doi:10.1002/jbm.a.31615
Lwigale PY, Cressy PA, Bronner-Fraser M 2005 Corneal keratocytes retain neural crest progenitor cell properties. Dev Biol 288: 284–293
Warnke PH, Wiltfang J, Springer I, Acil Y, Bolte H, Kosmahl M, Russo PA, Sherry E, Lutzen U, Wolfart S, Terheyden H 2006 Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials 27: 3163–3167
Wang CC, Chen CH, Lin WW, Hwang SM, Hsieh PC, Lai PH, Yeh YC, Chang Y, Sung HW 2008 Direct Intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res 77: 515–524. Doi:10.1093/cvr/cvm046
Derynck R, Akhurst RJ 2007 Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol 9: 1000–1004
Dragoo JL, Carlson G, McCormick F, Khan-Farooqi H, Zhu M, Zuk PA, Benhaim P 2007 Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng 13: 1615–1621
Pierret C, Spears K, Maruniak JA, Kirk MD 2006 Neural crest as the source of adult stem cells. Stem Cells Dev 15: 286–291
Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, Warburton D, Atala A, De Filippo RE 2007 Renal differentiation of amniotic fluid stem cells. Cell Prolif 40: 936–948
Golubev A, Khrustalev S, Butov A 2003 An in silico investigation into the causes of telomere length heterogeneity and its implications for the Hayflick limit. J Theor Biol 225: 153–170
Blagosklonny MV 2006 Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 5: 2087–2102
Hlinka D, Dudas M, Herman M, Kalina I 2001 Experimental attempts to extend the current preimplantation genetic diagnosis with individual karyotypization of human blastomeres. Reprod Nutr Dev 41: 91–106
Shkumatov A, Kuznyetsov V, Cieslak J, Ilkevitch Y, Verlinsky Y 2007 Obtaining metaphase spreads from single blastomeres for PGD of chromosomal rearrangements. Reprod Biomed Online 14: 498–503
Findikli N, Candan NZ, Kahraman S 2006 Human embryonic stem cell culture: current limitations and novel strategies. Reprod Biomed Online 13: 581–590
El Sherif A, Yano F, Mittal S, Filipi CJ 2006 Collagen metabolism and recurrent hiatal hernia: cause and effect?. Hernia 10: 511–520
Greenhalgh DG 1998 The role of apoptosis in wound healing. Int J Biochem Cell Biol 30: 1019–1030
Bellini A, Mattoli S 2007 The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 87: 858–870
Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM 2007 Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem 282: 22910–22920
Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani G, Martin MD, Haaksma CJ, Hinz B 2006 Contraction of myofibroblasts in granulation tissue is dependent on Rho/Rho kinase/myosin light chain phosphatase activity. Wound Repair Regen 14: 313–320
Virag JA, Rolle ML, Reece J, Hardouin S, Feigl EO, Murry CE 2007 Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol 171: 1431–1440
Desmouliere A, Chaponnier C, Gabbiani G 2005 Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13: 7–12
Sandulache VC, Parekh A, Dohar JE, Hebda PA 2007 Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng 13: 2791–2801
Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY 2006 Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet 2: e119
Watterson KR, Lanning DA, Diegelmann RF, Spiegel S 2007 Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen 15: 607–616
Roy S, Khanna S, Rink T, Radtke J, Williams WT, Biswas S, Schnitt R, Strauch AR, Sen CK 2007 p21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Mol Biol Cell 18: 4837–4846
Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG 2007 Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 46: 1246–1256
Goldberg MT, Han YP, Yan C, Shaw MC, Garner WL 2007 TNF-alpha suppresses alpha-smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Invest Dermatol 127: 2645–2655
Dvorak HF 1986 Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659
Chang HY, Cotsarelis G 2007 Turning skin into embryonic stem cells. Nat Med 13: 783–784
Farahani RM 2007 Hidden cameras for lifelong injurious stimuli: a hypothetic approach toward “wound memory”. Med Hypotheses 69: 472–473
McNulty AK, Schmidt M, Feeley T, Kieswetter K 2007 Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. Wound Repair Regen 15: 838–846
Ghazarian A, Garner WL, Ehrlich HP 2000 Memory of past exposure to the chemokine IL-8 inhibits the contraction of fibroblast-populated collagen lattices. Exp Mol Pathol 69: 242–247
Karamichos D, Lakshman N, Petroll WM 2007 Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest Ophthalmol Vis Sci 48: 5030–5037
Wang JH, Thampatty BP, Lin JS, Im HJ 2007 Mechanoregulation of gene expression in fibroblasts. Gene 391: 1–15
Park JS, Huang NF, Kurpinski KT, Patel S, Hsu S, Li S 2007 Mechanobiology of mesenchymal stem cells and their use in cardiovascular repair. Front Biosci 12: 5098–5116
Thiery JP, Sleeman JP 2006 Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW 2007 Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol 213: 374–383
Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A 2005 TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 16: 1987–2002
Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G 2007 Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320
Fu Q, Deng CL, Liu W, Cao YL 2007 Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int 99: 1162–1165
Brandner JM, Zacheja S, Houdek P, Moll I, Lobmann R 2008 Expression of matrix metalloproteinases, cytokines and connexins in diabetic and non-diabetic human keratinocytes before and after transplantation into an ex-vivo wound healing model. Diabetes Care 31: 114–120
Rimmer S, Johnson C, Zhao B, Collier J, Gilmore L, Sabnis S, Wyman P, Sammon C, Fullwood NJ, Macneil S 2007 Epithelialization of hydrogels achieved by amine functionalization and co-culture with stromal cells. Biomaterials 28: 5319–5331
Sherman RA, Shapiro CE, Yang RM 2007 Maggot therapy for problematic wounds: uncommon and off-label applications. Adv Skin Wound Care 20: 602–610
Schaden W, Thiele R, Kolpl C, Pusch M, Nissan A, Attinger CE, Maniscalco-Theberge ME, Peoples GE, Elster EA, Stojadinovic A 2007 Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res 143: 1–12
Chong AK, Satterwhite T, Pham HM, Costa MA, Luo J, Longaker MT, Wyss-Coray T, Chang J 2007 Live imaging of Smad2/3 signaling in mouse skin wound healing. Wound Repair Regen 15: 762–766
Askenasy N, Farkas DL 2003 In vivo imaging studies of the effect of recipient conditioning, donor cell phenotype and antigen disparity on homing of haematopoietic cells to the bone marrow. Br J Haematol 120: 505–515
Sorg H, Krueger C, Vollmar B 2007 Intravital insights in skin wound healing using the mouse dorsal skin fold chamber. J Anat 211: 810–818
Parnell LK, Ciufi B, Gokoo CF 2005 Preliminary use of a hydrogel containing enzymes in the treatment of stage II and stage III pressure ulcers. Ostomy Wound Manage 51: 50–60
Telgenhoff D, Lam K, Ramsay S, Vasquez V, Villareal K, Slusarewicz P, Attar P, Shroot B 2007 Influence of papain urea copper chlorophyllin on wound matrix remodeling. Wound Repair Regen 15: 727–735
Teot L, Lambert L, Ourabah Z, Bey E, Steenman C, Wierzbiecka E, Malikov S, Charles JP, Vives F, Bohbot S 2006 Use of topical negative pressure with a lipidocolloid dressing: results of a clinical evaluation. J Wound Care 15: 355–358
Yates CC, Whaley D, Babu R, Zhang J, Krishna P, Beckman E, Pasculle AW, Wells A 2007 The effect of multifunctional polymer-based gels on wound healing in full thickness bacteria-contaminated mouse skin wound models. Biomaterials 28: 3977–3986
Barbul A, Uliyargoli A 2007 Use of exogenous arginine in multiple organ dysfunction syndrome and sepsis. Crit Care Med 35: S564–S567
Hopf HW, Rollins MD 2007 Wounds: an overview of the role of oxygen. Antioxid Redox Signal 9: 1183–1192
Brem H, Tomic-Canic M 2007 Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117: 1219–1222
Lansdown AB, Sampson B, Rowe A 2001 Experimental observations in the rat on the influence of cadmium on skin wound repair. Int J Exp Pathol 82: 35–41
Chung JH, Eun HC 2007 Angiogenesis in skin aging and photoaging. J Dermatol 34: 593–600
Kleban J, Mikes J, Szilardiova B, Koval J, Sackova V, Solar P, Horvath V, Hofmanova J, Kozubik A, Fedorocko P 2007 Modulation of hypericin photodynamic therapy by pretreatment with 12 various inhibitors of arachidonic acid metabolism in colon adenocarcinoma HT-29 cells. Photochem Photobiol 83: 1174–1185
Grizzi F, Colombo P, Taverna G, Chiriva-Internati M, Cobos E, Graziotti P, Muzzio PC, Dioguardi N 2007 Geometry of human vascular system: is it an obstacle for quantifying antiangiogenic therapies?. Appl Immunohistochem Mol Morphol 15: 134–139
Badiavas EV, Ford D, Liu P, Kouttab N, Morgan J, Richards A, Maizel A 2007 Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen 15: 856–865
Malda J, Klein TJ, Upton Z 2007 The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng 13: 2153–2162
Nangaku M, Izuhara Y, Takizawa S, Yamashita T, Fujii-Kuriyama Y, Ohneda O, Yamamoto M, van Ypersele de Strihou C, Hirayama N, Miyata T 2007 A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler Thromb Vasc Biol 27: 2548–2554
Callegari A, Bollini S, Iop L, Chiavegato A, Torregrossa G, Pozzobon M, Gerosa G, De CP, Elvassore N, Sartore S 2007 Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 28: 5449–5461
Shujia J, Haider HK, Idris NM, Lu G, Ashraf M 2008 Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res 77: 525–533. Doi:10.1093/cvr/cvm077
Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P 2007 Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13: 1299–1312
Li X, Zheng L, Peng F, Qi C, Zhang X, Zhou A, Liu Z, Wu S 2007 Recombinant thymosin beta 4 can promote full-thickness cutaneous wound healing. Protein Expr Purif 56: 229–236
Aird WC 2007 Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100: 174–190
Naik-Mathuria B, Gay AN, Zhu X, Yu L, Cass DL, Olutoye OO 2007 Age-dependent recruitment of neutrophils by fetal endothelial cells: implications in scarless wound healing. J Pediatr Surg 42: 166–171
Sheehan P 2006 Early change in wound area as a predictor of healing in diabetic foot ulcers: knowing “when to say when”. Plast Reconstr Surg 117: 245S–247S
Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma-Bryant L 2006 Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 14: 680–692
Fife CE, Buyukcakir C, Otto G, Sheffield P, Love T, Warriner R III 2007 Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen 15: 322–331
Spilker RL, Feinstein JA, Parker DW, Reddy VM, Taylor CA 2007 Morphometry-based impedance boundary conditions for patient-specific modeling of blood flow in pulmonary arteries. Ann Biomed Eng 35: 546–559
Souza BR, Cardoso JF, Amadeu TP, Desmouliere A, Costa AM 2005 Sympathetic denervation accelerates wound contraction but delays reepithelialization in rats. Wound Repair Regen 13: 498–505
Rossi EL 2003 Gene expression, neurogenesis, and healing: psychosocial genomics of therapeutic hypnosis. Am J Clin Hypn 45: 197–216
Meyer SC 2003 DNA and the origin of life: information, specification, and explanation. In: Campbell JA, Meyer SC (eds) Darwinism, Design, and Public Education. Michigan State University Press, East Lansing, pp 1–544
Acknowledgements
We thank Dr. M. Goldirova of Safarik University and colleagues from the Saban Research Institute for consultations and a review of the text.
Author information
Authors and Affiliations
Corresponding author
Additional information
This interdisciplinary work was supported by the following independent research funds across different authors' institutions: K99DE018459 (NIDCR, NIH), R01GM055081 (NIGMS, NIH), 1/4320/07 (VEGA, SK), LPP-0155-06 (APVV, SK).
Rights and permissions
About this article
Cite this article
Dudas, M., Wysocki, A., Gelpi, B. et al. Memory Encoded Throughout Our Bodies: Molecular and Cellular Basis of Tissue Regeneration. Pediatr Res 63, 502–512 (2008). https://doi.org/10.1203/PDR.0b013e31816a7453
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31816a7453
This article is cited by
-
Discussing the final size and shape of the reconstructed tissues in tissue engineering
Journal of Artificial Organs (2023)
-
Regenerative tissue filler for breast conserving surgery and other soft tissue restoration and reconstruction needs
Scientific Reports (2021)
-
Adipose stem cells from type 2 diabetic mice exhibit therapeutic potential in wound healing
Stem Cell Research & Therapy (2020)
-
Cutaneous wound healing: recruiting developmental pathways for regeneration
Cellular and Molecular Life Sciences (2013)
-
A preliminary study of differentially expressed genes in expanded skin and normal skin: implications for adult skin regeneration
Archives of Dermatological Research (2011)