Abstract
Cytochrome P450 (CYP) inhibition with cimetidine reduces hyperoxic lung injury in young lambs. Nitric oxide (NO), also a CYP inhibitor, has been shown to either aggravate or protect against oxidant stress depending on experimental context. The objective of this study was to determine whether NO, like cimetidine, would protect young lambs against hyperoxic lung injury, and whether its effect was associated with CYP inhibition. Three groups of lambs were studied: 1) room air exposure, 2) >95% O2, and 3) >95% O2 plus inhaled NO. After 72 h, hyperoxia alone resulted in a significant increase in arterial Pco2 and number of polymorphonuclear leukocytes in bronchoalveolar lavage (BAL), and a significant decrease in arterial/alveolar O2 tension (a/A). The addition of inhaled NO significantly decreased the hypercarbia and BAL polymorphonuclear cellular response associated with hyperoxia but had no beneficial effect on a/A ratio. There were no significant differences in F2-isoprostanes or isofurans (markers of lipid peroxidation) measured in BAL or lung tissue among study groups. No intergroup differences were detected in BAL epoxyeicosatrienoic acid levels (index of CYP activity). The results of this study indicate that hypercarbia and inflammation accompanying hyperoxic lung injury in young lambs can be attenuated by inhaled NO. However, this study provides no direct evidence that NO is inhibiting CYP-mediated oxidant lung injury.
Similar content being viewed by others
Main
Previous work has shown that administration of a CYP inhibitor, cimetidine, would reduce hyperoxic lung injury in young lambs (1). In that work, lambs that breathed high oxygen concentrations exhibited impaired gas exchange (elevated Pco2 and lower than expected Po2) and evidence of an increase in pulmonary vascular permeability as indicated by a marked increase in lung lymph flow and protein clearance after 72–74 h of continuous hyperoxia. These effects of hyperoxia were attenuated by the administration of cimetidine before beginning high oxygen exposure. In further studies (2), oxygen breathing significantly increased lung CYP1A1 mRNA levels in vivo, and this increase preceded the increase in isozyme activity. Oxygen exposure also promptly increased CYP1A1 mRNA levels in cultured lamb lung microvascular endothelial cells in these studies. As a monooxygenase, the catalytic activity of CYP is accompanied by the production and release of free radicals (3), and there is a variety of evidence linking CYP to oxidant lung injury (4). Also, the products of arachidonic acid metabolism catalyzed by the CYP system have biologic actions that could cause or aggravate lung injury (5). Taken together, these findings are consistent with the hypothesis that the CYP system in the lung provides a source of damaging oxygen free radicals and catalyzes the production of certain arachidonic acid metabolites which may cause or aggravate lung injury.
NO, an agent that mediates a wide variety of biologic actions, is used clinically as a vasodilator in the management of persistent pulmonary hypertension of the newborn (6,7). NO is also a potent CYP inhibitor that reacts with a heme moiety that is common to all CYP isoforms (8). The purpose of this study was to determine whether inhaled NO would have the same beneficial effects as cimetidine on gas exchange in young lambs exposed to hyperoxia. This study was designed to answer the following questions: 1) Will inhaled NO improve pulmonary gas exchange in young lambs exposed to high oxygen for 72 h; 2) Will inhaled NO decrease lung lipid peroxidation in young lambs exposed to high oxygen for 72 h; 3) Will inhaled NO decrease the production and release of CYP arachidonic acid metabolites into the airway of young lambs exposed to high oxygen for 72 h; 4) Will inhaled NO decrease lung inflammation in young lambs exposed to high oxygen for 72 h.
METHODS
Twenty-three 3- to 7-d-old full-term lambs delivered spontaneously were studied according to a protocol approved by the Institutional Animal Care and Use Committee of the Vanderbilt University Medical Center. Before study, catheters were placed into a lateral saphenous vein and a cranial tibial artery. The lambs were placed in a Plexiglas chamber (0.9 m high × 0.7 m wide × 1.2 m deep) designed to provide an environment of either room air or ≥95% O2 when the chamber was flushed with a constant flow of either compressed air or 100% O2 at 20 L/min. Additional CO2 removal was accomplished with a soda lime CO2 scrubber placed in the chamber. Lambs were housed in the chamber for 72 h. Three to four times each day the lambs were removed from the chamber to nurse from their mothers for 5 min. A sample of arterial blood for blood gas analysis was taken in room air before exposure to the test gases and after exposure to the test gases for 72 h.
The 23 lambs were arbitrarily assigned to the following three groups: 1) RA: room air exposure (n = 5); 2) O2: ≥95% O2 exposure (n = 9); 3) O2-NO: ≥95% O2 exposure plus NO at 5–10 ppm (n = 9).
Sample sizes selected were similar to those in the previous study demonstrating the effects of cimetidine on oxidant lung injury in lambs (1). More lambs were included in the O2 and O2-NO groups than the RA group based on the assumption that any observed effect of NO on oxidant lung injury would be less than the effect of high O2 exposure alone. These sample sizes were sufficient to detect a difference in BAL F2-isoprostanes of 15 pg/mL between the RA and O2 groups and 10 pg/mL between the O2 and O2-NO groups. They were also sufficient to detect between-group differences in BAL total EET levels of 1.2 ng/mL and 0.75 ng/mL, respectively. These differences were detectable with a power of 80% and a Type I error of 0.05.
Target NO concentrations in the chamber were achieved using a flow of 70–230 mL/min of a NO source containing 400 ppm NO (kindly provided by INO Therapeutics, Clinton, NJ). O2, NO, NO2, and CO2 concentrations in the chamber were recorded just before removing the lamb from the chamber for feeding by the mother. Oxygen concentrations in the chamber were measured using an oxygen analyzer by Ventronics (Temecula, CA). NO and NO2 concentrations were measured using a PulmoNOx II RT NO analyzer, Pulmonox Medical Corporation (Tofield, Alberta, Canada) kindly provided by INO Therapeutics. Carbon dioxide concentrations were measured using a LB-2 Medical Gas Analyzer by Beckman (Schiller Park, IL). Arterial pH, Po2, and Pco2 determinations were made using a model 248 pH/Blood Gas Analyzer (Chiron Diagnostics Ltd., Halstead, Essex, UK).
The lambs were killed by a rapid intravenous injection of thiamylal, 200 mg/kg, after 72 h exposure to the assigned gas mixture. After opening the chest, BAL was carried out on the left lung after clamping bronchi leading to the right lung. A portion of the right lower lobe was frozen in liquid nitrogen for studies detailed below.
BAL was carried out by infusing 10 mL/kg normal saline into the left lung followed immediately by aspiration of the lavage. One minute elapsed between the beginning of the infusion and the end of the aspiration, and 58 ± 12% (mean ± SD) of the infused saline was recovered in the aspirate. An uncentrifuged 10-mL aliquot of BAL was placed in a plastic tube containing 40–50 mg triphenylphosphene, a peroxide-reducing agent that prevents auto-oxidation of lipids during processing of the BAL fluid. This aliquot was thoroughly mixed, and stored at –70°C for later analysis of CYP metabolites. After removing a small sample for cell count, the remaining fluid was centrifuged at 2500 rpm for 10 min. The supernatant was stored at –70°C for later analysis of F2-isoprostanes. A differential count of nucleated cells in the pellet was carried out using the cytospin technique (9)
F2-isoprostanes, which serve as a highly sensitive marker of lipid peroxidation, were measured in BAL fluid and lung tissue with an assay employing stable isotope dilution techniques utilizing NICI-GCMS. The assay has been shown to be a highly accurate method to quantify oxidant injury in vivo (10). It has a precision within 6% and an accuracy of 96%. Isofurans, another highly sensitive marker of lipid peroxidation, were measured in lung tissue by NICI-GCMS as previously described (11).
CYP arachidonic acid metabolites (8,9-, 11,12-, and 14,15-EET) were assayed using methods previously described (5). The analysis of endogenous EET in BAL fluid involved preparation of a lipid extract of the fluid in the presence of an equimolar mixture of synthetic 14C-labeled 8,9-, 11,12-, and 14,15-EET as internal standards. Alkaline hydrolysis was followed by HPLC purification, pentafluorobenzyl esterification, repurification of the pentafluorobenzyl esters, and regioisomer quantification by NICI-GCMS (5).
Statistical methods.
Data analysis was carried out using Systat Version 8 (Systat Software, Richmond, CA). To take into account skewed data, differences among study groups were assessed using the Kruskal-Wallis test which is the nonparametric analog of a one-way analysis of variance. Differences between groups at a p-value < 0.05 were considered statistically significant. Graphical data are presented as box plots which show the median, and the range within which the central 50% of the values fall, with the box edges at the first and third quartiles. More details are available in the Graphics Reference Manual for Systat 8.
All results are reported as the median and range for each group. F2-isoprostanes in BAL that were bld were assigned a value of zero for analysis and designated bld in Table 2. Results of all measurements were available for all 23 lambs, except that values for F2-isoprostanes and isofurans in lung tissue were available from only 9 lambs from the three groups because these tests were not undertaken until late in the study, when it became obvious that the anticipated differences in BAL F2-isoprostanes were not being seen. Also, measurements of CYP arachidonic acid metabolites were limited to 18 lambs in the three groups due to the intensity of labor and resources required coupled with the lack of any emerging trend toward intergroup differences.
RESULTS
The three groups of lambs were comparable with regard to arterial pH, Pco2, Po2, body weight, hematocrit, and respiratory rate before being placed into the chamber (data not shown). CO2 concentrations in the chamber were 0.37% (0.17–0.68%) [median (range)]. There were no significant differences in inspired CO2 concentrations across the study groups. Measured Fio2 in the groups receiving high oxygen was always 1.0. NO concentrations in the chamber were 7 (5–14 ppm) [median (range)] for the O2-NO group. NO2 concentration in the chamber was 0 (0–0.2 ppm) [median (range)] for the O2-NO group. Lambs in the two combined groups receiving high oxygen had less weight gain after 72 h than lambs in the RA group: 0.3 (–0.5–1.0 kg) versus 1.0 (0.4–1.4 kg) [median (range)], p < 0.05. Weight gain after 72 h was similar between the two groups receiving high oxygen. There were no differences in respiratory rate across the three groups after 72 h: RA, 76 (46–80); O2, 60 (44–80); O2-NO, 58 (40–120) [median (range)].
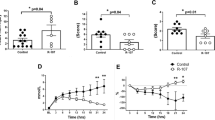
Arterial Pco2 values after 72 h in the chamber are shown for the three groups in Figure 1. Lambs in the O2 group had a significantly higher Pco2 than lambs in the RA group (p < 0.05). This increase in Pco2 was no longer significant in lambs that received NO in addition to high oxygen exposure (p = 0.641 for the O2-NO group versus the RA group). Also, Pco2 in the O2-NO group was significantly lower than Pco2 in the O2 group (p < 0.05).
a/A oxygen ratios after 72 h in the chamber are shown for the three groups in Figure 2. As expected, high oxygen exposure had an adverse effect on oxygenation, resulting in a significant decrease in a/A oxygen ratio for the O2 group when compared with the RA group (p < 0.01). No protective effect against the O2-associated decrease in a/A ratio was seen with NO in that the a/A ratio for the O2-NO group remained significantly decreased when compared with the RA group (p < 0.01). Complete arterial blood gas results are shown in Table 1.
Total, mononuclear, and PMN cell counts are shown in Figure 3 for the three study groups of lambs after 72 h exposure to study gases. In general, lambs exposed to high oxygen (groups O2 and O2-NO) had higher cell counts (both total and fractionated) than the RA group. There were no significant differences in either total cells or mononuclear cells between the two groups exposed to high oxygen. However, the PMN cell count was significantly decreased in the lambs that received O2 in combination with NO (Group O2-NO) compared with the lambs that received high O2 alone (Group O2).
There were no differences in F2-isoprostane levels in BAL fluid across the three study groups (Table 2). There were also no differences in lung tissue content of F2-isoprostanes or isofurans (data not shown). Finally, there were no differences in CYP arachidonic acid metabolites in BAL fluid across the three study groups (Table 3).
DISCUSSION
The results of this study indicate that hypercarbia and inflammation accompanying hyperoxic lung injury in young lambs can be attenuated by inhaled NO. Lambs in the O2 group had significantly increased arterial Pco2 tensions, significantly decreased a/A oxygen tension ratios, and significantly increased BAL PMN compared with the RA group. Treatment with inhaled NO significantly attenuated the effects of hyperoxia on arterial Pco2 and BAL PMN, but not on a/A oxygen tension ratios.
These effects of NO on hyperoxic lung injury are similar to the effects of cimetidine that were observed in a previous study of hyperoxic lung injury in lambs (1). These findings are interesting in that both NO and cimetidine are inhibitors of CYP. However, this study provides no direct evidence that NO is inhibiting CYP-mediated oxidant injury in that 1) no increase in the markers of lipid peroxidation or in CYP-catalyzed arachidonic acid metabolites was seen in the lambs breathing high oxygen concentrations versus the control lambs breathing room air, and 2) no effect of NO on these markers was seen when the O2-NO group was compared with either the RA group or the O2 group.
The two effects of inhaled NO on hyperoxic lung injury observed in this study were a reduction in the hypercarbia and a decrease in the number of PMN in BAL fluid compared with lambs exposed to high oxygen without NO. There are several explanations why inhaled NO might result in a decrease in arterial Pco2: 1) decreased pulmonary edema as a result of a protective effect of NO against endothelial and epithelial damage mediated by oxidant and inflammatory mechanisms (12); 2) reduced alveolar dead space consequent to normalizing the perfusion of ventilated alveoli (13); 3) improved minute ventilation secondary to counteracting the bronchoconstrictive effect of prolonged hyperoxia (14); and 4) protection of surfactant activity against free radical oxidants (15). It is possible that all of these mechanisms played a role in the beneficial effect of inhaled NO on hypercarbia in hyperoxic lung injury seen in this study.
The decrease in a/A ratio of oxygen tension in lambs breathing a high oxygen concentration was not affected by inhaled NO (Fig. 2). One explanation for the discordant effects of inhaled NO on Pco2 and a/A ratio in lambs exposed to high oxygen concentrations is that inhaled NO may impair hypoxic regulation of the matching of perfusion to ventilation in poorly ventilated atelectatic areas of the lung. This effect has been demonstrated in humans with chronic obstructive pulmonary disease (16), in dogs with experimentally induced intrapulmonary shunt and ventilation-perfusion mismatch (17), and in horses during general anesthesia (18).
The finding that inhaled NO significantly reduced the PMN count in BAL compared with lambs breathing high oxygen without NO implies that NO may exert an anti-inflammatory effect in the context of this study. Increased pulmonary capillary permeability is a well-established component of hyperoxic lung injury and appears, at least in part, to be mediated by neutrophils (19). Inhaled NO has been shown to reduce lung neutrophil accumulation in severe experimental hyaline membrane disease (20), and to prevent neutrophil-mediated, oxygen radical–dependent leak in isolated rat lung (21) and in lung of intact rabbits (22). Other studies have shown that endogenous NO also has an inhibitory effect on neutrophil function (23).
Not all studies of the effects of inhaled NO on lung injury in prolonged hyperoxia have shown protective effects, and some have shown effects that are potentially damaging. For example, inhaled NO may prevent up-regulation of superoxide dismutase and catalase activity in piglets during prolonged hyperoxia (24). In another study by the same group, inhaled NO had no effect on pulmonary matrix degradation and the increased lung collagen content seen in piglets exposed to prolonged hyperoxia (25). Even though survival was improved, inhaled NO had no effect on lung neutrophil accumulation in rats exposed to hyperoxia for 60 h (26). These differences seen in NO effect from study to study may not necessarily override the clinical efficacy of inhaled NO to prevent or ameliorate lung injury. In a randomized, double-blind, placebo-controlled study of the effect of inhaled NO during the first week of life in premature infants who were undergoing mechanical ventilation for the respiratory distress syndrome, infants receiving NO therapy had a significant reduction in the incidence of death or chronic lung disease compared with the placebo group (27). This promising finding awaits confirmation by other trials presently underway.
We had expected the markers of lipid peroxidation to be elevated with exposure to high oxygen concentrations for several reasons. F2-isoprostanes and isofurans have been shown to be highly sensitive and specific markers of lipid peroxidation in other contexts (10). Newborn infants with lung disease requiring treatment with high concentrations of inspired oxygen have elevated F2-isoprostane levels in tracheal aspirate fluid when compared with infants not being treated with added oxygen (unpublished data). Measurements of F2-isoprostanes in plasma have been used as evidence of oxidative stress in full-term healthy infants (28), and plasma 8-isoprostane has been shown to predict bronchopulmonary dysplasia and periventricular leukomalacia in premature infants (29). In addition, F2-isoprostanes in BAL have been used as a biomarker of oxidative stress in patients with interstitial lung disease (30). It is possible, however, that a hyperoxia-induced increase in these compounds did occur in the present study, but that the increase was transient and their levels returned to baseline before the BAL and lung tissue samples were obtained. It has also been shown that in contrast to isofuran formation, F2-isoprostane formation becomes limited in high oxygen tension (11), a phenomenon that may limit the value of the F2-isoprostanes as a marker of oxidant lung injury.
We had also expected to find elevated levels of CYP arachidonic acid metabolites in BAL fluid from lambs in the O2 group compared with lambs in the RA group. Newborn premature infants with lung disease requiring oxygen therapy have evidence of increased CYP activity in that their tracheal fluid aspirates have elevated levels of certain EET derivatives compared with infants not requiring oxygen therapy (unpublished data). It is not clear why the lambs in the O2 group in this study did not also have elevated EET levels in BAL fluid compared with lambs in the RA group. Again, it is possible that the EET levels were only transiently elevated before obtaining the BAL samples. In regard to this possibility, it is interesting that in lamb lung, the oxygen-induced increase in CYP1A1 gene expression in vitro and in vivo was back to baseline levels by 48 h after peaking at 24 h of high oxygen exposure (2).
A limitation of this study is the small number of lambs from which lung tissue levels of F2-isoprostanes and isofurans were measured. On the other hand, the analyses of BAL F2-isoprostanes and EETs were sufficiently powered to detect differences in these markers that were seen previously in tracheal aspirates from premature infants exposed to either room air or added oxygen (unpublished data).
In conclusion, hypercarbia and inflammation accompanying hyperoxic lung injury in young lambs can be attenuated by inhaled NO. There was no direct evidence that these beneficial effects of NO resulted from the inhibition of CYP-mediated oxidant injury to the lung inasmuch as the biochemical markers of CYP activity and lipid peroxidation did not change with either oxygen exposure or inhaled NO.
Abbreviations
- BAL:
-
bronchoalveolar lavage
- bld:
-
below limits of detection
- CYP:
-
cytochrome P450
- EET:
-
14,15-epoxyeicosatrienoic acids
- NICI-GCMS:
-
negative ion-chemical ionization-gas chromatography-mass spectrometry
- NO:
-
nitric oxide
- PMN:
-
polymorphonuclear leukocytes
References
Hazinski TA, France M, Kennedy KA, Hansen TN 1989 Cimetidine reduces hyperoxic lung injury in lambs. J Appl Physiol 67: 2586–2592
Hazinski TA, Noisin E, Hamon I, DeMatteo A 1995 Sheep lung cytochrome P450IA1 (CYP1A1): cDNA cloning and transcriptional regulation by oxygen tension. J Clin Invest 96: 2083–2089
Tindberg N, Ingelman-Sundberg M 1989 Cytochrome P-450 and oxygen toxicity. Oxygen-dependent induction of ethanol-inducible cytochrome P-450 (IIE1) in rat liver and lung. Biochemistry 28: 4499–4504
Bysani GK, Kennedy TP, Ky N, Rao NV, Blaze CA, Hoidal JR 1990 Role of cytochrome P-450 in reperfusion injury of the rabbit lung. J Clin Invest 86: 1434–1441
Zeldin DC, Plitman JD, Kobayashi J, Miller RF, Snapper JR, Falck JR, Szarek JL, Philpot RM, Capdevila JH 1995 The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest 95: 2150–2160
Kinsella JP, Abman SH 2000 Inhaled nitric oxide: current and future uses in neonates. Semin Perinatol 24: 387–395
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP 2000 Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 342: 469–474
Stadler J, Trockfeld J, Schmalix WA, Brill T, Siewert JR, Greim H, Doehmer J 1994 Inhibition of cytochromes P4501A by nitric oxide. Proc Natl Acad Sci U S A 91: 3559–3563
1990 Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. The BAL Cooperative Group Steering Committee. Am Rev Respir Dis 141: S169–S202
Roberts LJ, Morrow JD 1994 Isoprostanes. Novel markers of endogenous lipid peroxidation and potential mediators of oxidant injury. Ann N Y Acad Sci 744: 237–242
Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ 2nd 2002 Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A 99: 16713–16718
McElroy MC, Wiener-Kronish JP, Miyazaki H, Sawa T, Modelska K, Dobbs LG, Pittet JF 1997 Nitric oxide attenuates lung endothelial injury caused by sublethal hyperoxia in rats. Am J Physiol 272: L631–L638
Skimming JW, Banner MJ, Spalding HK, Jaeger MJ, Burchfield DJ, Davenport PW 2001 Nitric oxide inhalation increases alveolar gas exchange by decreasing deadspace volume. Crit Care Med 29: 1195–1200
Martin RJ, Mhanna MJ, Haxhiu MA 2002 The role of endogenous and exogenous nitric oxide on airway function. Semin Perinatol 26: 432–438
Hallman M, Bry K 1996 Nitric oxide and lung surfactant. Semin Perinatol 20: 173–185
Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R 1996 Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 347: 436–440
Hopkins SR, Johnson EC, Richardson RS, Wagner H, De Rosa M, Wagner PD 1997 Effects of inhaled nitric oxide on gas exchange in lungs with shunt or poorly ventilated areas. Am J Respir Crit Care Med 156: 484–491
Heinonen E, Nyman G, Merilainen P, Hogman M 2002 Effect of different pulses of nitric oxide on venous admixture in the anaesthetized horse. Br J Anaesth 88: 394–398
Lewis RE, Granger HJ 1986 Neutrophil-dependent mediation of microvascular permeability. Fed Proc 45: 109–113
Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH 1997 Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 41: 457–463
Guidot DM, Repine MJ, Hybertson BM, Repine JE 1995 Inhaled nitric oxide prevents neutrophil-mediated, oxygen radical-dependent leak in isolated rat lungs. Am J Physiol 269: L2–L5
Kang JL, Park W, Pack IS, Lee HS, Kim MJ, Lim CM, Koh Y 2002 Inhaled nitric oxide attenuates acute lung injury via inhibition of nuclear factor-kappa B and inflammation. J Appl Physiol 92: 795–801
Tavares-Murta BM, Machado JS, Ferreira SH, Cunha FQ 2001 Nitric oxide mediates the inhibition of neutrophil migration induced by systemic administration of LPS. Inflammation 25: 247–253
Ekekezie II, Thibeault DW, Zwick DL, Rezaiekhaligh MH, Mabry SM, Morgan RE, Norberg M, Truog WE 2000 Independent and combined effects of prolonged inhaled nitric oxide and oxygen on lung inflammation in newborn piglets. Biol Neonate 77: 37–44
Ekekezie II, Thibeault DW, Rezaeikhaligh MH, Mabry SM, Norberg M, Reddy GK, Youssef J, Truog WE 2000 High-dose inhaled nitric oxide and hyperoxia increases lung collagen accumulation in piglets. Biol Neonate 78: 198–206
Nelin LD, Welty SE, Morrisey JF, Gotuaco C, Dawson CA 1998 Nitric oxide increases the survival of rats with a high oxygen exposure. Pediatr Res 43: 727–732
Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P 2003 Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 349: 2099–2107
Friel JK, Friesen RW, Harding SV, Roberts LJ 2004 Evidence of oxidative stress in full-term healthy infants. Pediatr Res 56: 878–882
Ahola T, Fellman V, Kjellmer I, Raivio KO, Lapatto R 2004 Plasma 8-isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatr Res 56: 88–93
Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ 1998 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am J Respir Crit Care Med 158: 1524–1527
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants HL56697, GM42056, GM15431, and DK26657 from NIH and the National Institute of Environmental Health Sciences Division of Intramural Research
Rights and permissions
About this article
Cite this article
Cotton, R., Sundell, H., Zeldin, D. et al. Inhaled Nitric Oxide Attenuates Hyperoxic Lung Injury in Lambs. Pediatr Res 59, 142–146 (2006). https://doi.org/10.1203/01.pdr.0000191815.60293.cc
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000191815.60293.cc
This article is cited by
-
L-citrulline supplementation reverses the impaired airway relaxation in neonatal rats exposed to hyperoxia
Respiratory Research (2012)
-
Inhaled nitric oxide increases endothelial permeability in Pseudomonas aeruginosa pneumonia
Intensive Care Medicine (2007)