Abstract
Classical phenylketonuria (PKU) is caused by deficiency of phenylalanine hydroxylase, resulting in an accumulation of its upstream metabolite phenylalanine in brain tissue and cerebrospinal fluid of PKU patients. PKU is neuropathologically characterized by reduced dendritic arborization, loss of synapses, and neurodegeneration. We investigated whether increased concentrations of phenylalanine cause reduced synaptic density and alter dendritic branching. We treated primary cortical neurons differentiated for 21 d in vitro with 5 mM phenylalanine in the presence of all essential amino acids. Immunocytochemical analysis of 12 and 21 d in vitro primary neurons revealed no changes of dendritic morphology or neuronal viability but a significant difference in synaptic density, suggesting that elevated concentrations of extracellular phenylalanine cause an impairment of synaptogenesis. Although impairment of cerebral energy metabolism has been identified as an important pathophysiological principal in many diseases, respiratory chain function has not been extensively studied in PKU before. We investigated whether phenylalanine inhibits respiratory chain complexes I–V. In vitro analysis revealed no inhibitory effect of phenylalanine on complexes I–V, but an inhibition of pyruvate kinase, a key enzyme of glycolysis, catalyzing the formation of pyruvate. Pyruvate kinase is part of the enzyme assay to investigate enzyme activity of mitochondrial complex V and it remains to be elucidated whether this finding is relevant in vivo. In conclusion, elevated concentrations of phenylalanine might be involved in mechanisms underlying impaired synaptogenesis in PKU, supporting the common therapeutic strategy to reduce phenylalanine concentrations in the brain to prevent neurodegeneration.
Similar content being viewed by others
Main
Classical PKU (MIM # 261600) is the most frequent inborn error of metabolism and follows an autosomal recessive pattern of inheritance. Untreated patients characteristically develop severe mental retardation, microcephaly, behavioral abnormalities, seizures, and sometimes also spasticity and hyperreflexia. PKU is caused by deficiency of PAH (EC 1.14.16.1), which is located in the liver and kidney, resulting in an accumulation of phenylalanine. In untreated patients, millimolar concentrations of phenylalanine can be reached in plasma and other body fluids. Inclusion of PKU to newborn screening programs has allowed for early diagnosis and treatment of affected patients with a phenylalanine-restricted diet before clinical symptoms, however, the precise mechanisms underlying neurologic disease in untreated patients are not yet completely understood.
The majority of previous studies have considered phenylalanine as the main neurotoxin (1). High plasma concentrations (≥1.3 mM) of phenylalanine induce acute impairment in neuronal functions, such as EEG abnormalities or impaired neuropsychological function (2,3), whereas at lower concentrations (0.3–0.6 mM) phenylalanine possibly induces chronic white matter abnormalities (4). Phenylalanine competes with the transport and distribution of other amino acids, in particular the great neutral amino acids. This competition results in cerebral depletion of essential branched-chain amino acids, sequestration of phenylalanine in peripheral tissues, and an accumulation of phenylalanine in cerebrospinal fluid (CSF) and brain tissue (5). Additional alternative pathologic metabolites, such as phenyllactate, phenylacetate, and phenylpyruvate are not found in pathophysiologically relevant concentrations in the CNS of affected patients (6) or the PAHenu2 mouse, a transgenic mouse model for PKU (7), thus virtually excluding a relevant role for PKU neuropathology (8).
Alterations of brain histology and neuronal cell development have been studied in different models for PKU. Histopathological investigations of untreated PKU patients revealed severe impairment of brain architecture and maturation, with abnormalities in myelination, cell density and cell organization. Dendritic arborization was altered and the number of synaptic spines was decreased (9). A more recent autopsy study of an untreated patient reports neuronal loss reduced size of neurons and decreased dendritic processes of Purkinje cells (10). Exposure of rodents to high doses of phenylalanine and pharmacological inhibition of phenylalanine hydroxylase induced changes in brain architecture, such as a decreased number and span of dendritic basilar processes of large pyramidal cells from deeper cortical cell layers (11). In PAHenu2 mice, hypomyelination and cortical gliosis have been demonstrated. It has been hypothesized that elevated phenylalanine activates myelinating oligodendrocytes to adopt a nonmyelinating phenotype (12).
Although impairment of cerebral energy metabolism has been identified as an important pathophysiological principal in many diseases, respiratory chain function has not been extensively studied in PKU before. Rech et al. (13) report a reduced activity of mitochondrial complexes I–III using a combined test system in brain homogenates from rats. Moreover, 31P magnetic resonance spectroscopy revealed an acute effect of phenylalanine on cerebral energy metabolism (14). A subtle abnormality such as elevated ADP concentration under steady-state conditions was accentuated by phenylalanine loading of PKU patients.
The molecular mechanisms causing hypomyelination and reduced synaptogenesis in PKU are still unknown. In this study, we investigated whether phenylalanine affects synaptic density in mixed cortical cultures from mice in the presence of essential amino acids, and whether it affects mitochondrial energy metabolism.
METHODS
Cell culture and phenylalanine treatment.
Mouse embryos [embryonic day (E)14] from (129Sv × C57BL/6 F(x)) were prepared as described for rat hippocampal neurons (15), except that the whole cerebral cortex of mice instead of the rat hippocampus was used. Experimental animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and experiments were announced to the local animal experimentation committee. The cells were plated on poly-l-lysine-coated 15 mm coverslips (Marienfeld, Germany, Marienfeld, Cauda-Uönigshafen) in 3.5-cm dishes containing 3 mL of serum-free N2 medium with or without 5 mM phenylalanine (15). Neurons were cultivated at 37°C in standard atmosphere with 5% CO2. Every second day, half of the medium was exchanged with fresh N2 medium with or without 5 mM phenylalanine. Immunocytochemical analysis of the neurons was performed after 12 or 21 DIV.
Immunocytochemistry.
Mixed cortical neurons were fixed in 4% paraformaldehyde and processed for immunofluorescence as described previously (16). Mouse anti-MAP2 antibodies (1:200; Sigma Chemical Co., Germany), rabbit anti-MAP antibodies (1:200; Santa Cruz, Germany), rabbit anti-GFAP (GFAP glial fibrillary acidic protein) antibodies (1:500; Dako) and mouse anti-synaptophysin antibodies (1:100; Sigma Chemie, Deisenhofen, Germany) were used at the indicated dilutions. The secondary antibodies (Alexa Fluor 488 and 594) were purchased from Molecular Probes (Breda, Netherlands). Fixed neurons could be visualized using a fluorescence microscope (Leica, Wetzlar, Germany). Dendrites with a diameter between 1 and 3 μm and a minimal length of 20 μm were considered for analysis, and each synaptophysin stain along a particular dendrite was marked and counted by special software (Openlab from Improvision, version 3.0.4, Improvision, Coventry, England). Dendrite length was also measured by the same software and synaptic density was subsequently calculated. Evaluation of experiments was performed without knowledge of treatment.
Quantitative analysis of synaptic density.
At DIV 12 and 21, synaptic density was calculated as number of counts per line length and was expressed as increase in synaptic density in comparison to controls (DIV 21, without phenylalanine treatment). All experiments were performed in sister cultures. All data are expressed as mean ± SEM. After proving normal distribution with Kolmogorov-Smirnov test, t test for two groups, or one-way ANOVA with post hoc Scheffé test for three or more groups was calculated using SPSS for Windows software, Version 11.0 (SPSS Inc., Chicago, IL). A value of p < 0.05 was considered significant.
Spectrophotometric analysis of single respiratory chain complexes I–V.
SMP are a well-characterized model to study the activity of each single enzyme complex of the mitochondrial respiratory chain and the influence of organic compounds on the respiratory chain. SMP were prepared as previously described (17). Protein was determined according to Lowry et al. (18) with modifications of Helenius and Simons (19) using BSA as standard. The catalytic activities of respiratory chain complexes I–V were investigated in SMP as previously described (20,21). In brief, steady-state activities of mitochondrial complexes I–V were recorded using a computer tunable spectrophotometer (Versamax Microplate Reader 384, Molecular Devices, Sunnyvale, CA) operating in the dual-wavelength mode. Samples were analyzed in thermostated 96-well plates in a final volume of 300 μL. Mean standard activities (U/mg total protein, n = 3–6 experiments) were as follows and previously described (22): complex I (1.24 ± 0.02), complex II (0.97 ± 0.02), complex III (24.0 ± 0.5), complex IV (15 ± 0.2), and complex V (0.5 ± 0.01). The addition of standard inhibitors of single respiratory chain complexes [complex I: 1 μM DQA (2-n-decylquinazolin-4-yl-amine); complex II: 5 mM malonate; complex III: 2 μM stigmatellin; complex IV: 2 mM NaCN; complex V: 80 μm oligomycin] revealed a significant inhibitory response [residual activity (% control ± SD), complex I: 3 ± 1%; complex II: 4 ± 1%; complex III: 6 ± 1%; complex IV: 3 ± 1%; complex V: 20 ± 2%]. The effects of phenylalanine and alanine on single complex activities were subsequently investigated (n = 3–6 experiments).
Spectrophotometric measurements of pyruvate kinase and lactate dehydrogenase activity.
The activity of complex V was measured indirectly by analyzing the NADH oxidation that results from an enzymatic reaction cas-cade including pyruvate kinase and lactate dehydrogenase (LDH). Thus, inhibition of one of these reactions by phenylalanine would also decrease the measured activity. Pyruvate kinase activity was determined in purified enzyme from rabbit muscle (Roche Molecular Biochemicals, Mannheim, Germany) in a reaction buffer containing 0.1 M Tris-HCl, 10 mM MgCl2, 75 mM KCl, 0.16 mM NADH, LDH, ADP, 0.1% Triton X-100 at 37°C in a final volume of 300 μL using the same spectrophotometer as mentioned above operating in a dual-wavelength mode (340–400 nm). Phenylalanine (0.1–10 mM) was added to the reaction buffer before the reaction was started by addition of 1 mM phosphoenolpyruvate. Because alanine antagonizes an inhibition of pyruvate kinase by phenylalanine (22), we co-incubated 10 mM phenylalanine with alanine (1–10 mM). To exclude secondary inhibition by an inhibition of the second enzyme in the assay, i.e. LDH, we also investigated the influence of phenylalanine (up to 10 mM) on LDH activity. Purified LDH (Roche Molecular Biochemicals) from bovine heart was used according to a modified method of Lumeng et al. (24). LDH was applied in a reaction buffer at 37°C in a final volume of 300 μL, containing 50 mM potassium phosphate, 5.25 mM NAD, and 1.55 M D, L-lactic acid (adjusted to pH 7.5) in a spectrophotometer as mentioned above operating in a dual-wavelength mode (340–400 nm).
RESULTS
Phenylalanine reduces the synaptogenesis between DIV 12 and 21 in mixed cortical cultures from mice.
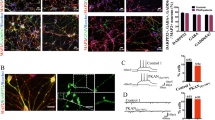
To investigate whether high concentrations of phenylalanine in brain of PKU patients might directly affect neuronal viability, morphology, or the rate of synaptogenesis, we cultivated primary cortical neurons in standard medium and medium with enhanced concentration of phenylalanine (5 mM). Half of the medium was exchanged every second day to hold the phenylalanine concentration on a constant level. The primary cultures were fixed at DIV 12 and 21 and subjected for immunocytochemistry using anti-MAP2, anti-GFAP, and anti-synaptophysin antibodies. MAP2 is a neuron-specific protein, which is localized to the somatodendritic compartment, whereas GFAP is only expressed in astrocytes. Immunocytochemical analyses demonstrated 20–30% of glial cells and 70–80% of neurons in our culture system. The ratio of glial to neuronal cells was not affected by enhanced concen-trations of phenylalanine. Furthermore, comparison of nontreated and phenylalanine-treated cultures at DIV 12 and 21 revealed no difference in the cell density or morphology of neuronal cells, suggesting that phenylalanine does not affect the neuronal viability nor the dendritic branching of neurons during this time period. In contrast, phenylalanine-treated neurons revealed only a minimal increase in synaptic density between DIV 12 and 21 compared with untreated controls (Figs. 1 and 2).
Phenylalanine reduces the synaptic density in mixed cortical cultures from mice. Neurons from whole cerebral cortex of mice on DIV 12 (A, C) stained by mouse anti-synaptophysin antibodies reveal a significant difference in synaptic density after continuous treatment without (A, B) or with (C, D) 5 mM phenylalanine on DIV 21 but no differences in dendritic morphology.
Quantitative analysis of the phenylalanine-mediated decrease in synaptogenesis of cultured cortical neurons from mice. Cultivated neurons from whole cerebral cortex of mice (n = 158) were stained by mouse anti-synaptophysin antibodies on DIV 12 and 21. Total synaptic density is expressed as counts per micrometer dendrite length on DIV 12 and 21 [mean ± SD] (A). Increase in synaptic density was calculated as the difference of synaptic density on DIV 12 and DIV 21 for nontreated (○) and treated (•) neurons (5 mM phenylalanine). Data are expressed as percentage increase of synaptic density on DIV 21 [mean ± SD]. Controls (□) on DIV 21 were taken as 100%. Treated (▪, 5 mM phenylalanine) (B). *p = 0.05 (t test).
Phenylalanine does not inhibit enzyme activity of single respiratory chain complexes I–V but reduces the activity of pyruvate kinase.
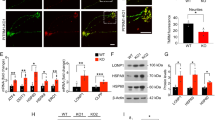
Several lines of evidence indicate that cerebral energy metabolism in PKU is impaired. This may also be relevant for our observation of reduced synaptogenesis in phenylalanine-treated neuronal cultures. Therefore, we determined the enzyme activities of respiratory chain complexes I–V in SMP. Phenylalanine (0.1–10 mM) did not exert any inhibitory effect on respiratory chain complexes I–IV, but an apparent inhibition of complex V (Fig. 3). As the activity of complex V was measured indirectly by analyzing the NADH oxidation, which results from an enzymatic reaction cascade, including pyruvate kinase and LDH, we could not exclude that the measured reduced activity might result from inhibition of one of these two enzymes. Therefore, we used two independent assays to test the influence of phenylalanine on pyruvate kinase and LDH activity. Phenylalanine (1–10 mM) had no effect on LDH activity (data not shown), whereas we observed a significant inhibition of pyruvate kinase (Fig. 4A).
Effect of phenylalanine on respiratory chain complexes I–V. Effects of phenylalanine on respiratory chain complex I–V activity in SMP revealed a false inhibition of complex V caused by concentration-dependent inhibition of pyruvate kinase in the assay. The activity of complexes I–IV is significantly reduced only by specific inhibitors, demonstrating the specificity and reliability of the assay system, but not by phenylalanine in pathophysiologically relevant concentrations. Complex V activity is expressed as percentage of control activity (SD = 1–2%, n = 3–6 experiments). Mean standard activities (U/mg total protein, n = 3–6 experiments) were as fol-lows: complex I (1.24 ± 0.02), complex II (0.97 ± 0.02), complex III (24.0 ± 0.5), complex IV (15 ± 0.2), and complex V (0.5 ± 0.01).
Inhibitory effect of phenylalanine on pyruvate kinase activity in purified enzyme. Phenylalanine (•) showed a concentration-dependent inhibition of pyruvate kinase (PK) activity. Alanine (□) revealed no inhibitory effect (A). Phenylalanine-induced inhibition of pyruvate kinase was reversed by co-incubation with alanine in a concentration-dependent manner (B). Data are expressed as mean ± SD of four independent experiments. *p = 0.05 (t test).
Phenylalanine-induced inhibition of pyruvate kinase was reversed by co-incubation with alanine in a concentration-dependent manner (Fig. 4B). Thus, the apparent inhibition of complex V activity in our assay system was most likely secondary to a phenylalanine-induced inhibition of pyruvate kinase.
DISCUSSION
The major finding of the present study was a reduced synaptic density in mixed cortical cultures of mice after incubation with phenylalanine, suggesting a reduced synapse formation or a loss of synapses. Furthermore, we demonstrated a phenylalanine-induced inhibition of pyruvate kinase, a key enzyme of the glycolytic pathway.
Our findings are in line with former investigations, demonstrating abnormalities in gray matter development and architecture as one of the two important neuropathological features in PKU (25), resulting in a reduced density of cortical neurons and a pertubated neuroanatomical structure of the cortex. These changes correspond to clinical key features of untreated PKU, i.e. mental retardation and seizures (25). Notably, such changes usually escape standard neuropathologic evaluation and can only be proven by subtle quantitative analyses as performed in our study (25). In two animal models for PKU, dendritic development has been studied: In the neocortex of phenylketonuric rats, changes in the structural organization of the cerebral cortex and a decreased number of span and basilar processes of large pyramidal cells were found (11). Another study showed decreased synaptogenesis in the cerebral cortex of rats using chemical markers to visualize synaptogenesis (26). Similar features can be seen in fetal hazard due to elevated maternal phenylalanine levels. Since the first reports in 1957, it has been well known that maternal hyperphenylalaninemia can lead to a distinct metabolic embryo-fetal syndrome, which is characterized by impaired cognitive development, microcephaly, and hypoplasia of the corpus callosum (27–29). In a rat model of maternal PKU, sparsity and malformation of dendritic spines of cortical pyramidal cells have been shown, which has been interpreted as impaired synaptic development (30).
Our results are in accordance with these findings, reveal-ing a decreased synaptic density in phenylalanine-treated cortical cultures. Furthermore, we have shown that phenylalanine itself directly affects this neuronal function, excluding side effects of inhibitors or defects in uptake of large neutral amino acids. In addition, our test system allows a standardized investigation of phenylalanine in the presence of essen-tial amino acids and, per se, excludes phenylalanine-induced effects on amino acid transport across the blood-brain bar-rier. This is important because phenylalanine and the LNAA (tyrosine, tryptophan, threonine, isoleucine, leucine, valine, methionine, and histidine) share a common transporter to the brain and compete with one another (31). Because phenylalanine has the lowest Km among these competitors, it is preferentially transported into the CNS (32,33). Therefore, elevated levels of phenylalanine in plasma inhibit the uptake of LNAA, resulting in a decrease of these amino acids and their metabolites in CSF (34). In contrast, increased concentrations of LNAA decrease phenylalanine uptake. Thus, the idea to implement LNAA into treatment of PKU as neuroprotective strategy has been generated (1).
Phenylalanine was reported to affect neuronal morphology and viability (11,25,26). In our primary neuronal cultures, we did not observe any changes in cell density, dendritic arborization, or dendritic length and diameter after 21 d of incubation. However, we cannot exclude that longer incubation periods, which cannot be investigated in our model, might have caused these changes. In the developing brain of PAHenu2 mice, the function and expression of NMDA (N-methyl-d-aspartate) and AMPA (amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors is altered with a high phenylalanine diet (35). Considering the important role of the glutamatergic system in brain development, these findings may offer an explanation for our results.
In the second part of the study, we investigated whether phenylalanine influenced mitochondrial energy metabolism. In contrast to previous studies using combined test systems and brain homogenates (13), single enzyme activity of respiratory chain complexes I–IV were not affected in presence of phenylalanine up to 10 mM in our system. We demonstrated concentration-dependent inhibition of purified pyruvate kinase by phenylalanine. Because pyruvate kinase, an enzyme of the anaerobic glycolysis catalyzing the conversion of phosphoenolpyruvate to pyruvate, is important for cerebral energy metabolism, reduced activity of this enzyme by phenylalanine might contribute to the pathophysiology of PKU (36). It has been suggested before that phenylalanine might compete with the physiologic substrates phosphoenolpyruvate and ADP (37). Notably, impaired glucose utilization and cerebral energy metabolism have been described in PKU patients and models: phenylalanine inhibited glucose metabolism in rat brain slices during differentiation (38), and brain areas with white matter abnormalities in PKU patients revealed an impaired glucose metabolism (39), whereas an impairment of cortical glucose metabolism remained unclear.
In conclusion, we have shown that phenylalanine reduced synaptic density and pyruvate kinase activity, suggesting that phenylalanine directly interacts with important mechanisms of brain development and cerebral energy metabolism. These results support the notion that phenylalanine is directly involved in the pathomechanisms of PKU but do not argue against the LNAA hypothesis.
Abbreviations
- DIV:
-
days in vitro
- GFAP:
-
glial fibrillary acidic protein
- LNAA:
-
large neutral amino acids
- MAP:
-
microtubule-associated protein
- PAH:
-
phenylalanine hydroxylase
- PKU:
-
phenylketonuria
- SMP:
-
submitochondrial particles from bovine heart
References
Kaufman S 1976 Phenylketonuria: Biochemical mechanisms. In: Agranoff BW, Aprison MH, (eds) Advances in Neurochemistry. Plenum Press, New York, pp 1–132
Krause W, Halminski M, McDonald L, Dembure P, Salvo R, Freides D, Elsas L 1985 Biochemical and neuropsychological effects of elevated plasma phenylalanine in patients with treated phenylketonuria. A model for the study of phenylalanine and brain function in man. J Clin Invest 75: 40–48
Michel U, Schmidt E, Batzler U 1990 Results of psychological testing of patients aged 3-6 years. Eur J Ped 149: S34–S38
Bick U, Fahrendorf G, Ludolph AC, Vassallo P, Weglage J, Ullrich K 1991 Disturbed myelination in patients with treated hyperphenylalaninemia: evaluation with magnetic resonance imaging. Eur J Pediatr 150: 185–189
Choi TB, Pardridge WM 1986 Phenylalanine transport at the human blood-brain barrier. Studies with isolated human brain capillaries. J Biol Chem 261: 6536–6541
Kaufman S 1989 An evaluation of the possible neurotoxicity of metabolites of phenylalanine. J Pediatr 114: 895–900
Sarkissian CN, Scriver CR, Mamer OA 2000 Measurement of phenyllactate, phenylacetate, and phenylpyruvate by negative ion chemical ionization-gas chromatography/mass spectrometry in brain of mouse genetic models of phenylketonuria and non-phenylketonuria hyperphenylalaninemia. Anal Biochem 280: 242–249
Scriver CR, Kaufman S 2001 Hyperphenylalaninemia: phenylalanine Hydroxylase Deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, pp 1667–1724
Bauman ML, Kemper TL 1982 Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta Neuropathol 58: 55–63
Kornguth S, Gilbert-Barness E, Langer E, Hegstrand L 1992 Golgi-Kopsch silver study of the brain of a patient with untreated phenylketonuria, seizures and cortical blindness. Am J Med Gen 44: 443–448
Cordero ME, Trejo M, Colombo M, Aranda V 1983 Histological maturation of the neocortex in phenylketonuric rats. Early Hum Dev 8: 157–173
Dyer CA, Kendler A, Philibotte T, Gardiner P, Cruz J, Levy HL 1996 Evidence for central nervous system glial cell plasticity in phenylketonuria. J Neuropathol Exp Neurol 55: 795–814
Rech VC, Feksa LR, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM 2002 Inhibition of the mitochondrial respiratory chain by alanine in rat cerebral cortex. Neurochem Res 27: 353–357
Pietz J, Rupp A, Ebinger F, Rating D, Mayatepek E, Boesch C, Kreis R 2003 Cerebral energy metabolism in phenylketonuria: findings by quantitative in vivo 31P MR spectroscopy. Pediatr Res 53: 654–662
Goslin K, Banker GA 1991 Rat hippocampal neurons in low density culture. In: Banker GA and Goslin K (eds) Culturing Nerve Cells. MIT Press, Cambridge, MA, pp 251–281
Kins S, Betz H, Kirsch J 2000 Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci 3: 22–29
Okun JG, Lümmen P, Brandt U 1999 Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase). J Biol Chem 274: 2625–2630
Lowry OH, Roseborough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Helenius A, Simons K 1972 The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem 247: 3656–3661
Okun JG, Hörster F, Farkas LM, Feyh P, Hinz A, Sauer S, Hoffmann GF, Unsicker K, Mayatepek E, Kölker S 2002 Neurodegeneration in methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting excitotoxicity. J Biol Chem 277: 14674–14680
Kölker S, Schwab M, Hörster F, Sauer S, Hinz A, Wolf NI, Mayatepek E, Hoffmann GF, Smeitink JA, Okun JG 2003 Methylmalonic acid, a biochemical hallmark of methylmalonic acidurias but no inhibitor of mitochondrial respiratory chain. J Biol Chem 278: 47388–47393
Kölker S, Okun JG, Hörster F, Assmann B, Ahlemeyer B, Kohlmüller D, Exner-Camps S, Mayatepek E, Krieglstein J, Hoffmann GF 2001 3-Ureidopropionate contributes to the neuropathology of 3-ureidopropionase deficiency and severe propionic aciduria: a hypothesis. J Neurosci Res 66: 666–673
Feksa LR, Cornelio AR, Rech VC, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM 2002 Alanine prevents the reduction of pyruvate kinase activity in brain cortex of rats subjected to chemically induced hyperphenylalaninemia. Neurochem Res 27: 947–952
Lumeng J, Blickenstaff L, Miller J 1971 A rapid spectrophotometric system for measurement of lactic acid dehydrogenase activity. Am J Clin Pathol 55: 471–474
Huttenlocher PR 2000 The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 159: S102–S106
Loo YH, Fulton T, Miller K, Wisniewski HM 1980 Phenylacetate and brain dys-function in experimental phenylketonuria: synaptic development. Life Sci 27: 1283–1290
Levy HL, Lobbregt D, Barnes PD, Poussaint TY 1996 Maternal phenylketonuria: magnetic resonance imaging of the brain in offspring. J Pediatr 128: 770–775
Mabry CC, Denniston JC, Nelson TL, Son CD 1963 Maternal phenylketonuria. A cause of mental retardation in children without the metabolic defect. N Engl J Med 269: 1404–1408
Mabry CC, Denniston JC, Coldwell JG 1966 Mental retardation in children of phenylketonuric mothers. N Engl J Med 275: 1331–1336
Lacey DJ 1986 Cortical dendritic spine loss in rat pups whose mothers were prenatally injected with phenylacetate (“maternal PKU” model). Brain Res 392: 283–285
Oldendorf WH, Szabo J 1976 Amino acid assignment to one of three blood-brain amino acid carriers. Am J Physiol 230: 94–98
Pardridge WM 1982 Blood-brain barrier amino acid transport: clinical implications. In: Cockburn F, Gitzelmann R (eds) Society for the Study of Inborn Errors of Metabolism, Inborn Errors of Metabolism in Humans: Monograph Based upon Proceedings of the International Symposium Held in Interlaken, Switzerland, September 2–5, 1980. MTP Press Limited, Lancaster, pp 87–99
Hargreaves KM, Pardridge WM 1988 Neutral amino acid transport at the human blood-brain barrier. J Biol Chem 263: 19392–19397
McKean CM 1972 The effects of high phenylalanine concentrations on serotonin and catecholamine metabolism in the human brain. Brain Res 47: 469–476
Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Sumners C, Laipis PJ, Embury JE, Baker SP, Otero DH, Dennis DM, Seubert CN, Martynyuk AE 2005 Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain 128: 300–307
Weber G 1969 Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpyruvate: possible relevance to phenylketonuric brain damage. Proc Natl Acad Sci U S A 63: 1365–1369
Feksa LR, Cornelio AR, Dutra-Filho CS, de Souza Wyse AT, Wajner M, Wannmacher CM 2003 Characterization of the inhibition of pyruvate kinase caused by phenylalanine and phenylpyruvate in rat brain cortex. Brain Res 968: 199–205
Weber G, Glazer RI, Ross RA 1970 Regulation of human and rat brain metabolism: inhibitory action of phenylalanine and phenylpyruvate on glycolysis, protein, lipid, DNA and RNA metabolism. Adv Enzyme Regul 8: 13–36
Hasselbalch S, Knudsen GM, Toft PB, Hogh P, Tedeschi E, Holm S, Videbaek C, Hendriksen O, Lou HC, Paulson OB 1996 Cerebral glucose metabolism is decreased in white matter changes in patients with phenylketonuria. Pediatr Res 40: 21–24
Acknowledgements
The authors thank Annette Trutzel and Inge Tomic for excellent technical assistance. We also thank Prof. Dr. Ulrich Brand (Department of Biochemistry I, Molecular Bioenergetics, University of Frankfurt, Germany) for the kind gift of DQA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hörster, F., Schwab, M., Sauer, S. et al. Phenylalanine Reduces Synaptic Density in Mixed Cortical Cultures from Mice. Pediatr Res 59, 544–548 (2006). https://doi.org/10.1203/01.pdr.0000203091.45988.8d
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000203091.45988.8d
This article is cited by
-
Whole-exome sequencing reveals genetic variants that may play a role in neurocytomas
Journal of Neuro-Oncology (2024)
-
Rat Hair Metabolomics Analysis Reveals Perturbations of Unsaturated Fatty Acid Biosynthesis, Phenylalanine, and Arachidonic Acid Metabolism Pathways Are Associated with Amyloid-β-Induced Cognitive Deficits
Molecular Neurobiology (2023)
-
Genetic etiology and clinical challenges of phenylketonuria
Human Genomics (2022)
-
A culture model for the assessment of phenylalanine neurotoxicity in phenylketonuria
In vitro models (2022)
-
Phenylketonuria
Nature Reviews Disease Primers (2021)