Abstract
Fetal growth impairment can occur in pregnancy complicated by diabetes. Although several studies have focused the effects of nutritional status on intrauterine development, the long-term impact of maternal diabetes on vascular and renal function in the offspring is poorly investigated. In the present study, blood pressure profiles and renal function parameters were investigated in the offspring of diabetic rats (DO). Female rats were made diabetic throughout gestation with a single dose of streptozotocyn (STZ) 10 d before mating. After weaning, the offspring had free access to food and water. Arterial pressure was evaluated every 15 d. Functional and morphometric kidney studies were performed in newborn, 3, 6 and 12-mo-old male rats in DO and in controls, C. Although maternal diabetes did not affect nephron number in the young adult rat, glomerular hypertrophy developed from 3 mo on. Glomerular Filtration Rate and Renal Plasma Flow were observed to be significantly decreased in DO when compared with C, from 3 mo on. In DO, hypertension was observed from 8 wk on and persisted elevated throughout the experimental period (12 mo). Vascular reactivity, evaluated in mesenteric arterial bed showed a decreased endothelium-dependent vasodilatation in 12-mo-old DO animals, while preserved response to sodium nitroprusside was demonstrated. Our data show that exposure to intrauterine diabetes induced by STZ does not affect nephron number in the young offspring but can cause permanent changes in Nitric Oxide (NO)-related vascular response, which, in turn may accelerate the natural age-related nephron loss.
Similar content being viewed by others
Main
Maternal status can affect several physiologic functions of the newborn. In addition, correlation between fetal growth conditions and susceptibility to a number of adult chronic diseases, including coronary heart disease, stroke and hypertension have been identified (1–4). Recently, we have demonstrated that maternal undernutrition promoted development of adult hypertension, impairment of the renal and endothelium functions, decreased absolute number of the nephrons and hypertrophy of the remaining glomeruli in the adult offspring (5–7). Although several experimental studies have focused the effects of the maternal undernutrition on fetal “programming” of adulthood disease, less attention has been paid to the possible in utero late effects of maternal diabetes. In fact, diabetes mellitus can impose several threats both to the mother and the offspring. Experimental and clinical studies have demonstrated that diabetic pregnancy increases the risk of intrauterine death, prematurity, perinatal mortality and congenital malformations (8–12). Indeed, Chugh et al. (13) have demonstrated that a sustained exposure of the fetus to elevated concentration of glucose may result in diabetic embryopathy, which is characterized by a multitude of congenital birth defects, including those of the nervous, cardiovascular, skeletal, and renal systems. These malformations result from defects occurring in early organogenesis including failure of neural tube closure, caudal regression syndrome, and urogenital abnormalities, which can be as severe as renal agenesis (5,14–16). Amri et al. (17) have demonstrated that exposure to hyperglycemia in utero impairs nephrogenesis in the rat, leading to a reduced number of nephrons. This nephron deficit could be a risk factor for the development of chronic renal disease and hypertension in adulthood. Other studies have shown that the offspring of diabetic mothers have higher incidence of glucose intolerance, obesity, insulin resistance, and hypertension in later life. Manderson et al. (18) showed that offspring of diabetic mothers might be at an increased risk for the development of vascular disease in later life. Holemans et al. (19) have suggested that maternal diabetes may also have lasting adverse consequences on cardiovascular function of the next generation, particularly because offspring of diabetic pregnant rats demonstrate overt insulin resistance in adulthood. On the other hand, aging is associated with loss of renal mass which, by itself, has little impact on overall renal function but may increase the vulnerability of the kidney to other injuries (20). The functional changes include a rise in renal vascular resistance, a decline in GFR, and a rise in filtration fraction (21,22). Age-related increases in renal fibrosis and in reactive oxygen species production were also demonstrated (23,24)
The present experiments were designed to investigate the possible effects of maternal diabetes on renal and vascular function at different ages of the offspring. In addition, renal morphometry and blood pressure profiles were also evaluated. Our data show that glomerular hypertrophy and hypertension developed in an early period of life in the offspring might be related to vascular disfunction in this experimental model. In the aged diabetic offspring, besides hypertension, intensification of nephron loss was also observed.
METHODS
All procedures used in this study were approved and performed in accordance with guidelines of the Ethics Committee of Biomedical Institute, Federal University of São Paulo and conformed to the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (National Institutes of Health Publication No.85-23, revised 1996). Wistar rats from our colony (Federal University of São Paulo) were maintained in a room at 22 ± 1°C with a 12 h light cycle and 60% humidity.
Animals.
Diabetes mellitus was induced by streptozotocyn (50 mg/kg), given by a single intraperitoneal injection to female Wistar rats (250–300 g). Control animals were given an equivalent amount of citrate buffer. Diabetic state was confirmed 48 h after, by measuring blood glucose. Blood glucose concentration was evaluated every three days. Only those animals with glycemic levels above 250 mg/dl were considered for mating. The diabetic rats were caged overnight with a male. Vaginal smears were taken in the following morning and a positive smear was considered as day 0 of gestation. All dams were housed and fed individually with the same diet. Maternal body weight gain was measured every 3 d. After birth, each litter, consisting of 6 male rats, was left with the mother for 28 d; when the male number was not enough to complete 6, females were used but discarded at weaning. The offspring were divided into two groups: a) group C—offspring of control mothers; b) group DO—offspring of diabetic mothers. After weaning, the rats were placed in individual cages, with free access to food and water until the time for the experiments. Animals from both C and DO groups were used in a randomized manner for the studies detailed below. Glycemia was measured in newborn rats (NB), 12 h after delivery. In 3, 6 and 12 mo-old rats, glycemia was measured in 12 h-fasted animals. Renal function was evaluated in 3, 6, and 12 mo-old rats. In addition, kidney morphometry was performed at the same mentioned ages and also in NB rats.
Renal function studies.
The animals were anaesthetized with sodium thiopental (30 mg/kg) and placed on a heated table to maintain body temperature at 37°C. Tracheotomy was followed by insertion of polyethylene catheters into the jugular vein for infusions, and into the carotid artery for blood sampling. Urine was collected from a catheter inserted into the bladder. After the surgical procedure, one hour stabilization period was allowed before the beginning of three collection periods. The animals were primed with 1 mL of saline containing inulin (300 mg/kg, and sodium para-aminohippurate (PAH, 2 mg/rat) and then submitted to continuous infusion of a saline solution containing inulin (15 g/L) and PAH (4 mg/L) at 0.08 mL/min. Plasma and urine inulin and PAH concentrations were measured by colorimetry for estimation of GFR (GFR) and renal plasma flow (RPF). Blood and urine Na+ were measured using ion selective electrode (Ciba-Corning 614 Na+/K+ analyzer). Net acid excretion (NAE) was calculated using the formula: NAE = TA + NH4+ − HCO3−, where TA is titratable acidy in urine measured by microtitration with 0.01 M sodium hydroxide, NH4+, excreted amount of ammonium evaluated by colorimetry and HCO3− is the net bicarbonate excretion calculated with a Blood Gas Analyzer (Ciba Corning, model 248). For protein excretion determination, rats were placed in metabolic cages and a 24-h urine collection was performed. Proteinuria was measured from 2 mo on, every 4 wk. Protein concentration was measured by precipitation with 3% sulfosalicylic acid.
All drugs and reagents were purchased from Sigma Chemical Co.-Aldrich, St. Louis Missouri, USA.
Morphometric study.
The morphologic evaluation was obtained by the following methodology: both kidneys from rats of each group were dissected out rapidly, cleaned of connective tissue, weighed and fixed in Bouin. Kidneys longitudinally cut were wax embedded and histologic sections (5 μm width) near renal hilus were performed. Sections cut were stained with hematoxilin and eosin to morphologic analysis. Glomerular area and diameters were measured using an image analysis program, Image-Pro PLUS, Media Cybernetica, MD, USA. Images were acquired in microscope (Leica DMLB, Wetzlar, Germany) connected to a microcomputer by a video camera (Sony-CCD-IRIS). Ten different images (fields) in 10 different slides were analyzed for each group. Each image had an area of 285 000 μm2. The number of glomeruli was also evaluated in these same fields. Total glomeruli number was calculated taking into account the glomeruli number per field, the number of studied fields, the glomerular diameter and the section thickness. Two different investigators who were unaware of the origin of the specimens performed this study.
Measurement of systolic blood pressure.
Systolic blood pressure was determined in conscious rats from both groups by an indirect tail-cuff method (Harvard Apparatus, UK). Before the effective measurements, rats were adequately trained; briefly, animals were placed in the restrainers for several times. They were covered with a dark tissue; after this step, they remained calm and the pressure records were made after a 15 to 20 min of quietude. The ambient was silent, with constant temperatures and the measures were always performed by the same person. Three consecutive measures were taken and if a great variability was found, all the values were discarded and in the following day, the procedures were repeated Rats were preheated at 40°C for 5 min, and then three stable consecutive measurements of blood pressure were averaged.
Vascular reactivity in vivo in mesenteric microvessels.
At 12 mo of age, males from both C and DO groups were used in a randomized manner for the vascular study. Rats were anesthetized with chloral hydrate (450 mg/kg, s.c), and the mesentery was exteriorized and arranged for microscopic observation in situ according to Zweifach (23) with slight modifications by Fortes et al. (24). Changes in the diameter of second order arterioles were measured following the application of vasoactive drugs: ACh (300 μg/mL); BK (3.0 μg/mL) and SNP (1.0 mg/mL) in a standard volume of 0.01 mL and were removed by washing out with the warmed Ringer-Locke solution. For each animal, at least three different microscopic fields were observed and the arteriole diameter measured. The rats were killed by excessive anesthetic dose.
Statistical analysis.
Statistical analysis was performed by unpaired t test, Mann-Whitney U-test or by analysis of variance, followed by the Scheffé test, where appropriate. Values that were not normally distributed were subjected to the Kruskal-Wallis test, followed by Dunn's posttest, when necessary. Statistical significance was defined as p < 0.05. The results are reported as means ± SEM.
RESULTS
Effect of maternal diabetes on body weight, glycemia and blood pressure levels in the adult offspring.
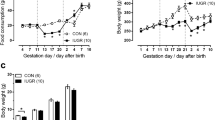
As shown in Table 1, in NB, no differences were observed between C and DO concerning body weight. However, from 6 mo on, DO tended to increase the body weight, which at 12 mo, was significantly higher, as shown in Fig. 1. As also shown in Table 1, from NB period until 12 mo, renal weight increased both in C and DO. However, the increment in renal weight assumed different profiles; in C, the maximum weight was attained at 3 mo while after this period a slight decline was observed. In DO, kidney weight continued to increase until 12 mo. Glycemia remained at normal levels in all the studied groups. In DO animals, from 3 mo on, significantly higher pressures were observed when compared with the respective control animals (Fig. 2). Hypertension lasted until the end of the observation period.
Effect of maternal diabetes on blood pressure levels in the offspring. Maternal diabetes promoted a significant increase in the offspring BP from 3 mo on. Comparisons were made among animals of the same age. Number of measurements: C (▪, n = 20) and DO (▴, n = 18). Significance level: p < 0.05 (* C vs. DO);
Effect of maternal diabetes on renal function.
Proteinuria remained unchanged in DO when compared with age-matched controls (data not shown). As shown in Table 2, with age, GFR and RPF tended to decrease in both C and DO. However, in DO, mean values for these parameters were significantly decreased in all the studied periods, when compared with age matched controls. As also shown in this table, absolute net acid excretion (NAE) decreased in DO while fractional excretion of Na+ increased in both 6 and 12-mo-old DO rats, suggesting impairment in Na+ conservation. As seen in Fig. 3, when NAE values were calculated as a function of GFR, in C, with age, values tended to increase while in DO they were accentuately decreased, suggesting an impairment in tubular acid excretion. Taken together these results suggest that maternal diabetes may accentuate the natural age-related decay, acting as a negative adjuvant in the physiopathology of the aging process.
Effect of maternal diabetes on renal morphology.
As expected, from newborn period until 3 mo after birth, increased glomerular diameters were observed both in C and DO groups. However, a significant increase in glomerular area was observed in 3 mo-old DO rats, when compared with respective age-matched control, which persisted until the end of the experimental observation; these data suggest that glomerular hypertrophy was an early event in this DO model.
As shown in Fig. 4, glomeruli number determined 3 mo after birth was the same for C and DO but in 12 mo-old rats, DO had about 20% fewer.
Numbers of glomeruli in the offspring from control (□) and diabetic mothers (▪). Maternal diabetes caused a significant decrease in glomeruli number in 12 mo-old DO rats. Number of measurements: 3 mo-old, C (n = 49) and DO (n = 50). 12 mo-old C (n = 50) and DO (n = 50). Significance level: p < 0.05 (* C vs. DO).
Effect of maternal diabetes on vascular function—Responses to Acetylcholine, Bradykinin and Sodium Nitroprusside.
There were no differences in the baseline diameter between the control and restricted offspring (18.65 ± 0.57 versus. 19.91 ± 0.65 μm). The magnitude of ACh (Fig. 5A) and BK (Fig. 5B) responses was significantly less in DO compared with control group. In contrast to the effects observed with these agents, SNP produced similar vasodilatation in the control and DO groups (Fig. 5C).
Percentage of change in vascular diameter of mesenteric arterial bed. Maternal diabetes caused an impaired response to endothelium-dependent agents Bar graphs shows the response of the mesenteric microvessels to: (A) acetylcholine (300 μg/mL); (B) bradykinin (3,0 μg/mL) and (C) sodium nitroprusside (1.0 mg/mL) in control (□, n = 06) and DO (▪ n = 09) groups. Values are expressed as mean ± SEM * p < 0.05 compared with control group.
DISCUSSION
Clinical and experimental studies point to the importance of intra-uterine environment in determining the metabolic, cardiovascular and endocrine disorders in the offspring. Maternal diabetes is recognized as an important cause of congenital anomalies, progeny hyper or hypoglycemic syndromes, with all the consequences of these metabolic disturbances (9,14,15).
In this study we have demonstrated that gestational diabetes mellitus promotes remarkable changes in both kidney function and vascular reactivity in the mature offspring. Our data concerning DO model point to an interesting model in which, although a normal nephron number is observed in the young rat, two important factors, which contribute to the progression of renal disease, systemic hypertension and glomerular hypertrophy are present. In our model, although decreased nephron number was not observed in young animals, a significant decrease in nephron number was observed in 12 mo-old DO. These findings suggest that maternal diabetes not only represent a new component to the growing list of causes that contribute to the fetal origins of adult-onset diseases but also can accelerate the aging process of nephron loss. In a previous study, Amri et al. (17) suggested that hyperglycemic environment is able to impair in vitro metanephrons development. The authors showed a decreased nephron number in the offspring of STZ-treated dams, induced to diabetes at the beginning of pregnancy. In our model, diabetes was induced before pregnancy, in a time were STZ is expected not to affect, directly, pancreatic development. On the other hand, in Amri studies, (17) the nephron count was performed by using glomeruli isolation. In our study, we have used a methodology in which glomeruli number is counted in 40 fields of both kidneys and glomerular diameter and area are calculated by using a computer program. Changes in renal function were also shown by Nelson et al. (25) in Pima Indians children, exposed to intrauterine diabetes. The authors showed high levels of urinary albumin excretion in that population, suggesting increased risk of renal disease. Although proteinuria was not observed in DO group, significant decrease in GFR, RPF and urinary flux suggest that the natural age-related decline in renal function, which was also present in C group, was accelerated in these rats; the increase in glomerular area shown in DO characterizes glomerular hypertrophy known to be the early event in the progression of glomerular damage (26–28).
Both experimental and clinical studies have indicated a decreased nephron number as a common denominator of hypertensive states (29–31). In some models of reduced nephron numbers, systemic hypertension and glomeruli hypertrophy are interpreted as compensatory mechanism to maintain renal function in adequate levels (32). In a model of renal ablation performed in newborn rats, Woods et al. (31) showed that hypertension developed from 8 wk on and proteinuria was significantly higher in the 20-wk-old group, suggesting that hypertension preceded glomerular damage, which was significant only in the older group. Our previous work in the aged offspring of food restricted mothers showed that glomerular involvement occurred independently of systemic hypertension, which was prevented by L-arg administration (33), similar to the findings from Floege et al., in Milan normotensive rats, that showed that an early podocyte damage was observed, preceding glomerulosclerosis, in the absence of systemic hypertension, hyperglycemia and glomerular hypertension (34). On the other hand, hypertension by itself plays an important role in the progression of renal disease (29,30). A persistent elevation in blood pressure can be transmitted to glomeruli leading to progressive injury, which in turn, can aggravate systemic hypertension. In the aging spontaneously hypertensive rats (SHR), Tolbert et al. (35), showed that at 9 mo of age, glomerular hypertension developed because of a small increase in systemic blood pressure and a decline in preglomerular vascular resistance, allowing transmission of elevated systemic pressure to the glomerular capillaries. In the present study, hypertension was an early event in DO suggesting that it could be the initial trigger factor of renal function impairment, which is reflected by glomerular hypertrophy and changes in renal function parameters. However, other hypertrophyc stimuli may be present in this model. For example, TGFβ is known to be increased in glomeruli of human and experimental diabetes (36). The possibility of transference of mother's phenotypic characteristics was demonstrated by Malathi et al. (37) that showed that offspring originated from hyperglycemic mothers had also several metabolic disturbances similar to the hyperglycemic dams. In their study, they concluded that a dietary modification in the early postnatal life of the mothers sets up a vicious cycle of spontaneous transfer of the phenotype to its progeny.
To investigate alterations of vascular reactivity in the adult offspring from diabetic mothers, responses to acetylcholine, bradykinin and sodium nitroprusside were investigated. Whereas the responses induced by endothelium-dependent vasodilators were decreased in mesenteric microvessels, no differences could be detected to sodium nitroprusside. Therefore, we may suggest that it is not the smooth muscle vasodilating capability that is reduced in mesenteric bed from offspring of diabetic rats, but some function related to the endothelium. Corroborating our in vivo data, in vitro studies have demonstrated that severe maternal diabetes can led to vascular dysfunction in adult offspring, characterized by enhanced sensitivity to noradrenaline and abnormal endothelium-dependent relaxation to acetylcholine and bradykinin in small mesenteric arteries (38). The origin of the vascular dysfunction induced in these offspring cannot be directly inferred from the present study. However, it seems that these animals had acquired an endothelial defect similar to that observed in arteries of diabetic adult rats (39) and in patients with diabetes, (40–43) suggesting that these disturbances, which were already described in diabetes, could be transferred to the fetus, changing blood pressure regulation and interfering in the developing organs.
The exact mechanism by which maternal diabetes impairs the endothelial function is not fully elucidated. Considering that nitric oxide (NO) is the main agonist responsible for the endothelium-dependent relaxation induced by acetylcholine or bradykinin, maternal diabetes may cause a reduction in NO synthesis and/or bioavailability, decreasing the endothelium-dependent vasodilation in the adult offspring. However, studies are necessary to evaluate the mechanisms involved in this alteration of the vascular function induced by maternal diabetes.
Although our data point to systemic hypertension as the primary cause of both glomerular hypertrophy and renal function impairment in DO model, the role of the kidney in perpetuating vascular alterations is a possibility that cannot be ruled out and need to be addressed in further investigations. To date, no study has specifically examined the late consequences of maternal diabetes on offspring blood pressure regulation and kidney function. Most observations have been found directly in the presence of diabetic status or have focused the disturbances in the hormonal control of glycemia in the offspring of diabetic mothers. Our data suggest that maternal diabetes can represent an important cause of hypertension and renal dysfunction latter in life, reinforcing the concept of fetal programming of adult diseases.
Abbreviations
- DO:
-
offspring from diabetic mother
- NAE:
-
net acid excretion
- NB:
-
newborn
- PAH:
-
para-aminohippurate
- RPF:
-
renal plasma flow
References
Barker DJ, Osmond C, Golding J, Kuhn D, Wadsworth ME 1989 Growth in utero, blood pressure in childhood and adult life and mortality from cardiovascular disease. BMJ 298: 564–567
Barker DJ 1995 Fetal origins of coronary heart disease. BMJ 311: 171–174
Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM 1996 Diet in pregnancy and the offspring's blood pressure 40 years later. Br J Obstet Gynaecol 103: 273–280
Barker DJ 1998 In utero programming of chronic disease. Clin Sci 95: 115–128
Lucas SR, CostaSilva VL, Miraglia SM, Zaladek Gil F 1997 Functional and morphometric evaluation of offspring kidney after intrauterine undernutrition. Pediatr Nephrol 11: 719–723
Alves GM, Barão MA, Odo LN, Nascimento Gomes G, Franco MCP, Nigro D, Lucas SR, Laurindo FR, Brandizzi LI, Zaladek Gil F 2002 L-Arginine effects on blood pressure and renal function of intrauterine restricted rats. Pediatr Nephrol 17: 856–862
Lucas SR, Miraglia SM, Zaladek Gil F, Coimbra TM 2001 Intrauterine Food Restriction as a Determinant of Nephrosclerosis. Am J Kidney Dis 37: 467–476
Pedersen LM, Tygstrup I, Pedersen J 1964 Congenital malformations in newborn infants of diabetic women. Correlation with maternal diabetic vascular complications. Lancet 13: 1124–26
Martinez-Friaz ML 1994 Epidemiological analysis of outcomes of pregnancy in diabetic mothers: identification of the most characteristic and most frequent congenital anomalies. Am J Med Genet 51: 108–113
Travers JP, Pratten MK, Beck F 1989 Effects of low insulin levels on rat embryonic growth and development. Diabetes 38: 773–778
Boloker J, Gertz SJ, Simmons RA 2002 Gestational diabetes leads to the development of diabetes in adulthood in the rat. Diabetes 51: 1499–1506
Roberts AB, Pattison NS 1990 Pregnancy in women with diabetes mellitus, twenty years experience 1968-1987. N Z Med J 103: 211–213
Chugh SS, Wallner EI, Kanwar YS 2003 Renal development in high-glucose ambience and diabetic embryopathy. Semin Nephrol 23: 583–592
Nold JL, Georgieff MK 2004 Infants of diabetic mothers. Pediatr Clin North Am 51: 619–637
Garcia Carrapato MR 2003 The offspring of gestational diabetes. J Perinat Med 31: 5–11
Lynch SA, Wright C 1997 Sirenomelia, limb reduction defects, cardiovascular malformation, renal agenesis in an infant born to a diabetic mother. Clin Dysmorphol 6: 75–80
Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelièvre-Pegorier M 1999 Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48: 2240–2245
Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR 2002 Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia 45: 991–996
Holemans K, Gerber RT, Meurrens K, De Clerck F, Poston L, Van Assche FA 1999 Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia 42: 81–89
Melk A, Halloran PF 2001 Cell senescence and its implications for nephrology. J Am Soc Nephrol 12: 385–393
Anderson S, Brenner BM 1986 Effects of aging on the renal glomerulus. Am J Med 80: 435–442
Dodane V, Chevalier J, Bariety J, Pratz J, Corman B 1991 Longitudinal study of solute excretion and glomerular ultrastructure in an experimental model of aging rats free of kidney disease. Lab Invest 64: 377–391
Cruz CI, Ruiz-Torres P, del Moral RG, Rodriguez-Puyol M, Rodriguez-Puyol D 2000 Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol 278: F123–F129
Fortes ZB, Becker C, Oliveira MA, Scivoletto R 1996 Influence of aldose reductase inhibition on the microvascular reactivity in experimental diabetes. Gen Pharmacol 27: 917–921
Nelson RG, Morgenstern H, Bennett PH 1998 Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes 47: 1489–1493
Yoshida Y, Fogo A, Ichikawa I 1989 Glomerular hemodynamic changes vs. hypertrophy in experimental glomerular sclerosis. Kidney Int 35: 654–660
Fogo A, Hawkins EP, Berry PL, Glick AD, Chiang ML, MacDonell RC Jr, Ichikawa I 1990 Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int 38: 115–123
Miller PL, Rennke HG, Meyer TW 1991 Glomerular hypertrophy accelerates hypertensive glomerular injury in rats. Am J Physiol 261: F459–F465
Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J 1996 Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18
Marcantoni C, Jafar TH, Oldrizzi L, Levey AS, Maschio G 2000 The role of systemic hypertension in the progression of nondiabetic renal disease. Kidney Int 75: S44–S48
Woods LL, Weeks DA, Rasch R 2001 Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension 38: 337–342
Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM 2001 Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol 12: 1315–1325
Zaladek Gil F, Lucas SR, Gomes GN, Cavanal Mde F, Coimbra TM 2005 Effects of intrauterine food restriction and long-term dietary supplementation with L-arginine on age-related changes in renal function and structure of rats. Pediatr Res 57: 724–731
Floege J, Hackmann B, Kliem V, Kriz W, Alpers CE, Johnson RJ, Kuhn KW, Koch KM, Brunkhorst R 1997 Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int 51: 230–243
Tolbert EM, Weisstuch J, Feiner HD, Dworkin LD 2000 Onset of glomerular hypertension with aging precedes injury in the spontaneously hypertensive rat. Am J Physiol Renal Physiol 278: F839–F846
Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA 1993 Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 90: 1814–1818
Srinivasan M, Aalinkeel R, Song F, Patel MS 2003 Programming of islet functions in the progeny of hyperinsulinemic/obese rats. Diabetes 52: 984–990
Holemans K, Gerber RT, Meurrens K, De Clerck F, Poston L, Van Assche FA 1999 Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia 42( 1): 81–89
Taylor PD, Graves JE, Poston L 1995 Selective impairment of acetylcholine-mediated endothelium-dependent relaxation in isolated resistance arteries of the streptozotocin-induced diabetic rat. Clin Sci 88: 519–524
McNally PG, Watt PA, Rimmer T, Burden AC, Hearnshaw JR, Thurston H 1994 Impaired contraction and endothelium-dependent relaxation in isolated resistance vessels from patients with insulin-dependent diabetes mellitus. Clin Sci 87: 31–36
McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR 1992 Impaired endothelium-dependent and independent vasodilation in patients with type-2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35: 771–776
Pieper GM, Peltier BA 1995 Amelioration by L-arginine of a dysfunctional arginine/ nitric oxide pathway in diabetic endothelium. J Cardiovasc Pharmacol 25: 397–403
Özçelikay AT, Tay A, Güner S, Tasyaran V, Yildizoglu-Ari N, Dincer UD, Altan VM 2000 Reversal effects of L-arginine treatment on blood pressure and vascular responsiveness of streptozotocin-diabetic rats. Pharmacol Res 41: 201–209
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Fundação de Amparo Å Pesquisa do Estado de São Paulo, FAPESP.
Rights and permissions
About this article
Cite this article
Rocha, S., Gomes, G., Forti, A. et al. Long-Term Effects of Maternal Diabetes on Vascular Reactivity and Renal Function in Rat Male Offspring. Pediatr Res 58, 1274–1279 (2005). https://doi.org/10.1203/01.pdr.0000188698.58021.ff
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000188698.58021.ff
This article is cited by
-
Perinatale Programmierung des Typ-2-Diabetes
Der Diabetologe (2016)
-
Acute kidney injury and progression of renal failure after fetal programming in the offspring of diabetic rats
Pediatric Research (2015)
-
Fetale und perinatale Programmierung der Nierenfunktion
Gynäkologische Endokrinologie (2014)
-
Sex-specific programming of hypertension in offspring of late-gestation diabetic rats
Pediatric Research (2012)
-
Fetal programming of renal function
Pediatric Nephrology (2012)