Abstract
Treatment with phototherapy or with the lipase inhibitor orlistat decreases plasma unconjugated bilirubin (UCB) concentrations in hyperbilirubinemic Gunn rats. We investigated the mechanism(s) underlying the effects of orlistat, phototherapy, and combined treatment, using steady-state 3H-UCB kinetics. After three weeks of treatment with orlistat (200 mg/kg chow), phototherapy (19 μW/cm2/nm) or combined treatment, tracer 3H-UCB was administered IV to treated and untreated (control) Gunn rats. Plasma samples and feces were collected every 12h for 60h, and bile for 30 min at 60h. The following results were obtained: 1) each treatment decreased plasma bilirubin levels compared with controls: orlistat– 19%, phototherapy–32%, combined treatment–53%; 2) plasma bilirubin concentrations were strongly, negatively correlated with fractional bilirubin turnover; 3) orlistat treatment induced net transmucosal excretion of UCB into the intestinal lumen, whereas phototherapy increased biliary UCB excretion rate; 4) all treatments profoundly increased the enterohepatic circulation of UCB derivatives, indicating enhanced metabolism by intestinal bacteria. In conclusion, orlistat and phototherapy lower plasma bilirubin concentrations in Gunn rats by increasing (net) intestinal influx of UCB, either by transmucosal excretion (orlistat), or increased biliary secretion (phototherapy). The mechanism of phototherapy and orlistat treatment involves increasing the availability of UCB in the intestinal lumen for fecal excretion and for metabolism by intestinal bacteria.
Similar content being viewed by others
Main
Severe unconjugated hyperbilirubinemia can cause bilirubin-induced neurologic dysfunction (BIND), kernicterus, and death (1). Conventional treatment for unconjugated hyperbilirubinemia involves phototherapy, which induces photoisomerization of unconjugated bilirubin (UCB) to more water-soluble derivatives that can be excreted into bile (2). Short-term phototherapy has relatively minor side effects and is considered safe (3).
Crigler-Najjar disease is characterized by permanent, unconjugated hyperbilirubinemia due to a genetic deficiency in bilirubin glucuronidation (4). Conventional treatment for Crigler-Najjar disease (type I) involves life-long daily phototherapy. Disadvantages of long-term phototherapy are a declining efficacy with age and a profound impact on the quality of (social) life (5,6). Since the introduction of phototherapy, serious long-term effects have not been reported. However, there have been reports of phototherapy-induced DNA damage to human cell lines in vitro and bilirubin was found to enhance this damage (7,8).
An alternative treatment strategy for unconjugated hyperbilirubinemia is based on intestinal capture of UCB. UCB can diffuse across the intestinal mucosa from the blood into the intestinal lumen (9,10) and vice versa (11). Capture of UCB in the intestine, by orally administered unabsorbable binders of UCB, can prevent its re-absorption into the enterohepatic circulation. Such agents, like agar, activated charcoal, and cholestyramine, are no longer used for treatment of unconjugated hyperbilirubinemia because of inconsistent clinical results and side effects. Intestinal capture of UCB by calcium phosphate was very effective in Gunn rats (12), a well-established animal model for Crigler-Najjar disease (13). In Crigler-Najjar patients however, the decrease in plasma UCB concentration was less pronounced, and occurred only in patients treated simultaneously with phototherapy (5). Phototherapy profoundly enhances the very limited biliary secretion of UCB that can occur in the absence of conjugation (14), thus increasing the amount of UCB available for capture in the intestine.
Recently, we demonstrated in Gunn rats that dietary supplementation with the lipase inhibitor orlistat decreased plasma UCB levels, parallel to an increase in fecal fat excretion (15,16). Plasma UCB concentrations were strongly, negatively correlated with the amount of fat excreted via the feces. Orlistat treatment was equally effective as continuous phototherapy in Gunn rats, and combined treatment was more effective than either treatment alone (16), which suggests that the two treatments operate by different mechanisms. The mechanism by which orlistat treatment reduces plasma bilirubin levels has not been elucidated so far. In the present study, we used steady-state 3H-bilirubin kinetics to assess the mechanism(s) underlying the effects of orlistat, phototherapy, and combined treatment in Gunn rats.
METHODS
Animals.
Homozygous male Gunn rats (RHA/jj, 220–320g), were obtained from our breeding colony (University Medical Center Groningen, The Netherlands). Animals were housed in an environmentally controlled facility, were fed ad libitum, and were caged individually or, in case of phototherapy, per treatment group. The Ethics Committee for Animal Experiments (University of Groningen, The Netherlands) approved experimental protocols.
Phototherapy lamps.
Two phototherapy devices were developed according to the prototype designed by Ostrow (2). Each device consisted of two blue phototherapy lamps (Philips, TL-20W/03T) suspended in a reflective canopy 20 cm above the bottom of the cage. Phototherapy (19 μW/cm2/nm; 380–480 nm; measured by an Elvos-LM-1010 Lux meter at 20 cm distance), was administered continuously to Gunn rats, shaven on their backs and flanks.
Chemicals.
2,3-3H-labeled 5-aminolevulinic acid (specific activity 2.0 Ci/mmol) was obtained from Amersham Biosciences (Piscataway, NJ). Heptadecanoic acid (C17:0) and bilirubin were purchased from Sigma Chemical Co. (St. Louis, MO). Orlistat was from Roche Nederland (Woerden, The Netherlands).
Preparation of 3H-labeled UCB.
3H-labeled UCB (specific activity 48.8 × 103 dpm/μg = 12.9 μCi/μmol) was prepared by biosynthetic labeling of bilirubin in dog bile from precursor labeled 2,3-3H-5-aminolevulinic acid (17). The 3H-UCB was isolated and recrystalized twice (18).
Preparation of 3H-UCB solution for IV administration.
Under dim light, immediately before injection into the Gunn rats, 1.5 mg of 3H-UCB was dissolved in 1.0 mL DMSO. Then, 12.5 mL of Wistar rat plasma was slowly added with continuous, thorough mixing. 0.2 mL/100g body weight (BW), containing 22 μg UCB/100g BW, and 0.49 μCi/100g BW, was administered to each Gunn rat via the penile vein.
Diets.
Hope Farms (Woerden, The Netherlands) produced the semisynthetic, purified control diet (code 4063.02), that contained 13 energy% fat / 5.2 wt% long-chain fatty acids. As in previous studies, all Gunn rats were fed the control diet for a run-in period of 4 wk (15,16). The orlistat-supplemented diet was prepared by thorough mixing of orlistat (Xenical®, 200 mg/kg chow) into the control diet.
Study Design: Pilot experiment to determine nontoxic limit of injected DMSO.
To study 3H-UCB kinetics under steady-state conditions, it was essential to prevent DMSO-induced hemolysis, by minimizing the volume of administered DMSO, without compromising solubilization of bilirubin. IV administration of 0.3 or 0.15 mL of DMSO (diluted 1:4 with Wistar rat plasma) to ∼300g Gunn rats caused transient hemolysis, characterized by increased plasma UCB concentrations, decreased Hb and hematocrit (Ht), and reticulocytosis (data not shown). By contrast, when an 8 mM solution of bilirubin in DMSO was diluted 1:16 with Wistar rat plasma, and 0.2 mL/100g BW was administered IV to three adult Gunn rats, samples of tail vein blood obtained 0, 6, 12, 24, 30 and 48 h after injection revealed no evidence of hemolysis. Thus, in an adult male Gunn rat, IV administration of ∼0.04 mL of DMSO, diluted 1:16 with rat plasma, should not alter steady-state 3H-UCB kinetics.
Effects of orlistat and/or phototherapy on steady-state bilirubin kinetics.
After 4 wk run-in on the control diet, Gunn rats were randomly assigned to one of four groups (n = 4–6 per group), to receive either the control diet, the orlistat-supplemented diet, continuous phototherapy, or the combination of orlistat-supplemented diet and continuous phototherapy. Previously, we showed that 2 wk of orlistat treatment is sufficient to reach steady-state plasma UCB concentrations and fecal UCB excretion (15). After 3 wk of treatment (T0h), heparinized samples of tail vein blood were obtained under isoflurane anesthesia. We then administered 3H-UCB (∼0.49 μCi/100g BW) via the penile vein and collected blood samples every 12h for 60h to determine plasma 3H and bilirubin concentration. After ∼60h, under pentobarbital anesthesia, bile was collected for 30 min under light-protected conditions after cannulation of the common bile duct. Bile flow was determined gravimetrically, assuming a density of 1 g/mL. After bile collection, blood was obtained by vena cava inferior puncture for determination of Hb, Ht, reticulocytes, aspartate-aminotransferese activity (AST), and alanine-aminotransferase activity (ALT). Three days before T0h, feces were collected for 72h to determine steady-state fecal fat excretion. From T0h onwards, feces were collected every 12h for 60h to determine fecal 3H excretion.
Calculation of fluxes based on steady-state 3H-UCB kinetics.
Fractional turnover of 3H-UCB (%/h) was calculated from semi-logarithmic plots of 3H-UCB specific activity. Bilirubin pool size was calculated by dividing the calculated specific activity at T0h (Y-axis intercept) by the administered dose of 3H-UCB radioactivity. Total bilirubin turnover was calculated as the product of fractional turnover and pool size (19) and assumed to equal steady-state fecal excretion of UCB and derivatives. The data allowed estimation of steady-state fluxes of UCB and derivatives in the enterohepatic circulation during the various treatments. Individual fluxes were calculated as follows. Biliary excretion of UCB was measured by HPLC (see Analytical Methods section). With biliary excretion of UCB and specific activity in plasma at the time of bile collection, the percentage of 3H in bile which originated from 3H-UCB was calculated. Biliary excretion of derivatives was calculated by subtracting 3H-UCB radioactivity from total biliary 3H-radioactivity measured by liquid scintillation, and expressed as equivalent percent of the exchangeable bilirubin pool excreted per time period. Net transmucosal flux of UCB was estimated by subtracting biliary UCB excretion from fractional UCB turnover. Total fecal excretion of UCB and its derivatives was calculated by dividing the total amount of dpm in feces between T12 and T60 hours, by the mean specific activity of UCB in plasma between T12 and T60 hours.
Analytical Methods: Plasma.
All analytical procedures were performed in dim light. Bilirubin levels were determined by reflectance spectrophotometry on a Vitros-950 analyzer (Johnson & Johnson, Tilburg, The Netherlands), and were confirmed by HPLC analysis. Hb, Ht, and reticulocytes were determined on a Sysmex-XE-2100 hematology analyzer (Goffin Meyvis, Etten-Leur, The Netherlands). AST, and ALT were determined on a Mega analyzer (Merck, Darmstadt, Germany). 3H content was determined by liquid scintillation (Tri-Carb 2500-TR Liquid Scintillation Analyzer, Packard Bioscience, Meriden, CT), using two ml of Ultima Gold-XR (Packard Bioscience) scintillation fluid added to 100 μL of plasma. Samples were counted for 10 min and quench corrected using 133Barium as external standard.
Bile.
For UCB measurement, bile was submitted to alkaline methanolysis and chloroform extraction. After evaporation under nitrogen, the residue was re-dissolved in chloroform and analyzed by reversed-phase HPLC, as described previously (16) 3H content in 100 μL of bile was determined by liquid scintillation as described above.
Feces.
Feces were freeze-dried and mechanically homogenized. For determination of fatty acids, aliquots of feces were extracted, hydrolyzed and methylated (20), using methanol/hexane. Resulting fatty acid methyl esters were determined by gas chromatography (HP-Ultra-1 column, Hewlett-Packard, Palo-Alto, CA). Fatty acid contents were calculated in molar amounts, using C17:0 as internal standard. For duplicate determination of 3H content, two aliquots (∼100 mg) of feces were decolorized by incubation with 0.6 mL of NaOCl for 30 min at 37°C. Then 60 μL of NH3 was added to neutralize free chloride. After vortexing, 10 mL of scintillation fluid was added and thoroughly mixed. After 24h, 3H content was determined by liquid scintillation.
Statistical Analyses.
Analyses were performed using SPSS 11.0 for Windows (SPSS Inc., Chicago, IL). Results are expressed as mean ± SD. Based on a normal distribution of plasma bilirubin levels in large groups of Gunn rats (15), parametric tests were used for statistical analysis. ANOVA with posthoc Bonferroni correction was performed for comparisons among groups. The relationship between fractional 3H-UCB turnover or pool size, and plasma bilirubin concentration was analyzed by linear regression analysis. p values <0.05 (two-tailed) were considered significant.
RESULTS
Effects of orlistat and/or phototherapy on body weights and food intake.
Body weights at the start and end of 3 wk of treatment were not significantly different among groups. All animals increased their body weight in 3 wk (controls, 7 ± 2%; orlistat, 14 ± 5%; phototherapy (PT), 12 ± 8%, orlistat+PT, 7 ± 4%; not significant). As observed previously (15), orlistat treated animals appeared to compensate fecal fat loss by increasing their food intake, compared with controls (25.6 ± 5.1 versus 20.0 ± 2.4 g/24h, respectively; p < 0.05).
Effects of orlistat and/or phototherapy on plasma bilirubin concentrations.
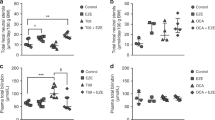
Figure 1 shows that after 3 wk of treatment (T0h) with orlistat or phototherapy, plasma bilirubin concentrations were significantly lower than in controls (−19%, p < 0.05; and −32%, p < 0.01, respectively). Efficacies of orlistat and phototherapy were not significantly different. Combined treatment with orlistat and phototherapy induced a more profound decrease in plasma bilirubin concentrations than either treatment alone (−53%, p < 0.001). After IV 3H-UCB administration, plasma UCB levels at 12h intervals did not change significantly compared with T0h values (except at T24h in the combined treatment group), in accordance with steady-state conditions. Also, none of the treatments significantly affected relevant hematological and liver function parameters (Table 1), confirming absence of significant hemolysis.
Steady-state plasma bilirubin concentrations after IV administration of 3H-UCB. Four groups of Gunn rats (n = 4–6 per group) were either untreated (controls ♦), or treated for 3 wk with orlistat (□), continuous phototherapy (PT ▴), or combined treatment (orlistat+PT ×). After 3 wk (T0h) 3H-UCB was injected IV; blood samples were taken at 12h intervals. Data represent mean± SD. *p < 0.05, **p < 0.01, § p < 0.001, T0h data compared with controls.
Effects of orlistat and/or phototherapy on steady-state bilirubin kinetics.
Table 2 shows biliary UCB secretion and results of the 3H-UCB kinetics study. Similar to previous observations (15), orlistat treatment did not significantly affect bile flow compared with control Gunn rats (4.2 ± 0.9 and 3.2 ± 0.7 μL/min per 100g BW, respectively), and neither did phototherapy or combined treatment (3.9 ± 0.3 and 4.0 ± 0.5 μL/min per 100g BW, respectively). Biliary excretion rate of UCB tended to be higher during phototherapy (+25%), compared with controls, and lower during orlistat treatment (−12%) and combined treatment (−33%), but none of these differences were statistically significant.
Figure 2 shows a semi-logarithmic plot of plasma 3H-UCB specific activities during 60h after 3H-UCB administration. In each of the four groups, the logarithm of plasma 3H-UCB specific activity declined in a linear fashion with time, in accordance with steady-state kinetics. Table 2 summarizes the effects of orlistat and/or phototherapy on steady-state 3H-bilirubin kinetics. Bilirubin pool sizes, compared with controls, had decreased in each treatment group after 3 wk (orlistat, −21%; PT, −23%; orlistat+PT, −42%), but statistical significance was only reached with combined treatment (p < 0.01). Bilirubin pool sizes were strongly positively correlated with plasma bilirubin concentrations at T0h (r = 0.87, p < 0.001, not shown). Total bilirubin turnover was not significantly altered by any of the treatments, confirming steady-state conditions and absence of (DMSO-induced) hemolysis. The quantity of 3H-UCB injected was <1.5% of the total UCB pool in all groups, thus representing a true tracer dose labeling of the UCB pool. Fractional turnover of 3H-UCB, compared with controls, was increased by 54% during orlistat treatment (p = 0.058), by 69% during phototherapy (p < 0.05), and by 119% during combined treatment (p < 0.01). Figure 3 shows that fractional turnover of 3H-UCB and plasma bilirubin concentrations at T0h were negatively, linearly correlated (r = −0.87, p < 0.001), indicating that the differences in plasma bilirubin concentration between controls and treated groups were related to stimulation of fractional turnover of bilirubin in the treated animals.
Semi-logarithmic plots of 3H-UCB specific activity after IV administration of 3H-UCB to Gunn rats. Experimental groups, treatments and 3H-UCB administration as in Fig. 1. Blood samples were taken at 12h intervals. Data represent mean values for each group. ♦ controls, r = −1.00; □ orlistat, r = −1.00; ▴ PT, r = −0.99; × orlistat+PT, r = −0.99.
Negative linear relationship between fractional turnover of 3H-UCB and steady-state plasma bilirubin levels at T0h in Gunn rats. Experimental groups, treatments and 3H-UCB administration as in Fig. 1. Each symbol represents data obtained in an individual animal. r = −0.87, p < 0.001. ♦ controls, □ orlistat, ▴ PT, × orlistat+PT.
Effects of orlistat on fecal fat and 3H excretion.
In agreement with our previous data (15,16), orlistat treatment increased fecal fat excretion. Fatty acid concentration in feces collected for 72h was significantly higher in orlistat treated animals (0.44 ± 0.08 mmol/g freeze-dried feces; p < 0.001), compared with controls (0.04 ± 0.01). Orlistat treatment also increased dry fecal weight (3.7 ± 0.7 g/24h) compared with controls (2.5 ± 0.3; p < 0.01), and cumulative fecal 3H excretion between T0h and T60h (+31%, p < 0.05, Table 2).
Fecal fat excretion per individual animal could not be calculated for the phototherapy or combined treatment group because these animals were housed together per treatment group. In pooled fecal samples from these rats, fatty acid concentrations were respectively 0.32 mmol/g feces (combined treatment) and 0.05 mmol/g feces (phototherapy). Cumulative 3H excretion in the pooled fecal samples of the phototherapy group was comparable to the orlistat group. With combined treatment, cumulative 3H excretion was almost twice control values (Table 2).
DISCUSSION
We investigated in Gunn rats the mechanism(s) underlying the hypobilirubinemic effects of orlistat, phototherapy and combined treatment, using steady-state 3H-UCB kinetics. Our data indicate that orlistat treatment and phototherapy lower plasma bilirubin concentrations in Gunn rats by increasing intestinal influx of UCB, either by transmucosal excretion (orlistat), or increased biliary secretion (phototherapy). By increasing intestinal influx of UCB, it becomes available for fecal elimination and/or for metabolism by intestinal bacteria, both of which interrupt the enterohepatic circulation of UCB.
In the assessment of bilirubin turnover rates, it is crucial to work at steady-state conditions. Steady-state conditions were confirmed by the following observations: 1) in each group, plasma bilirubin concentrations remained stable during the 3H-UCB kinetics study (Fig. 1). Previously, we showed that plasma UCB concentrations in Gunn rats did not change significantly between 2 and 24 wk of orlistat treatment (15); 2) total bilirubin turnover was unaltered compared with controls (Table 2). Under steady-state conditions, total bilirubin turnover equals UCB production rate. Neither phototherapy nor orlistat treatment has been shown to affect UCB production rate (21); 3) relevant hematological parameters were stable (Table 1); and finally, 4) 3H-UCB specific activities declined in a semi-logarithmic fashion in each of the four groups (Fig. 2).
Not all individual parameters of bilirubin kinetics were significantly different between the various treatments and controls (Table 2). Yet, plasma bilirubin levels were strongly, negatively correlated with fractional turnover of 3H-UCB (Fig. 3). This observation indicates that phototherapy and orlistat treatment both decrease plasma UCB concentrations via stimulation of bilirubin turnover. The present study also showed a strong, positive correlation between plasma UCB concentrations and bilirubin pool size, indicating that plasma UCB concentrations closely reflected UCB pool sizes during these (steady-state) treatments. The decrease in UCB pool size during phototherapy (−23%) is almost identical to results reported by Cohen et al. (−22%) (21).
The present study allowed estimation of steady-state fluxes of UCB and its derivatives in the enterohepatic circulation under the four experimental conditions (Fig. 4). Calculations are based on the assumption that quantitative disposal of bilirubin and its derivatives from the body occurs exclusively via the feces; i.e., fecal excretion of UCB plus derivatives equals fractional bilirubin turnover from the UCB pool. This assumption seems reasonable, since, in Gunn rats, urinary secretion of labeled UCB and its derivatives (consisting of polar photoderivatives, urobilinoids and other bacterial metabolites) is limited (5%) (10,22). Our calculations are also based on the assumption that metabolism of UCB to UCB-derivatives quantitatively occurs only in the intestinal lumen. It does, therefore, not take into account oxidative catabolism of UCB that could occur, e.g. in the liver. This catabolism is, however, relatively small in the untreated Gunn rat (21,23). Net transmucosal flux of UCB from the intestinal lumen into the blood or vice versa was calculated by subtracting biliary UCB excretion from fractional UCB turnover. It needs to be emphasized that the estimate reflects the net difference between the unidirectional fluxes of UCB from and into the intestinal lumen, whereas individual fluxes may be substantially different between treatments.
Fractional steady-state biliary and fecal fluxes of UCB and derivatives, and calculated net transmucosal flux of UCB in Gunn rats after 3 wk of either no treatment (controls), or treatment with orlistat, continuous phototherapy (PT), or combined treatment (orlistat+PT). Fluxes of UCB and derivatives were determined from tracer 3H-UCB kinetic data, as described in Methods. UCB fluxes are expressed as % of the exchangeable (plasma+tissue) bilirubin pool excreted per hour. Derivatives fluxes are expressed as equivalent% of the exchangeable bilirubin pool excreted per hour. [A] fractional turnover of UCB; [B] fractional biliary excretion of UCB; [C] fractional biliary excretion of derivatives; [D] fractional fecal excretion of UCB + derivatives (equals [A] in a steady-state, under the assumption that turnover of plasma UCB is completely determined by fecal excretion of UCB and derivatives); [E] estimated net transmucosal flux of UCB, from intestinal lumen to blood or vice versa, calculated as [A]–[B]. EHC, enterohepatic circulation. Ovals in upper left of each panel show bilirubin pool size (μmol/100g BW; mean ± SD).
The main results obtained from the calculations are: 1) phototherapy increases biliary excretion of UCB and its derivatives; 2) orlistat treatment induces net transmucosal excretion of UCB into the intestinal lumen; 3) treatment with phototherapy and/or orlistat enhances catabolism of UCB to derivatives that undergo efficient enterohepatic circulation; 4) combined treatment with orlistat and phototherapy more effectively increases the amount of UCB in the intestinal lumen and its subsequent disposal than either treatment alone. The main results will now be discussed consecutively in more detail.
Phototherapy enhanced biliary excretion of UCB, as shown previously (2). A novel finding of the present study is that phototherapy also enhances biliary excretion of derivatives. The increased biliary excretion of derivatives in the phototherapy group is most likely the consequence of enterohepatic recirculation of urobilinogens and other metabolites due to enhanced supply of substrate in the intestinal lumen. Previously, Schmid et al. (10) and Kotal et al. (9,24) showed that labeled UCB derivatives in Gunn rat bile are mostly urobilinogen and other diazo-negative polar products. Interestingly, the fractional flux of derivatives undergoing enterohepatic circulation is increased during phototherapy, compared with controls. This suggests that enhanced conversion (metabolism) of UCB to derivatives in the intestine quantitatively contributes to the hypobilirubinemic effect of phototherapy in Gunn rats. Another novel finding of the present study is the highly efficient re-absorption and enterohepatic circulation of UCB-derivatives in all four groups. Our data do not permit calculation of derivative pool sizes or the exact percentages of derivatives that were reabsorbed into the enterohepatic circulation.
Detailed analysis of (radiolabeled) photoisomers was not performed. It has been shown previously that the contribution of photoisomers to plasma radioactivity, originating from tracer radiolabeled UCB administered IV to Gunn rats, is minor (2). This is probably due to much more rapid hepatic clearance and biliary excretion of photoisomers by the Gunn rat (25). That study also documented that the major photoisomer in bile was cyclized, stable photobilirubin II, whereas unstable photobilirubins IA&B rapidly reverted to UCB. There is no reason to believe that results in our Gunn rats would be different.
Our estimation of fluxes assumes that almost all 3H in blood is UCB. Others have shown previously with 14C labeled UCB that only a very small amount of label is present in plasma urobilinogen. Our steady-state turnover rates are comparable with those found with 14C labeled UCB (2,10). The exchange rate of 14C with other C atoms is much lower than that of 3H with H-atoms, e.g. H2O. Furthermore, the fact that we measure more radioactivity in bile relative to plasma turnover indicates that UCB derivatives apparently undergo rapid enterohepatic circulation, but do not enter the systemic circulation, presumably due to a rapid first-pass effect in the liver. Finally, 3H-label exchange with water is not likely considering the following. We used 3H-UCB prepared biosynthetically in dogs by administration of 2,3-3H-5-aminolevulinic acid (17) based on the method of Howe et al. (26). As stated by them, all tritium atoms in the labeled UCB are in stable positions on the side chains. They documented this directly by showing that, after simultaneous IV administration of 3H-UCB and 14C-UCB to humans, plasma disappearance curves of 3H and 14C radioactivity were superimposable for periods ranging from 12 h to 16 d. Comparable results were obtained by Lester and Klein(27) with the supposedly less stable 3H-UCB prepared from 3,5-3H-5-aminolevulinic in rats; the 3H/14C-ratio of the two labeled UCB excreted in rat bile was constant over a period of 24 h.
Orlistat reduces absorption of dietary fats through inhibition of gastrointestinal lipases (28). Based on a strong negative correlation between the amount of fat excreted via the feces and plasma UCB concentrations in Gunn rats, we hypothesized that orlistat treatment diminishes the enterohepatic circulation of UCB via intestinal capture of UCB by unabsorbed fat (15,16). In the two groups treated with orlistat (± phototherapy), there was indeed net transmucosal passage of UCB from the blood into the intestinal lumen, and enhanced fecal excretion of UCB and its derivatives, compatible with the proposed concept. In addition, however, the hypobilirubinemic effect of orlistat appears to result from increased metabolism of UCB to derivatives. As with phototherapy, the increased intraluminal pool of UCB during orlistat treatment appears to provide increased substrate for intestinal metabolism. Vitek et al. (29) recently showed that plasma UCB concentration increases significantly in Gunn rats in which conversion of UCB to urobilinoids by anaerobic intestinal flora was prevented by treatment with antibiotics. We cannot exclude the possibility that phototherapy or orlistat alters the composition of the intestinal microflora and/or their capacity to metabolize UCB, in addition to increasing the amount of UCB available in the intestinal lumen for microbial metabolism.
In the combined treatment group, the increased influx of UCB into the intestine appeared to be due mainly to enhanced net transmucosal UCB excretion, since biliary UCB secretion was lower in the combined treatment group than in the phototherapy group. This may be related to the greater decrease in steady-state plasma UCB concentration in the combined treatment group, since phototherapy is less effective at lower plasma UCB concentrations due to depletion of the bilirubin pool in the skin, which is the main target of phototherapy (21,30). In addition, one could speculate that orlistat treatment induces net transmucosal transport of photoisomers of UCB, although these compounds are considered relatively water-soluble.
CONCLUSION
Orlistat treatment and phototherapy lower plasma bilirubin concentrations in Gunn rats by increasing intestinal influx of UCB, either by transmucosal excretion (orlistat), or increased biliary secretion (phototherapy). Combined treatment has an additive effect. The mechanism of phototherapy and of orlistat treatment involves increasing the availability of UCB in the intestinal lumen for subsequent fecal excretion and for metabolism by intestinal bacteria. We speculate that manipulation of the metabolizing capacity of the intestinal flora (antibiotics, probiotics) will influence the hypobilirubinemic effects of phototherapy, orlistat treatment, or combined treatment.
Abbreviations
- BW:
-
body weight
- PT:
-
phototherapy
- UCB:
-
unconjugated bilirubin
References
Shapiro SM 2005 Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND). J Perinatol 25: 54–59
Ostrow JD 1971 Photocatabolism of labeled bilirubin in the congenitally jaundiced (Gunn) rat. J Clin Invest 50: 707–718
Tan KL 1991 Phototherapy for neonatal jaundice. Clin Perinatol 18: 423–439
Crigler JF, Najjar VA 1952 Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics 10: 169–180
Van Der Veere CN, Jansen PL, Sinaasappel M, Van Der Meer R, Van der Sijis H, Rammeloo JA, Goyens P, Van Nieuwkerk CM, Oude Elferink RP 1997 Oral calcium phosphate: a new therapy for Crigler-Najjar disease?. Gastroenterology 112: 455–462
Yohannan MD, Terry HJ, Littlewood JM 1983 Long term phototherapy in Crigler-Najjar syndrome. Arch Dis Child 58: 460–462
Rosenstein BS, Ducore JM 1984 Enhancement by bilirubin of DNA damage induced in human cells exposed to phototherapy light. Pediatr Res 18: 3–6
Sideris EG, Papageorgiou GC, Charalampous SC, Vitsa EM 1981 A spectrum response study on single strand DNA breaks, sister chromatid exchanges, and lethality induced by phototherapy lights. Pediatr Res 15: 1019–1023
Kotal P, Van Der Veere CN, Sinaasappel M, Elferink RO, Vitek L, Brodanova M, Jansen PL, Fevery J 1997 Intestinal excretion of unconjugated bilirubin in man and rats with inherited unconjugated hyperbilirubinemia. Pediatr Res 42: 195–200
Schmid R, Hammaker L 1963 Metabolism and disposition of C14-bilirubin in congenital nonhemolytic jaundice. J Clin Invest 42: 1720–1734
Lester R, Schmid R 1963 Intestinal absorption of bile pigments. I. The enterohepatic circulation of bilirubin in the rat. J Clin Invest 42: 736–746
Van Der Veere CN, Schoemaker B, Bakker C, Van Der Meer R, Jansen PL, Elferink RP 1996 Influence of dietary calcium phosphate on the disposition of bilirubin in rats with unconjugated hyperbilirubinemia. Hepatology 24: 620–626
Chowdhury JR, Kondapalli R, Chowdhury NR 1993 Gunn rat: a model for inherited deficiency of bilirubin glucuronidation. Adv Vet Sci Comp Med 37: 149–173
Zenone EA, Stoll MS, Ostrow JD 1982 The effect of elimination of environmental light on the metabolism of unconjugated bilirubin in the Gunn rat. Dig Dis Sci 27: 1117–1120
Nishioka T, Hafkamp AM, Havinga R, vn Lierop PP, Velvis H, Verkade HJ 2003 Orlistat treatment increases fecal bilirubin excretion and decreases plasma bilirubin concentrations in hyperbilirubinemic Gunn rats. J Pediatr 143: 327–334
Hafkamp AM, Havinga R, Sinaasappel M, Verkade HJ 2005 Effective oral treatment of unconjugated hyperbilirubinemia in Gunn rats. Hepatology 41: 526–534
Bayon JE, Pascolo L, Gonzalo-Orden JM, Altonaga JR, Gonzalez-Gallego J, Webster C, Haigh WG, Stelzner M, Pekow C, Tiribelli C, Ostrow JD 2001 Pitfalls in preparation of (3)H-unconjugated bilirubin by biosynthetic labeling from precursor (3)H-5-aminolevulinic acid in the dog. J Lab Clin Med 138: 313–321
Webster C, Tiribelli C, Ostrow JD 2001 An improved method for isolation of unconjugated bilirubin from rat and dog bile. J Lab Clin Med 137: 370–373
Zilversmit DB 1960 The design and analysis of isotope experiments. Am J Med 29: 832–848
Lepage G, Roy CC 1986 Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27: 114–120
Cohen AN, Kapitulnik J, Ostrow JD, Webster CC 1986 Effect of combined treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and phototherapy on bilirubin metabolism in the jaundiced Gunn rat. Hepatology 6: 490–494
Tiribelli C, Ostrow JD 2005 Intestinal flora and bilirubin. J Hepatol 42: 170–172
Kapitulnik J, Gonzalez FJ 1993 Marked endogenous activation of the CYP1A1 and CYP1A2 genes in the congenitally jaundiced Gunn rat. Mol Pharmacol 43: 722–725
Kotal P, Fevery J 1990 Urobilinogen-i is a major derivative of bilirubin in bile of homozygous Gunn rats. Biochem J 268: 181–185
Stoll MS, Zenone EA, Ostrow JD 1981 Excretion of administered and endogenous photobilirubins in the bile of the jaundice gunn rat. J Clin Invest 68: 134–141
Howe RB, Berk PD, Bloomer JR, Berlin NI 1970 Preparation and properties of a specifically labeled radiochemically stable 3H-bilirubin. J Lab Clin Med 75: 499–502
Lester R, Klein PD 1966 Biosynthesis of tritiated bilirubin and studies of its excretion in the rat. J Lab Clin Med 67: 1000–1012
Guerciolini R 1997 Mode of action of orlistat. Int J Obes Relat Metab Disord 21: S12–S23
Vitek L, Zelenka J, Zadinova M, Malina J 2005 The impact of intestinal microflora on serum bilirubin levels. J Hepatol 42: 238–243
Rubaltelli FF, Carli M 1971 The effect of light on cutaneous bilirubin. Biol Neonate 18: 457–462
Acknowledgements
The authors would like to thank Prof.Dr. Ronald P.J. Oude Elferink for valuable discussions and Herman Velvis for technical support regarding HPLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant support by the ‘Najjar Fonds,' by the Fondo Studi Fegato-ONLUS (FCRT 01/00) and the Italian Ministry of Education and Research is gratefully acknowledged.
Rights and permissions
About this article
Cite this article
Hafkamp, A., Havinga, R., Ostrow, J. et al. Novel Kinetic Insights into Treatment of Unconjugated Hyperbilirubinemia: Phototherapy and Orlistat Treatment in Gunn Rats. Pediatr Res 59, 506–512 (2006). https://doi.org/10.1203/01.pdr.0000203180.79636.98
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000203180.79636.98