Abstract
Severe obesity is a major problem in patients suffering from craniopharyngioma (CP), a benign tumor located in pituitary and hypothalamic regions. In this study, the hypothesis that hypothalamic damage leads to a reduction in overall sympathetic tone was tested. Catecholamines, as well as their metabolites homovanillic acid (HVA) and vanillylmandelic acid (VMA), markers of catecholamine turnover, were measured in morning voided urine of 109 patients participating in a German pediatric CP study, and their physical activity was analyzed using a questionnaire. HVA and VMA results were compared with age-matched HVA and VMA in urine of patients proven to not have a catecholamine-secreting tumor. Patients with the most severe obesity displayed the lowest urine HVA and VMA values. Patients with hypothalamic CP had 3.2-fold higher BMI values (p < 0.0001), lower HVA (0.72-fold, p < 0.001), and VMA (0.84-fold, p < 0.01) values, and significantly lower activity scores than those without hypothalamic involvement, but their epinephrine- and norepinephrine/creatinine ratios were not significantly different, possibly due to low levels. The low HVA and VMA values suggest decreased sympathetic outflow contributing to reduced physical activity and severe obesity, especially in patients with a hypothalamic tumor. In further studies investigating treatment options for hypothalamic obesity, disturbed sympathetic tone should be considered.
Similar content being viewed by others
Main
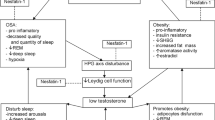
CP is a benign tumor located in pituitary and hypothalamic regions. In the German CP study “KRANIOPHARYNGEOM 2000,” the majority of patients with CP diagnosed during childhood suffer from obesity after tumor extirpation, likely resulting from destruction of parts of the medial hypothalamus (1–4). An association between surgery and irradiation affecting hypothalamic structures and hypothalamic obesity is known (5,6). Disturbed regulation between the hypothalamus, pituitary, and peripheral hormonal production necessitates hormonal substitution involving thyroid hormone, growth hormone, desmopressin acetate, hydrocortisone, and gonadal steroids. However, many patients receiving hormonal substitution suffer from obesity exacerbated by reduced physical activity, increased daytime sleepiness, and unmodulated hunger (5–7). Hypothalamic structures integrate afferent hormonal feedback signals from the body such as leptin, ghrelin, peptide YY (PYY), insulin, glucose, and energy homeostasis–regulating signals released from neurons like neuropeptide Y, agouti-related peptide, alpha-melanocyte-stimulating hormone (α-MSH), and cocaine-amphetamine regulated transcript (8). The ventromedial nucleus of the hypothalamus (VMH) also regulates efferent autonomic vagal and sympathetic nervous activity (9).
We suspected that parameters regulating the overall sympathetic tone are reduced in CP patients. To address this question, we measured dopamine and epinephrine and norepinephrine, as well as the major catecholamine metabolites, HVA and VMA, in morning voided urine and also analyzed the patients' self-estimated physical activity.
PATIENTS AND METHODS
Patients.
All 212 patients involved in the German pediatric CP study “KRANIOPHARYNGEOM 2000” were asked to voluntarily participate after receiving an information letter. Informed consent was obtained from all patients. Data from 109 (59 male, 50 female) out of 123 participating patients were evaluable after their completed questionnaires, written consent, and urine samples had been received. Using the questionnaire, the patients were asked to record their current treatment, their heart rate at morning in bed before standing up, and three questions about their self-estimated physical activity. They were also asked to document their most recent blood pressure values measured by their attending physician up to 6 wk before the urine sample collection. All 109 patients had undergone cranial tumor surgery. In 69% of these patients, hypothalamic involvement, assessed by intraoperative microscopic inspection and/or imaging, was present. A complete tumor excision had been obtained in 36% of patients, whereas 42% of all 109 patients (66% of 70 patients with noncomplete resection) received percutaneous cranial irradiation. In 30% of 109 patients, the tumor relapsed.
Body weight of patients and controls was evaluated by calculating the body mass index (BMI): BMI = weight (kg)/ height2 (m2) and expressed as a SD score (SDS) (10). Severe obesity was defined as BMI >4 SDS, nonsevere obesity as BMI 2–4 SDS, and nonobesity as BMI <2 SDS. CP patients were assessed for hormonal deficiencies and were adequately treated as required (Table 1). The study protocol and the questionnaire were approved by the local standing committee for clinical studies and the committee on ethical practice. The investigations were conducted according the principles expressed in the Declaration of Helsinki. Written parental and/or patient consent was obtained in all cases.
Catecholamine metabolites in urine of CP patients.
The urine specimens of the second morning voided urine were obtained at home and sent by mail at room temperature. Urine pH was checked before analysis and showed no evidence of bacterial overgrowth in the samples. In the first part of the study, epinephrine, norepinephrine, and dopamine were simultaneously measured using an Agilent Series 1100 HPLC (Agilent Technologies, Inc., Santa Clara, CA) and a commercially available reversed-phase HPLC assay (urinary catecholamines by HPLC; Bio-Rad, Munich, Germany) coupled with electrochemical detection (Bio-Rad) according to the manufacturer's instructions. Briefly, after addition of the internal standard, the weakly basic amines were bound to a disposable column, which was filled with a cation-exchange resin. Epinephrine, norepinephrine, and dopamine were then specifically eluted from the column with boric acid. Subsequently, the eluate was separated into its individual components by ion-pair chromatography on a reversed0phase cartridge. The mobile phase was set at a flow rate of 0.5 mL/min.
In the second part of the study, we focused on urinary HVA and VMA concentrations performed by gas chromatography/mass spectrometry (11). HVA and VMA were detectable in the urine in all patients. Urinary concentrations for HVA and VMA of children and adolescents proven not to have a catecholamine-secreting tumor served as controls. In all patients as well as 540 same-age controls, VMA/creatinine and HVA/creatinine ratios were calculated. In a second step, ratios of individual CP patients were divided by mean VMA/creatinine and HVA/creatinine values of age-matched controls (unpublished data). The lower detection limits for HVA and VMA were 0.02 nmol/μmol creatinine, which is about 50 times less than the lowest concentration found in any sample we have ever seen. The interassay and intraassay coefficients of variance are 6.2%/5.7% and 4.6%/6.5% for HVA and VMA, respectively.
Functional capacity and quality of life.
To assess functional capacity, a German ability scale, FMH, was used as previously described (12,13). The FMH scale assesses the functional capability for daily activities using 56 items. The average time for answering the questionnaire is 4.5 min. The questionnaire is in German and all participants of the study were native German speakers. It was standardized using 971 persons (45% female), aged 0–102 y, resulting in age-dependent percentiles with a retest reliability coefficient of 0.99. The validity of the FMH scale has been tested in brain tumor patients (13).
Physical activity scores.
Before this study, questionnaire results were validated using data from a mixed collective of 102 patients who were consecutively seen in the outpatient unit of the Department of General Pediatrics at the University Hospital in Bonn, Germany. Using this questionnaire, the self-estimated daily physical activity was recorded using scores ranging from –2.0 to 2.0 in 0.5 increments: “activity much less” was scored –2.0, “comparable” was scored 0, and “activity much more” was scored 2.0. Scores were described in the questionnaire as values relative to healthy people of same age for categorical variables of overall physical activity, speed in daily procedures, and walking speed.
Values of the 102 normal weight controls showed a normal distribution with a median value of 0 for all three assessments. Patients with acute or severe diseases, untreated hormonal deficiencies, corticoid therapy, and obesity were disqualified from participating. In all 109 CP patients, self-estimated activity data were recorded and analyzed using this questionnaire assessing comparative pretreatment versus posttreatment variables. In a further analysis, questionnaire results of two BMI- and gender-matched subgroups of CP patients, one with and the other without hypothalamic tumor, were compared with 22 healthy same-age overweight patients of same sex distribution without CP. The latter group was examined in the outpatient clinic for obesity before obesity treatment.
Calculations and statistics.
In cases of nonparametric values, statistical analysis was performed using Mann-Whitney test or Kruskal-Wallis test followed by Dunn's test for comparison of two or multiple groups, respectively. Categorical FMH data were analyzed using Fisher's exact test. Although activity scores are discrete numerical data, we considered them as continuous for statistical analyses and analyzed them by one-way ANOVA followed by Bonferroni's multiple comparisons test. All analysis differences were considered significant if p < 0.05 using two-tailed tests. The analysis was performed using the Prism program (GraphPad Software, San Diego, CA).
RESULTS
Clinical data and hormonal substitution of the different BMI groups.
In 44 patients, the SDS score for BMI was >4 SDS (severely obese), in 28 patients was 2–4 SDS (moderately obese), and in 37 patients was <2 SDS (nonobese) (Table 1). In the three different BMI groups, rates of hormonal substitution were similar, but hypothalamic tumors were significantly (p < 0.0001) more frequent in overweight patients (91% and 81% of patients with BMI >4 SDS or BMI 2–4 SDS, respectively) than in patients of normal weight (30% of patients with BMI <2 SDS). The functional capacity as assessed by FMH scores was reduced in 23.3% of patients with a BMI >4 SDS, compared with 4.6% and 10.3% of patients with BMI 2–4 SDS or <2 SDS, respectively. This difference was statistically not significant (Table 1).
HVA and VMA concentrations in urine.
In the first part of the study, in 50% of the patients epinephrine and dopamine levels in urine were below the detection limit of 2 μg/L and 6.5 μg/L, whereas in all but nine patients, norepinephrine was clearly above the lower detection limit of 2 μg/L. In all patients, values for catecholamines and catecholamine metabolites were related to creatinine concentration in the same urine samples. In patients with hypothalamic involvement, norepinephrine/creatinine ratios were not lower than in patients without hypothalamic tumor: 0.367 ± 0.041 versus 0.333 ± 0.038 μg/mg, mean ± SEM respectively. No correlation was found between norepinephrine and HVA or VMA.
In the second part of the study, HVA and VMA were normalized in relation to urinary creatinine from CP patients and were compared with age-matched controls proven not to have a catecholamine-secreting tumor, e.g. CP-patient's HVA (or VMA) expressed as nmol/μmol creatinine divided by HVA (or VMA) nmol/μmol creatinine of controls (mean normal value = 1.00). Normalized HVA and VMA values were significantly lower in obese CP patients than in CP patients with normal BMI (Fig. 1). Patients with hypothalamic CP had higher BMI-SDS values (p < 0.0001) and lower normalized HVA (p < 0.001) and VMA (p < 0.01) values than CP patients without hypothalamic involvement (Fig. 2, A–C). Normalized HVA and VMA values of patients who received cranial irradiation (RX) versus patients who received no irradiation (no RX) did not differ significantly for HVA (p = 0.62) or VMA (p = 0.32). Furthermore, HVA and VMA values did not differ between patients who underwent total excision and those who underwent subtotal resection [95% confidence interval (CI): HVA: 0.63–0.87 versus 0.81–1.04, p = 0.06; VMA: 0.68–0.91 versus 0.71–0.85, p = 0.87; patients with complete excision versus incomplete resection, respectively]. Neither did they differ between patients with tumor recurrence and patients without recurrence (95% CI: HVA: 0.75–0.96 versus 0.72–1.06, p = 0.74; VMA: 0.70–0.91 versus 0.73–0.95, p = 0.64; patients with versus without tumor recurrence, respectively).
We checked for differences in urinary HVA and VMA values between those patients with normal adrenal function and those with adrenal insufficiency (and therefore treated with hydrocortisone) and found no significant differences (95% CI: HVA: 0.79–0.99 versus 0.60–1.09, p = 0.75; VMA: 0.75–0.94 versus 0.62–1.09, p = 0.93; patients with versus without secondary adrenal insufficiency, respectively). In the patients with CP, correlation between normalized HVA and VMA ratios was significant (Fig. 3).
Physical activity scores.
Activity scores were significantly lower in CP patients with high BMI compared with CP patients with normal BMI (self-estimated overall physical activity BMI-SDS >4 (severely obese): –1.36 ± 0.12; BMI-SDS 2–4 (moderately obese): –0.88 ± 0.13; versus BMI-SDS <2 (nonobese): –0.21 ± 0.17; mean ± SEM, p < 0.0001 and p < 0.01, respectively). Patients with hypothalamic involvement of CP had significantly lower activity scores compared with those without hypothalamic involvement (Fig. 4). Analysis of comparable BMI groups (22 patients in each group, mean BMI-SDS 2.12–2.29) showed lower overall physical activity scores in patients with HI compared with CP patients without HI (no HI) and overweight controls (Table 2).
Scores of overall physical activity (OA), speed in daily procedures (SP), and walking speed (WS) are shown in CP patients with (HI, n = 67) and without (no HI, n = 42) hypothalamic involvement. Bars show mean values ±SEM; ‡p < 0.001; †p < 0.05 compared with the respective parameter in patients without hypothalamic involvement.
Heart rate and blood pressure comparisons.
The morning heart rate was documented in 62 patients and the blood pressure values were recorded in 70 patients. To compare heart rate and blood pressure scores, we formed three same-sized groups of patients according to their VMA values and according to their HVA values, respectively: group A being the group with the lowest values, group C with the highest values, and group B between groups A and C. We found in VMA group A, CP patients with the lowest VMA values (VMA patient/VMA control ratios), an average heart rate of 67.5 ± 2.5, compared with 71.8 ± 3.9 in VMA group B, and 75.0 ± 2.7 beats per minute in VMA group C, mean ± SEM, p < 0.05 between groups A and C. No significant differences were seen in the heart rates of the three HVA groups. The systolic and diastolic blood pressure values did not differ significantly between the groups with lowest, middle, or highest VMA or HVA values.
DISCUSSION
The results demonstrate a reduced sympathetic tone as indicated by urine catecholamine metabolites in the majority of our CP patients suffering from severe obesity. In a previous study, we speculated that there might be a causal link between disturbance of the hypothalamic regulation of the sympathetic nervous system, reduced spontaneous physical activity, increased daytime sleepiness, unmodulated hunger, and severe obesity in CP patients (7). In the present study, we found markedly lower physical activity scores in CP patients with hypothalamic involvement which is consistent with our hypothesis of disturbed sympathoadrenergic feedback mechanism and its consequences.
Plasma HVA and VMA are filtrated in the kidneys, contributing to urine HVA and VMA. The origin of HVA is the catecholamine precursor dopamine, whereas the main origin of VMA is epinephrine, which is synthesized and stored in the adrenal medulla and released into the systemic circulation, as well as norepinephrine, which is synthesized and stored in peripheral nerve endings (14). In recent years, understanding of the catecholamine metabolism has significantly improved. One important discovery is that metabolization of catecholamines mostly takes place within the same cells where amines are synthesized. Also, most neuronal catecholamines are metabolized intraneuronally after leakage from stores. It has also been clearly demonstrated that deamination of norepinephrine and epinephrine produces a reactive aldehyde that is reduced to form 3,4-dihydroxyphenylglycol (DHPG). The major pathway of VMA formation is via oxidation of 3-methoxy-4-hydroxyphenylglycol (MHPG) formed by O-methylation of (DHPG) in the liver (15). It has also been shown that most MHPG is derived from DHPG formed in peripheral sympathetic nerves, that MHPG sulfate reflects norepinephrine turnover in the gastrointestinal tract, and that urinary dopamine is derived mainly from plasma DOPA. Physiologically, urinary VMA is much more abundant than urinary epinephrine or norepinephrine due to the metabolic pathway mentioned above. In our study, CP patients had low urinary epinephrine, norepinephrine, and dopamine concentrations. Norepinephrine levels did not show any differences between patients with and without hypothalamic tumor, whereas there were clear differences in VMA and HVA values between these two groups and there was a clear correlation between normalized urinary HVA and VMA values.
We found evidence for reduced physical activity, especially in patients who suffered from CP with hypothalamic involvement leading to severe obesity. To rule out that the higher degree of obesity alone is the reason for decreased physical activity, we analyzed activity scores from subgroups of patients with comparable BMI, e.g., CP patients, one subgroup with and one without hypothalamic tumor, and one subgroup of overweight patients without CP. Only in the subgroup of CP patients with hypothalamic tumor were physical activity scores significantly lower than in the other two subgroups. It is possible that, in these patients, further factors such as visual disorders contribute to reduced physical activity (2). In patients with BMI >4 SDS, the functional capacity and quality of life as assessed by FMH scores were more frequently reduced than in patient groups with BMI 2–4 SDS or <2 SDS. A possible explanation is the higher percentage of large tumors, which, in the majority of cases, affect hypothalamic structures as well as optic nerves/chiasm, and also the high BMI as a disabling factor itself (12).
Several neuroendocrine signals are known to influence afferent signals of body weight homeostasis. However, the sympathetic nervous system represents the main efferent signal path and determines energy expenditure as well as substrate utilization (8,9). Nuclei located in the medial hypothalamus play a key role in the control of feeding circuits and energy homeostasis and neurons of the arcuate nucleus (ARH) are primary targets for many peripheral metabolic signals of the energy homeostasis. In the ARH, these hormonal signals are transformed into neuronal signals that are transmitted to the periventricular hypothalamus (PVH). The PVH integrates signals from feeding circuits and regulates the hypothalamic hormone production as well as the autonomic outflow to the sympathetic regulation of energy expenditure (16). Augmented sympathetic activity and diminished food intake are mediated by norepinephrine at β2- and/or β3-receptors in the brain (9). Several animal models demonstrated that lesions of medial hypothalamic structures, especially of the VMH, promote hyperphagia and decrease thermogenesis in brown adipose tissue due to reduced β3-adrenergic sympathetic signaling pathway (17).
In a clinical study with adult patients, hypoglycemia-induced sympathoadrenal activation was impaired in five out of eight patients with hypothalamic CP. However, no link between defective sympathoadrenal counter-regulation and postoperative weight gain could be demonstrated in the study (18). In a recent pediatric CP study, reduced epinephrine concentrations were found in plasma and urine of patients with CP compared with controls (19). Treatment with the central nervous stimulant dextroamphetamine resulted in stabilization of weight gain in children who experienced obesity following surgical resection for CP. Furthermore, significant improvements in children's overall activity and attention were observed in this study (20). Results of these and the current study support our hypothesis of decreased sympathoadrenal regulation as an important contributing factor to obesity in CP. It is also important to note that Lustig et al. (5) reported reduction of weight gain in CP patients treated with octreotide in a double-blind, placebo-controlled study. The authors suggest that VMH damage disinhibits the efferent output of the vagal nerve, which acts on the pancreatic β-cell, leading to increased postprandial insulin secretion in patients with CP. Other factors, such as growth hormone deficiency and other pituitary hormonal deficiencies, must also be discussed in the context of obesity in craniopharyngioma (5,21). Obviously, in CP patients, a disturbed sympathoadrenergic regulation leading to reduced physical activity is a contributing factor to severe obesity, especially in patients with hypothalamic tumor involvement. Even though no direct causality has been proven yet, a disturbed sympathetic tone should be considered in further studies investigating treatment options for hypothalamic obesity.
There are several limitations of our study that have to be taken into account when interpreting the results. First, there might be a bias as only approximately half of all patients of the German pediatric CP study volunteered to participate in these investigations. Second, there were no age-related reference values for the values of urinary norepinephrine, epinephrine, and dopamine. Only values for HVS and VMS could be normalized by calculating ratios of the values of CP patients in comparison to age-matched unaffected controls, since a strong age dependency of normal HVS and VMS values exists. In patients with pheochromocytoma, the HPLC method used for the detection of norepinephrine, epinephrine, and dopamine is reliable for detecting increased catecholamine secretion, but for evaluating results in our CP patients, the metabolites HVS and VMS proved to be more informative than norepinephrine, epinephrine, and dopamine in detecting conditions connected to low sympathetic outflow. Third, patients reported their physical activity by answering a questionnaire. Even though this method is vulnerable to bias due to the subjective nature of self-reporting, due to the multicenter study setting we could not use accelerometers as we did in our previous study (7). Looking at results of the three VMA value-graduated groups and the three HVA value-graduated groups, we found a lower morning heart rate in the lowest VMA group compared with the highest VMA group. But there were no significant blood pressure differences between any of these groups. This might be due to the fact that the blood pressure measurements were not performed under controlled conditions nor were the values available for all patients. The higher BMI in patients with low VMA could potentially contribute to a relatively high blood pressure, which in turn could equalize a low blood pressure due to a sympathetic tone. Clearly, future studies under well-controlled clinical conditions are needed to address this anomaly.
These limitations not withstanding, we conclude that the lower HVA and VMA values in CP patients with hypothalamic tumor probably indicate a disturbed sympathoadrenergic regulation. Thus, the disturbance of the sympathoadrenergic regulation is at least empirically supported by the self-assessment survey of this study, and these lower values are conceivable contributing factors in the development of severe obesity and reduced physical activity in these patients. We also conclude that the detection of urinary epinephrine, norepinephrine, and dopamine might be more useful in diagnosing conditions of exaggerated catecholamine production, such as in patients with pheochromocytoma, where large amounts of catecholamines are leaking from tumor cells, than for reflecting conditions with reduced catecholamine production and secretion. The metabolites HVS and VMS are also useful for detecting conditions of low catecholamine output. Accordingly, we suggest that strategies for increasing the sympathetic tone should be considered in the overall treatment of hypothalamic obesity.
Abbreviations
- CP:
-
craniopharyngioma
- FMH:
-
“Fertigkeitenskala Münster–Heidelberg” scale to assess functional capacity and quality of life
- HI:
-
hypothalamic involvement
- HVA:
-
homovanillic acid
- VMA:
-
vanillylmandelic acid
References
Geffner M, Lundberg M, Koltowska-Haggstrom M, Abs R, Verhelst J, Erfurth EM, Kendall-Taylor P, Price DA, Jonsson P, Bakker B 2004 Changes in height, weight, and body mass index in children with craniopharyngioma after three years of growth hormone therapy: analysis of KIGS (Pfizer International Growth Database). J Clin Endocrinol Metab 89: 5435–5440
Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O, Shine B, Turner HE, Wass JA 2005 Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf) 62: 397–409
Kendall-Taylor P, Jonsson PJ, Abs R, Erfurth EM, Koltowska-Haggstrom M, Price DA, Verhelst J 2005 The clinical, metabolic and endocrine features and the quality of life in adults with childhood-onset craniopharyngioma compared with adult-onset craniopharyngioma. Eur J Endocrinol 152: 557–567
Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E 2004 Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 46: 220–229
Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, Rai SN, Lensing SY, Wu S, Xiong X 2003 Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab 88: 2586–2592
Müller HL, Emser A, Faldum A, Bruhnken G, Etavard-Gorris N, Gebhardt U, Oeverink R, Kolb R, Sörensen N 2004 Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J Clin Endocrinol Metab 89: 3298–3305
Harz KJ, Müller HL, Waldeck E, Pudel V, Roth C 2003 Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab 88: 5227–5231
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404: 661–671
Bray GA 2000 Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes Relat Metab Disord 24: S8–S17
Rolland-Cachera MF, Cole TJ, Sempe M, Tichet J, Rossignol C, Charraud A 1991 Body mass index variations: centiles from birth to 87 years. Eur J Clin Nutr 45: 13–21
Hunneman DH 1983 Mass fragmentographic determination of homovanillic and 4-hydroxy-3-methoxy mandelic acids in 50 μl plasma. Clin Chim Acta 135: 169–174
Müller HL, Bruhnken G, Emser A, Faldum A, Etavard-Gorris N, Gebhardt U, Kolb R, Sörensen N 2005 Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv Syst 21: 975–980
Wolff JE, Daumling E, Dirksen A, Dabrock A, Hartmann M, Jurgens H 1996 Munster Heidelberg Abilities Scale—a measuring instrument for global comparison of illness sequelae. Klin Padiatr 208: 294–298
Young JB, Landsberg L 1998 Catecholamines and the adrenal medulla. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR (eds) Williams Textbook of Endocrinology, 9th ed. WB Saunders Co, Philadelphia, pp 665–728
Eisenhofer G, Kopin IJ, Goldstein DS 2004 Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56: 331–349
Grove KL, Smith MS 2003 Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 79: 47–63
Ricquier D, Bouillaud F, Toumelin P, Mory G, Bazin R, Arch J, Penicaud L 1986 Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid beta-adrenoreceptor-mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem 261: 13905–13910
Schofl C, Schleth A, Berger D, Terkamp C, von zur Mühlen A, Brabant G 2002 Sympathoadrenal counterregulation in patients with hypothalamic craniopharyngioma. J Clin Endocrinol Metab 87: 624–629
Coutant R, Maurey H, Rouleau S, Mathieu E, Mercier P, Limal JM, Le Bouil A 2003 Defect in epinephrine production in children with craniopharyngioma: functional or organic origin?. J Clin Endocrinol Metab 88: 5969–5975
Mason PW, Krawiecki N, Meacham LR 2002 The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Arch Pediatr Adolesc Med 156: 887–892
Srinivasan S, Ogle GD, Garnett SP, Briody JN, Lee JW, Cowell CT 2004 Features of the metabolic syndrome after childhood craniopharyngioma. J Clin Endocrinol Metab 89: 81–86
Acknowledgements
The authors thank patients and their physicians participating in the KRANIOPHARYNGEOM 2000 study. We also thank Kevin Grove, Oregon Health Sciences University, Portland, OR, and M. Neff-Heinrich, Göttingen, Germany for their critical review of the paper, and R. Maslak, Bonn, for her kind support in collecting the samples. We thank Mrs. S. Vallana for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was supported by the German foundation “Deutsche Kinderkrebsstiftung,” Bonn.
Rights and permissions
About this article
Cite this article
Roth, C., Hunneman, D., Gebhardt, U. et al. Reduced Sympathetic Metabolites in Urine of Obese Patients With Craniopharyngioma. Pediatr Res 61, 496–501 (2007). https://doi.org/10.1203/pdr.0b013e3180332cd6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/pdr.0b013e3180332cd6
This article is cited by
-
Hypothalamic syndrome
Nature Reviews Disease Primers (2022)
-
Physical function, body mass index, and fitness outcomes in children, adolescents, and emerging adults with craniopharyngioma from proton therapy through five years of follow-up
Journal of Neuro-Oncology (2022)
-
Detailed assessment of hypothalamic damage in craniopharyngioma patients with obesity
International Journal of Obesity (2019)
-
Risk-adapted, long-term management in childhood-onset craniopharyngioma
Pituitary (2017)
-
Efficacy of growth hormone replacement on anthropometric outcomes, obesity, and lipids in children with optic nerve hypoplasia and growth hormone deficiency
International Journal of Pediatric Endocrinology (2016)