Abstract
To examine whether long-term consumption of fermented milk containing a specific Lactobacillus casei may improve the health status of preschool children suffering from allergic asthma and/or rhinitis a randomized, prospective, double blind, controlled trial was conducted in 187 children 2–5 y of age. The children received for 12 mo either fermented milk (100 mL) containing Lactobacillus casei (108 cfu/mL) or placebo. The time free from and the number of episodes of asthma/rhinitis after starting intervention were the outcome measures. The number of fever or diarrhea episodes and the change in serum immunoglobulin were further assessed. No statistical difference between intervention and control group occurred in asthmatic children. In children with rhinitis, the annual number of rhinitis episodes was lower in the intervention group, mean difference (95% CI), −1.6 (−3.15 to −0.05); the mean duration of an episode of diarrhea was lower in the intervention group, mean difference −0.81 (−1.52 to −0.10) days. While long-term consumption of fermented milk containing Lactobacillus casei may improve the health status of children with allergic rhinitis no effect was found in asthmatic children.
Similar content being viewed by others
Main
The rates of allergic diseases in childhood are increasing worldwide, mainly in industrialized countries (1) possibly as the immune system does not receive adequate stimulation early in life (2). Allergic diseases cause disability among children (3), and can lead to an impairment of quality of life and reduce effectiveness at work of parents, with public consequences (4). Interventions aimed to improve allergic diseases condition could be of practical relevance, for both clinical and social implications.
Probiotic bacteria, improving the intestinal microbial balance, may facilitate modulation of immune response. Differences exist in the intestinal flora composition of allergic and nonallergic children (5). In particular, the occurrence of Clostridia in intestinal flora is higher in allergic subjects, while occurrence of bifidobacteria is lower (5–6). Moreover, current lifestyle has changed the gut microflora composition, with prevalence of enterobacteria on lactobacilli and bifidobacteria (6). Interfering in the gut flora throughout ingestion of live microbiota (Lactobacilli), could favor the correct maturation of the immune system (7), and reduce development of allergy in childhood (7–9). Randomized controlled trials hypothesized an effect of lactobacilli on atopic dermatitis in children (10–13) and suggested that the lactobacilli could be beneficial for the respiratory tract (14). Indeed, Lactobacillus paracasei might improve the quality of life of adolescents with perennial allergic rhinitis (15–16). Few studies examined the effect of probiotics on rhinitis and results are controversial (17–19). Randomized studies need to clarify the effects of probiotic bacteria on allergy-related disorders. Lactobacillus casei may be a promising strain as it survives through the gastrointestinal tract (20), be metabolically active during transit (21), and promote recovery from diarrhea in young children (22,23).
The aims of the current study were to investigate whether the long-term daily consumption of fermented milk containing a specific Lactobacillus casei may reduce the occurrence and duration of episodes of asthma and allergic rhinitis and modify the immunologic profile of preschool children with allergic asthma and/or rhinitis.
SUBJECTS AND METHODS
Population.
This randomized, prospective, double-blind, placebo controlled study analyzed 187 children consecutively enrolled throughout a 12-month period in 8 care centers located in Milan and surroundings, Northern Italy. Enrolment was carried out between 01 April 2003 and 31 March 2004, and data collection ended on 31 March 2005. Inclusion criteria were: age 2–5 y inclusive; allergy proved by prick test; and diagnosis of allergic asthma and/or rhinitis. Exclusion criteria were: cow's milk allergy, lactose intolerance, severe food allergy or other severe chronic disease, perinatal respiratory problems, antibiotic use in the preceding 4 wk before starting intervention.
Skin prick tests were performed at enrolment and the end of the study in accordance with the Italian Society of Allergy and Clinical Immunology (24) and assessed pollens widespread in the examined area using standardized extracts (STALLERGÈNES France SA, Antony, France). As recommended by the Joint Council of Allergy, Asthma and Immunology (Palatine, IL), the prick test was considered positive when the wheal was at least 3 mm larger in diameter. Asthma was diagnosed and classified in accordance with the Global Initiative for Asthma (GINA) guidelines (25). Allergic rhinitis was diagnosed and classified in accordance with the Allergic Rhinitis and its Impact on Asthma (ARIA) (26).
The parents of eligible children received explanation about the aim of the study, and were requested to sign a consent form. The Ethics Committee of the coordinator center, the San Paolo Hospital, Milan, approved the study protocol.
Intervention.
The study protocol scheduled daily oral supplementation of either an intervention “product”, that is one pot (100 mL, 0.83 Kcal/g, 0.16% fat) of fermented milk containing two yoghourt cultures (Lactobacillus bulgaricus, 107 colony forming units [cfu]/mL, and Streptococcus thermophilus, 108 cfu/mL) and the probiotic strain Lactobacillus casei DN-114 001 (108 cfu/mL) or one pot (100 mL, 0.83 Kcal/g, 0.16% fat) of a non fermented milk (control), both providing the same dairy load. The intervention product was obtained mixing the yoghourt cultures and the probiotic strain plus the metabolites produced during fermentation. Milks, comparable in taste and texture, were bottled in identical opaque coded pots provided by Danone (Milan, Italy). All individuals involved in the trial were unaware of the milk supplied until codes were broken after the completion of the data analysis. Four trained health workers distributed milks twice monthly. At that time pots nonconsumed in the previous two weeks were got back. Administration of milks started 5 ± 1 d after enrolment. Consumption of other products containing probiotic bacteria was forbidden.
Baseline data collection and clinical examinations.
Baseline data were collected at enrolment. Prospective data were recorded daily by the parents on a diary; a methodology used in literature (14), and included solicited questions (yes/no) about occurrence of symptoms of asthma (wheezing, chest tightness, cough, difficult breathing) or rhinitis (rhinorrea, nasal obstruction, nasal itching, sneezing). Occurrence of abdominal symptoms (diarrhea, nausea/vomiting, abdominal pain, constipation) was assessed by a sequence of questions (yes/no) adapted from the Abdominal Symptom Questionnaire (27). Questions were illustrated to the parents, who were instructed as to evaluate symptoms. The parents further recorded whether the child experienced fever and was given antibiotic treatments. Occurrence of an asthma episode was defined as the child experienced an episode or an attack of asthma (28). This definition has been used in recent studies (29,30) and by the National Center for Health Statistics (Hyattsville, M.D.). Occurrence of a rhinitis episode was defined as the child experienced two or more symptoms for longer than one-hour day (31). The family pediatrician confirmed diagnosis of an allergic episode on a physical examination (32). Ambiguous cases had to refer to the care center for testing (32). A new episode was identified as at least 2 d free from symptoms of asthma or rhinitis elapsed since the previous episode. The definition of diarrhea was 2 or more watery or unusually loose bowel motions in 24 h (33). A new episode of diarrhea was defined as the occurrence of diarrhea after a period of 3 or more symptom-free days (33). Fever was defined as the armpit temperature was above 37.2° for two consecutive measurements taken at 4 h interval. A new episode of fever was defined as the occurrence of fever for at least 24 consecutive hours after a period of 2 or more fever-free days.
Children were visited at the care centers within 3 ± 1 d (baseline) after enrolment, and at 3, 6, 9 and 12 mo after starting intervention, when data from diaries were collected and discussed with the parents by the same pediatrician (one for center).
Fecal samples were taken at baseline, and at 6 and 12 mo of intervention in a random subsample of 45 children, 30 in the intervention group and 15 in the control group. Presence of Lactobacillus casei was identified using DNA PCR analysis (34,35). The amount of Lactobacillus casei was not enumerated.
Immunologic blood assessment.
Fasting blood samples were taken at 8.30 h ± 30 m at baseline and at 360 ± 5 d after starting intervention. Total serum immunoglobulins A (IgA), E (IgE), G (IgG) and M (IgM) were measured at the same clinical laboratory by an immunoturbidometric assay (Tina-Quant Roche Diagnostics SpA, Milan, Italy) on a Modular P800 analyzer (Roche Diagnostics).
Sample size and randomization.
The sample size was calculated to detect a reduction of 40% or more in the mean number of episodes of asthma and/or rhinitis occurring during the 12-mo intervention period. Recursive calculation was performed through the recruitment period. Admitting a two-tailed type I error level of 5% with a power of 80%, and assuming an expected annual number of episodes in controls equal to the one reported in the last year before intervention in children already recruited, 6o children with asthma and 66 with rhinitis needed in each group. Children were randomly assigned to the intervention or control group based on a computer generated, blocked randomization list by each center. A block size of four was used, stratified according to gender, age (< or ≥3 y) and diagnosis of asthma and/or rhinitis. For fecal analysis, 15 blocks were randomly (same chance) selected. Within each of these blocks the two children assigned to intervention group and one (same chance) of the two controls were considered.
Outcome measures.
The primary outcome measures were the time (number of days) free from episodes of asthma and/or rhinitis after starting intervention, and the cumulative number and duration (number of days) of episodes. Secondary outcome measures were the number and duration of episodes of diarrhea or fever and the change in immunologic profile.
Statistical analysis.
Log transformation was used for the cumulative number of episodes of asthma and/or rhinitis, duration of episodes, and immunoglobulin, as these variables were not normally distributed. Univariate comparison between groups was performed by the t test or the Mann-Whitney test, the χ2 test and ANOVA for repeated measures. Kaplan-Meier survival curves for the time free from episodes were constructed and compared by the log-rank test. Adjustment for potential confounders (age, severity of asthma and rhinitis at baseline) was performed using a Cox regression analysis. Logistic regression analysis was used to assess the effect of intervention on incidence of asthma and/or rhinitis, and estimate the corresponding odds ratio (OR). Analyses were performed on the intention to treat population. A significance level of 0.05 was used and the statistical tests are two-tailed. The SPSS software, version 12.0 (SPSS Inc., Chicago, IL), was used for the statistical analysis.
RESULTS
Of the 196 children randomized, 187 started the study receiving either fermented milk containing Lactobacillus casei (n = 92) or nonfermented milk (n = 95). Figure 1 details the progress of participants throughout the study. Table 1 reports baseline characteristics of the children. The block randomization resulted in a similar distribution of age, allergic disease and severity in the intervention and control groups. At the baseline, among children with allergic rhinitis (n = 131) the disease was, respectively, perennial or seasonal in 42/64 (65.6%) and 22/64 (34.4%) of children in the intervention group versus 37/67 (55.2%)and 30/67 (44.8%) in the control group (p = 0.224). No between groups difference at 12 mo was found in the skin prick tests (intervention group, positive 81/89 versus 89/93, control group). Before intervention, 37/62 (59.7%) and 33/57 (57.9%) of asthmatic children used asthma medications in the intervention and control group, respectively, while 40/64 (62.5%) and 40/67 (59.7%) children with rhinitis used allergic medications. No significant change in using medications occurred during the study period in intervention or control group.
Asthma and rhinitis episodes.
The time free from episodes of asthma/rhinitis was longer in the intervention group compared with the control group (mean [95% CI] 3.5 [2.7 to 4.3] versus 2.1 [1.5 to 2.7] months, p = 0.027) with an adjusted intervention:control ratio of 0.76 (0.56 to 1.03) (p = 0.082). In children with asthma, mean (95% CI) time free from episodes was 4.1 (3.1 to 5.0) months in the intervention group versus 3.3 (2.4 to 4.3) months in the control group (unadjusted p = 0.231; adjusted p = 0.405) (Fig. 2). In children with rhinitis the corresponding values were 6.2 (5.0 to 7.4) and 5.1 (4.0 to 6.3) (unadjusted p = 0.937; adjusted p = 0.937) (Fig. 2).
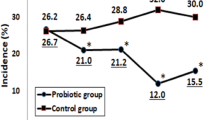
In the pooled data the cumulative number of asthma and rhinitis episodes was lower in the intervention than in the control group (median, inter quartile range [IQR], 5 [2 to 9] versus 7 [4 to 11], unadjusted p = 0.036; adjusted p = 0.073). In children with asthma no difference between groups was found (Fig. 3). In children with rhinitis difference between groups was significant (unadjusted p = 0.040; adjusted p = 0.053) with a mean number of episodes of respectively 3.2 (2.4 to 4.1) versus 4.8 (3.5 to 6.1), that is a mean difference of 1.6 episodes/y (Fig. 3). In the 3–6 mo of intervention occurrence of episodes of rhinitis in the period was lower in the intervention group (p < 0.01) with an adjusted odds ratio (95% CI) of 0.39 (0.19 to 0.82, p < 0.01). No significant difference between intervention and control group was found in the mean duration of an episode of asthma (mean difference [95% CI] −0.47 [−1.47 to 0.53] days) or rhinitis (mean [95% CI] 1.02 [−0.27 to 2.32] days).
Abdominal symptoms, diarrhea and fever episodes.
Abdominal symptoms were reported in 56.5% versus 64.2% of children in intervention and control groups, respectively (p = 0.282). In particular, 40 (43.5%) and 49 (51.6%) children, respectively, experienced diarrhea (p = 0.267). The corresponding number of children who reported episodes of fever was, respectively, 79 (85.9%) and 74 (77.9%) (p = 0.157). Antibiotics were administered to 66 (71.7%) and 65 (68.4%) children, respectively in intervention and control groups. Table 2 details the cumulative number of diarrhea and fever episodes throughout the study. In children with rhinitis, the mean duration of a single episode of diarrhea was lower in the intervention group compared with the control group (mean [95% CI] 1.04 [0.55 to 1.53] versus 1.85 [1.32 to 2.38] days) with an adjusted intervention:control ratio of 0.80 (0.63 to 0.99; p = 0.048).
Immunologic profile.
No overall statistical difference was found at baseline or at 12 mo of intervention between intervention and control groups for any examined immunologic variable, neither significant difference occurred when children with asthma and rhinitis were considered separately (Table 3).
Compliance.
Compliance estimated on the basis of nonconsumed pots was 70% and 74% in intervention and control groups, respectively. Compliance was higher in the first semester (80% in both groups), then declined to 59.9% and 68.0%, respectively. Fecal analysis showed that no child carried Lactobacillus casei at baseline. In the intervention group the rate of recovery of Lactobacillus casei was 78.6% and 77.8% at 6 and 12 mo, respectively. No control child showed presence of Lactobacillus casei in the fecal sample at 6 or 12 mo.
DISCUSSION
This study evaluated whether long-term consumption of a fermented milk containing a specific Lactobacillus casei (DN 114 001) may induce benefits on preschool children with allergic asthma and/or rhinitis. Compliance to treatment was good enough. However, based on nonconsumed pots, it was approximately 14% reduced during the second semester of intervention as compared with the first semester. This may be a limitation of the study that could have been prevented to find significant differences between groups. A methodological limitation is that the control used was a nonfermented milk. A better control would be fermented milk without the addition of the Lactobacillus casei or a sterilized fermented milk. Indeed, a control group similar to the one used in the present study was used in other studies (19). Only recently it was generally agreed that this control would be not acceptable (e.g., The First International DIA Workshop: “Developing Probiotics as Foods and Drugs – Scientific and Regulatory Challenges” Oct 16–17, 2006, Adelphi, M.D.; http://www.isapp.net/IS_news.htm). However, it should be noted that the present trial started in 2003, when appropriateness of the control group was controversial. The study demonstrated the effect of fermented milk containing a specific Lactobacillus casei strain, but it is not possible to conclude about the effect of Lactobacillus casei per se. Indeed, articles state that plain yoghurt may have impact on rhinitis and asthma (18). Due to these shortcomings caution should be exercised in drawing definitive conclusions.
The analysis of pooled data revealed that children receiving fermented milk containing Lactobacillus casei had 33% lower occurrence of rhinitis episodes/y as compared with the control group. No significant difference between groups was found in children with asthma for any outcome measure. In children with rhinitis number of annual episodes was lower in the intervention group, with an occurrence of episodes twice lower during the second quarter of intervention. The nonsignificance during the first quarter may suggest that improvement of balance and metabolic profile of intestinal microflora and modulation of immune response is not immediate. Indeed, Guerin-Danan et al. (36) found that after one month of consumption more than 50% of healthy infants consuming Lactobacillus casei exhibited significant increase in the fecal population of Lactobacillus, but it may not excluded that longer time needs in non healthy children. The vanishing effect during the second semester might be due, at least in part, to the reduced compliance.
Studies reported positive immunostimulatory effect of bacteria in preventing recurrent infections in children (e.g., 37). In the present study the fermented milk containing Lactobacillus did not exercise a significant effect on fever. It can be not excluded that this is due to the relatively low dose ingested daily (100 mL). Hatakka et al. hypothesized that the immunostimulatory effect of Lactobacillus may be dose dependent (14).
It has been suggested that assumption of Lactobacillus casei may reduce incidence and duration of diarrhea in infants and young children (22,23,38). In this study the annual incidence of diarrhea did not differ between the intervention and control groups but duration of an episode was lower in children receiving Lactobacillus (1.0 versus 1.7 d). Comparable results have been found in smaller studies (39).
In conclusion, within the limitations of the present study, one can infer that long-term consumption of fermented milk containing a specific Lactobacillus casei may improve the health status of children with allergic rhinitis but appear to do not exercise significant effect in children with asthma.
Abbreviations
- Cfu:
-
colony forming units
- IQR:
-
interquartile range
- IgA:
-
Immunoglobulin A
- IgE:
-
Immunoglobulin E
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- U:
-
arbitrary unit
References
Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H 2006 Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368: 733–743
Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E 2004 The hygiene hypothesis of atopic disease- an extended version. J Pediatr Gastroenterol Nutr 38: 378–388
Center for Disease Control 1995 Disabilities among children aged lower or equal 17 years - United States, 1991-1992. MMWR 44: 609–613.
Weiss KB, Sullivan SD 2001 The health economics of asthma and rhinitis. Assessing the economic impact. J Allergy Clin Immunol 107: 3–8
Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M 2001 Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 108: 516–520
Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E 2002 Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning?. Gut 51: 51–55
Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E 2001 Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357: 1076–1079
Viljanen M, Pohjavuori E, Haahtela T, Korpela R, Kuitunen M, Sarnesto A, Vaarala O, Savilahti E 2005 Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J Allergy Clin Immunol 115: 1254–1259
Kalliomaki MA, Isolauri E 2004 Probiotics and down-regulation of the allergic response. Immunol Allergy Clin North Am 24: 739–752
Rosenfeldt V, Benfeldt E, Dam Nielsen S, Michaelsen K, Jeppesen D, Valerius NH, Paerregaard A 2003 Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 111: 389–395
Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M 2005 Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy 60: 494–500
Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S 2000 Probiotics in the management of atopic eczema. Clin Exp Allergy 30: 1604–1610
Weston S, Halbert A, Richmond P, Prescott SL 2005 Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child 90: 892–897
Hatakka K, Savilahti E, Pönkä A, Meurman JH, Poussa T, Näse L, Saxelin M, Korpela R 2001 Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322: 1327–1329
Peng GC, Hsu CH 2005 The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennialallergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol 16: 433–438
Wang MF, Lin HC, Wang YY, Hsu CH 2004 Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy Immunol 15: 152–158
Ciprandi G, Vizzaccaro A, Cirillo I, Tosca A 2005 Bacillus clausii effects in children with allergic rhinitis. Allergy 60: 702–710
Aldinucci C, Bellussi L, Monciatti G, Passali GC, Salerni L, Passali D, Bocci V 2002 Effects of dietary yoghurt on immunological and clinical parameters of rhinopathic patients. Eur J Clin Nutr 56: 1155–1161
Tamura M, Shikina T, Morihana T, Hayama M, Kajimoto O, Sakamoto A, Kajimoto Y, Watanabe O, Nonaka C, Shida K, Nanno M 2006 Effects of Probiotics on Allergic Rhinitis Induced by Japanese Cedar Pollen: Randomized Double-Blind, Placebo-Controlled Clinical Trial. Int Arch Allergy Immunol 143: 75–82
Oozeer R, Leplingard A, Mater DD, Mogenet A, Michelin R, Seksek I, Marteau P, Dore J, Bresson JL, Corthier G 2006 Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl Environ Microbiol 72: 5615–5617
Oozeer R, Goupil-Feuillerat N, Alpert CA, van de Guchte M, Anba J, Mengaud J, Corthier G 2002 Lactobacillus casei is able to survive and initiate protein synthesis during its transit in the digestive tract of human flora-associated mice. Appl Environ Microbiol 68: 3570–3574
Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P 1999 The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int J Clin Pract 53: 179–184
Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P 2000 Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J Clin Pract 54: 568–571
Italian Society of Allergy and Clinical Immunology 1987 Italian Society of Allergy and Clinical Immunology: Memorandum on diagnosis of allergic diseases. Fed Med 40: 861–874
Global Initiative for Asthma 2003 Global Strategy for Asthma Management and Prevention. pp 1–180 http://www.ginasthma.com/search.asp?l1=9&l2=0; accessed April 4, 2007
Bousquet J, van Cauwenberge P, Khaltaev N, ARIA Workshop Group, World Health Organization 2001 Allergic Rhinitis and its Impact on Asthma. J Allergy Clin Immunol 108: S147–S334
Agreus L, Svardsudd K, Nyren O, Tibblin G 1993 Reproducibility and validity of a postal questionnaire. The abdominal symptom study. Scand J Prim Health Care 11: 252–262
Center for Disease Control 2000 Measuring childhood prevalence before and after the 1997. Redesign of the National Health Interview Survey - United States. MMWR 49: 908–911
Center for Disease Control 2005 Self-Reported asthma among high school students- United States, 2003. MMWR 54: 765–767
Swahn MH, Bossarte RM 2006 The associations between victimization, feeling unsafe, and asthma episodes among US high-school students. Am J Public Health 96: 802–804
International Rhinitis Management Working Group 1994 International consensus report on the diagnosis and management of rhinitis. International Rhinitis Management Working Group. Allergy 49: 1–34
Quillen DM, Feller DB 2006 Diagnosing rhinitis: allergic vs. nonallergic. Am Fam Physician 73: 1583–1590
Roberts L, Jorm L, Patel M, Smith W, Douglas RM, McGilchrist C 2000 Effect of infection control measures on the frequency of diarrheal episodes in child care: a randomized, controlled trial. Pediatrics 105: 743–746
Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, Morelli L 2005 Enteral nutrition and microflora in pediatric Crohn's disease. JPEN J Parenter Enteral Nutr 29: S173–S175
Tynkkynen S, Satokari R, Saarela M, Mattila-Sandholm T, Saxelin M 1999 Comparison of ribotyping, randomly amplified polymorphic DNA analyses, and pulsed-field gel electrophoresis in typing of Lactobacillus rhamnosus and L casei strains. Appl Environ Microbiol 65: 3908–3914
Guerin-Danan C, Chabanet C, Pedone C, Popot F, Vaissade P, Bouley C, Szylit O, Andrieux C 1998 Milk fermented with yogurt cultures and Lactobacillus casei compared with yogurt and gelled milk: influence on intestinal microflora in healthy infants. Am J Clin Nutr 67: 111–117
Collet JP, Ducruet T, Kramer MS, Haggerty J, Floret D, Chomel JJ, Durr F 1993 Stimulation of nonspecific immunity to reduce the risk of recurrent infections in children attending day-care centers. The Epicreche Research Group. Pediatr Infect Dis J 12: 648–652
Gonzales S, Albarracin G, Locascio de Ruiz Pesce M, Male M, Apella MC, Pesce de Ruiz Holgado A, Oliver G 1990 Prevention of infantile diarrhea by fermented milk. Microbiol Alim Nutr 8: 349–354
Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T 1991 A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 88: 90–97
Acknowledgements
The FELICITA study group comprised of Besana R, Biasucci G, Galluzzo C, Longhi R, Podestà A, Sterpa A, and contributed to the data collection.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
This study was supported in part by the DANONE GROUP (France).
Rights and permissions
About this article
Cite this article
Giovannini, M., Agostoni, C., Riva, E. et al. A Randomized Prospective Double Blind Controlled Trial on Effects of Long-Term Consumption of Fermented Milk Containing Lactobacillus casei in Pre-School Children With Allergic Asthma and/or Rhinitis. Pediatr Res 62, 215–220 (2007). https://doi.org/10.1203/PDR.0b013e3180a76d94
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3180a76d94
This article is cited by
-
Immunomodulatory effects of probiotic supplementation in patients with asthma: a randomized, double-blind, placebo-controlled trial
Allergy, Asthma & Clinical Immunology (2023)
-
Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma
Italian Journal of Pediatrics (2017)
-
Novel Microbiome-Based Therapeutics for Chronic Rhinosinusitis
Current Allergy and Asthma Reports (2015)
-
Probiotics in addition to antibiotics for the treatment of acute tonsillitis: a randomized, placebo-controlled study
European Journal of Clinical Microbiology & Infectious Diseases (2015)
-
Probiotics and food allergy
Italian Journal of Pediatrics (2013)