Abstract
Before birth, the peripheral chemoreceptors located in the carotid bodies (CB) are adapted to the low fetal Po2 and are relatively insensitive to hypoxia. After birth, the sensitivity of the CB to hypoxia is reset in response to the rise in Po2. The mechanism underlying this resetting, which requires several days to complete, remains unknown. We have investigated the possibility that the hypoxia-inducible factors HIF-1α and HIF-2α, which are activated by oxygen deprivation, are involved in this resetting process. Accordingly, we used immunostaining and densitometry to quantitate the levels of the HIF-1α and HIF-2α proteins in the rat CB during early perinatal life and after exposure to in vivo hypoxia during adolescence. Tyrosine hydroxylase (TH) was used as a marker for catecholaminergic neurons and oxygen-sensitive cells in the CB. Double-immunostaining revealed constitutive expression of HIF-1α in both glomus cells (TH+) and sustentacular cells (TH−) of the CB of adolescent rats. However, immunoreactivity toward HIF-2α was restricted to glomus cells. After exposure to hypoxia (8% O2, 6 h), the expression of HIF-1α was selectively up-regulated in glomus cells and apparent translocation of both HIF-1α and HIF-2α to the nucleus was observed. Both of these proteins were expressed constitutively in the CB during the perinatal transition period. During the first postnatal week, the intensity of immunostaining for HIF-1α in glomus cells decreased markedly, whereas the level of HIF-2α remained constant. We suggest that this selective down-regulation of HIF-1α may be involved in the postnatal maturation of CB responsiveness to hypoxia.
Similar content being viewed by others
Main
The CB constitute the first line of defense against hypoxia in mature mammals, triggering an increase in breathing and arousal whenever necessary (1). Before birth, the CB are relatively insensitive to hypoxia, being adapted to the chronically low Po2 in utero (2). The sudden rise in Po2 at the time of birth results in a progressive increase in CB sensitivity to hypoxia, a resetting process that takes approximately 2 wk to complete in rodents (3). If the normal postnatal rise in Po2 is delayed or accelerated, CB function can be significantly impaired (3–6).
Although the molecular mechanisms underlying this resetting of the CB are still unknown, catecholamines appear to play an important role in this process (7,8). The dopamine content and the level of mRNA encoding TH, the rate-limiting enzyme in catecholamine synthesis, are used as two principal indicators of catecholamine metabolism in the rat CB. Both of these parameters are relatively high in the fetus, but decrease rapidly after birth (7–10). Exposure of adult rats to hypoxia enhances the expression of TH mRNA in their CB and, therefore, it is proposed that fetal hypoxia results in a high level of TH mRNA in utero, which is reduced in response to the sudden rise in Po2 at birth (11,12).
The postnatal increase in Po2 and fall in the level of TH mRNA in the CB might involve hypoxia-inducible factors (HIF), members of the basic helix-loop-helix superfamily of eukaryotic transcription factors (13,14). The HIF-1 heterodimer consists of HIF-1α and HIF-1β (ARNT) (14), whereas HIF-2 contains HIF-2α and HIF-1β (15). The activities of these factors are regulated by the stability of their α subunits, which function as direct sensors of intracellular oxygen concentrations. Binding of HIF-1 or HIF-2 heterodimers to the hypoxia responsive element located in the promoter of the TH gene promoter (16,17) regulates transcription of this gene (18). Thus, there is strong evidence that HIF-1α plays a role in chemosensitivity in adult mice (19) and HIF-2α and TH are co-expressed in the CB of adult mice (20,21).
To determine whether HIF-1α or HIF-2α participate in the perinatal resetting of the CB, we first investigated here whether hypoxia in vivo induces expression of these proteins in the CB of adolescent rats [postnatal d 40 (P40)]. Thereafter, we analyzed developmental changes in the expression of these two factors in the CB of rats of different ages, ranging from embryonic d 20 (E20) to P14.
METHODS
Animals and treatment.
Male Sprague-Dawley rats (B & K Universal, Stockholm, Sweden) were studied on E20, at the time of birth (P0), and on P2, P7, P14, and P40, with birth occurring in all cases during the night following the E20. At least five to six pups of each age, randomly selected from different litters, were analyzed. All animals were housed at sea level in an air-conditioned room at 24°C, with a 12-h light-dark cycle and access to food and water ad libitum.
We also compared the CB parameters of five adolescent (P40) rats subjected to normobaric hypoxia (8% O2/92% N2) for 6 h or to normoxic conditions (five animals in each group). The CO2 level in the chamber was maintained at the same level as that of room air. The regional ethical committee for animal experimentation, which follows the regulations of the European Community, approved this study.
Immunostaining.
Pregnant rats were killed on E20 by cervical dislocation and casarean section was performed within 2 min thereafter. To minimize the time elapsed during removal of the pups and, thereby, the risk for alterations in the expression of HIF-1α or HIF-2α [both of which exhibit rapid turnover and short half-time decay (14)] due to an increase in O2 tension, only three pups from each mother were used. The carotid bifurcations of all rat pups were excised under deep anesthesia (50 mg pentobarbital/kg body weight) within 3 min following delivery, after which the animals were killed immediately by decapitation.
Adolescent rats (P40) exposed to hypoxia in vivo (6 h) were subsequently anesthetized directly with pentobarbital (50 mg/kg) and perfused transcardially with PBS (0.1 M, pH 7.4) containing 4% paraformaldehyde for 10 min (flow rate = 30 mL/min). To prevent reestablishment of normoxic conditions during this period, both anesthesia and fixation were performed under 8% O2. Subsequently, the carotid bifurcation was immediately fixed for 60 min in ice-cold 4% paraformaldehyde in PBS, followed by immersion in 30% sucrose overnight.
Cryosections with a thickness of 10–14 μm were prepared and allowed to adhere to SuperFrostPlus glass slides (Menzel-Glaser, Braunschweig, Germany). These sections were then permeabilized with 0.1% saponin and 0.1% TritonX, blocked with 7% normal goat (NGS) or donkey (NDS) serum, and incubated overnight at 4°C with the primary antibody in PBS containing 3.5% serum, 0.1% saponin, and 0.1% Triton X. Thereafter, the sections were washed, incubated with secondary antibody in the same PBS solution, washed again, and examined by confocal microscopy.
This procedure was used to examine a number of different parameters. TH immunoreactivity in the CB was used as a marker for glomus cells (22). The cellular hypoxic response and the localization of HIF-1α and HIF-2α at various stages of development were determined by double immunostaining (TH/HIF-1α or TH/HIF-2α). For this purpose, HIF-1α was probed with an affinity-purified mouse MAb (diluted 1:500) against human HIF-1α (Novus Biologicals, Inc., Littleton, CO) (23); HIF-2α with an affinity-purified goat polyclonal antibody (1:400) against human HIF-2α (sc-8712, Santa Cruz Biotechnology, Santa Cruz, CA) (24); and tyrosine hydroxylase with polyclonal sheep antibody (1:500) against rat tyrosine hydroxylase (Calbiochem-Novabiochem San Diego, CA) (25). Goat-anti-mouse Alexa 546 (1:200), rabbit-anti-goat Alexa 546 (1:200), and donkey-anti-sheep Alexa 488 (1:200) (all from Molecular Probes, Eugene, OR) were used as the secondary antibodies.
These same conditions were used to characterize the nuclear translocation of HIF-1α and HIF-2α following hypoxia in vivo in adolescent rats, except that in this case freshly prepared hydrogen peroxide (3%, 37°C, 10 min) was first used to block endogenous peroxidase activity. Exposure to the primary antibodies was followed by incubation with mouse anti-goat (Goat ExtrAvidin Peroxidase EXTRA-1 kit, Sigma Chemical Co., St. Louis, MO) or goat anti-mouse (Mouse ExtrAvidin Peroxidase EXTRA-2 kit; Sigma Chemical Co.) antibodies that had been biotinylated (1:15) for 1 h at room temperature. The tissue sections were incubated with ExtrAvidin-peroxidase (1:15) for 30 min at room temperature, after which sites of antigen-antibody interaction were visualized with diaminobenzidine tetrahydrochloride and 0.01% H2O2 in 2.5 mM Tris-Cl (pH 7.6) for 10 min (Fast DAB Kit; Sigma Chemical Co.). The slides were subsequently mounted in ProLong Antifade (Molecular Probes).
Controls in which the primary antibody was omitted were run for each immunocytochemical procedure to ensure that there was no unspecific immunoreactivity originating from the secondary antibodies. In the case of the double immunostaining, we carefully tested the level of cross-reactivity by performing two single immunostainings, switching the secondary antibodies, which clearly revealed that staining due to cross-reactivity was negligible (8% of the total staining). As an additional control, we omitted the secondary antibodies to determine the background level of autofluorescence.
Analysis.
The immunolabeled tissues were scanned with a Zeiss LSM410 or a Leica TCS SP inverted confocal scanning laser microscope using 63×/1.4 N.A. and 20×/0.75 N.A. objectives. Green fluorescence was produced by excitation at 488 nm and detected with a 515–540 nm band-pass filter. In the case of red fluorescence, excitation was at 543 nm and a 570 nm long-pass filter was used for detection.
Because of the small size of the CB, standard approaches to quantify the levels of different proteins in this organ are problematic. Therefore, we used immunoquantification of the HIF proteins. Densitometric analysis of the staining for HIF-1α and -2α was performed applying ImageJ software from the National Institutes of Health Internet site (http://rsb.info.nih.gov) to 15–30 TH+ cells in one image from each section. From each image, we also analyzed 15–30 TH− cells to determine the background level of staining, as well as staining for HIF-1α or HIF-2α in sustentacular cells (TH−). For each age and mode of treatment (normoxia/hypoxia), the mean level of staining for five or six sections from each of two or three CB was determined.
During perinatal maturation, the diameter and volume of the CB may increase, which would affect our calculations. However, our estimates of the diameter (122 ± 15.4 μm at E20 versus 146 ± 44.3 μm at P14), as well as of the volume (10.1 ± 1.25 × 104 mm3 at E20 versus 16.3 ± 2.56 × 104 mm3 at P14) of this organ indicated no significant alteration in these macroscopic parameters during the perinatal period investigated.
Statistical analysis.
To determine whether the responses varied with age or treatment (hypoxia/normoxia), the data were analyzed using one-way ANOVA. In cases where the difference was thus found to be significant, modified t tests with the Bonferroni correction were applied a priori for comparison of pairs of responses. A p value ≤0.05 was considered to be statistically significant.
RESULTS
HIF-1α and HIF-2α are induced in adolescent rats in response to hypoxia in vivo.
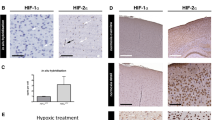
On P40, HIF-1α and HIF-2α were found to be expressed constitutively in the CB of rats maintained under normoxic conditions. When rats of the same age were exposed to 8% O2 for 6 h, immunostaining of the CB for HIF-1α was elevated 7-fold (Fig. 1). Intense nuclear and cytoplasmic immunostaining was observed, primarily in the glomus cells (as indicated by co-localization with the neural-specific marker TH) (Fig. 1). The level of HIF-1α staining in TH− cells was only half of that in TH+ cells. In addition, immunopositive, flat nuclei, most likely localized in endothelial cells, were observed in some of the blood vessels.
Expression of HIF-1α in the carotid body of adolescent rats after exposure to hypoxia (6 h, 8% O2) in vivo. (A) These photomicrographs (×10) illustrate the immunolocalization of the HIF-1α protein in a whole carotid body of adolescent rats (P40) after sustained hypoxia. We observed staining for HIF-1α in cell clusters and flat nuclei associated with blood vessels, probably located in endothelial cells (circle). (B) The arrows indicate strong nuclear and cytoplasmic immunostaining for HIF-1α under hypoxic conditions (×100). (C) Immunostaining for HIF-1α in normoxic and hypoxic carotid bodies. The bar indicates a distance of 100 μm. (D) Densitometric quantitation of the immunostaining for HIF-1α in hypoxic (n = 5) and normoxic (n = 5) CB, revealing a higher level in the hypoxic organ. *Statistically significant difference. (E) Double immunolabeling for HIL-1α and TH in the CB of adolescent rats (P40) exposed to hypoxia (6 h at 8% O2) in vivo. The localization of TH is depicted in green and that of HIF-1α in red. In the overlaid image, HIF-1α-positive cells appear red, TH-positive cells green, and cells expressing both of these markers yellow. Hypoxia induces the expression of HIF-1α primarily in TH+ cells. Internal scale bars = 100 μm.
In addition, expression of HIF-2α was strongly up-regulated in these hypoxic CB, as indicated by strong and specific nuclear and cytoplasmic immunostaining (Fig. 2). This immunoreactivity was observed in numerous cells arranged in clusters. No immunoreactivity was detected in CB sections processed in the same manner except for omission of the primary antibodies against HIF-1α, HIF-2α, or TH (not shown).
Photomicrographs depicting the expression and localization of the HIF-2α protein in the carotid bodies of adolescent rats (P40) exposed to normoxia (n = 3) or sustained hypoxia (6 h at 8% O2; n = 5) in vivo. (A) Immunostaining for HIF-2α in the carotid bodies of rats maintained under normoxic conditions (×10). (B) After sustained hypoxia, the carotid body exhibits strong immunostaining for HIF-2α (×10). (C) The arrows indicate nuclear and cytoplasmic immunostaining for HIF-2α in response to hypoxia (×100).
Expression of HIF-1α and HIF-2α in the rat CB at different stages of development.
TH immunoquantification was performed during the CB development (E20, 62.01 ± 10.6; P0, 71.97 ± 6.3; P2, 53.9 ± 4.21; P7, 55.68 ± 11.80; P14, 48.62 ± 8.30; P40, 45.8 ± 8.8). There was a general tendency (p = 0.07) for a general decrease in TH immunoquantification with age (regression: y = –0.4161x + 60.628). Immunostaining for HIF-1α was also present at all stages investigated, from E20 to P40. On E20, P0, and P2, this protein was shown using double-immunostaining (HIF-1α/TH) to be present in both glomus (TH+) and sustentacular cells (TH−). However, subsequent to the perinatal period (i.e. at P7 and P14), HIF-1α was still expressed in sustentacular cells, but deeply decreased from glomus cells (Fig. 3). Quantitation of these morphologic changes by densitometry confirmed that the levels of HIF-1α in TH+ and TH− cells were similar (with a ratio of approximately 1) at early developmental stages (E20, P0, and P2), but that this ratio was significantly lower (around 0.35) on P7 and P14 (Fig. 3).
Immunostaining for HIF-1α and TH during maturation of the rat carotid body, i.e. at E20, birth (P0), and P2, P7, and P14 (A) and after hypoxia in vivo (P40) (B). In the overlaid images, cells that are only positive for HIF-1α appear red, TH-positive (TH+) cells appear green, and cells expressing both of these markers appear yellow. In early stages of development (i.e. E20, P0, and P7; n = 5 or 6), HIF-1α is expressed by both TH+ and TH− cells. At later stages (P7 and P14; n = 5 or 6), staining for HIF-1α and TH is present in different cells. (B) This graph depicts the ratio of HIF-1 staining (as quantified by densitometry) in TH+ vs TH− cells. At early stages (E20, P0, and P2; n = 5 or 6), HIF-1α staining in TH+ and TH− cells is of the same intensity; whereas at later stages [P7, P14, and P40 normoxic (NX) rats; n = 5 or 6], HIF-1α is expressed primarily in the TH− cells. After exposure of P40 rats (n = 5) to hypoxia (HX) in vivo, expression of HIF-1α was strongly enhanced in TH+ cells. *Statistically significant difference compared with the ratio at E20. †Statistically significant difference compared with P40 rats exposed to normoxic conditions.
Immunoreactivity toward HIF-2α was present and of similar intensity in CB of all ages and was restricted to cells arranged in clusters. Double immunostaining (HIF-2α/TH) revealed that this protein was expressed in glomus cells (TH+) only and not in sustentacular cells (TH−) (Fig. 4). Indeed, all cells expressing HIF-2α also expressed TH.
DISCUSSION
Our major finding in the present investigation is that immunoreactivity toward HIF-1α is relatively high in the fetal CB and decreases substantially several days after birth. Moreover, we show that hypoxia in vivo induces the expression of both HIF-1α and HIF-2α in the CB of adolescent rats.
The high level of HIF-1α in the fetal CB is remarkable, because HIF are not usually expressed under normal conditions in healthy organs (26–28). Although the fetus is not regarded as being hypoxic, it is well known that the Po2 in utero is relatively low, which may explain our finding that HIF are constitutively expressed during this period.
Here, we demonstrate that severe hypoxia (8% O2) induces expression of both HIF-1α and HIF-2α in the CB of adolescent rats, relative to their basal levels of expression. Exposure to this degree of hypoxia represents a strong but tolerable challenge, which can be observed under certain physiologic or pathophysiological situations. Furthermore, we found a significant reduction in the intensity of immunostaining for HIF-1α, but not in HIF-2α during the first week of postnatal life.
This decrease in HIF-1α immunoreactivity was restricted to glomus cells, without any change with respect to sustentacular cells. The glomus cells are situated in the vicinity of capillaries and are thereby particularly sensitive to changers in blood parameters such as Po2, pH, CO2, and endocrine factors. Moreover, increased mitochondrial production of reactive oxygen species is required for stabilization of HIF-1α and such production may vary in different cell types (29). Indeed, large mitochondria with numerous cristae and a matrix of relatively low density are typically seen in glomus cells, whereas the mitochondria in sustentacular cells appear to be denser and smaller and to contain fewer cristae (30,31). This difference might also contribute to the difference in the levels of HIF-1α immunostaining in these two cell types.
Immunoreactivity toward HIF-1α was seen to decrease relatively slowly after birth, in contradiction to the suggestion that this decrease is a response to the immediate increase in Po2 experienced after birth. However, other factors related to the stress of being born, e.g. changes in the circulating levels of hormones such as angiotensin and insulin (32–35), might play a role in this context. For example, the levels of angiotensin and IGF1 in the blood of rat and human babies are very high during the first two postnatal days, after which they fall markedly (36,37). We propose that these high levels may compensate for the sudden loss of hypoxic stimulus and sustain a high level of HIF-1α expression during the first 2 d of postnatal life. In contrast, endocrine factors that influence the expression of HIF-2α have yet to be identified.
In adolescent rats, we found that severe and prolonged hypoxia stimulates the expression of both HIF-1α and HIF-2α in the CB, resulting in pronounced nuclear staining. The reason why such a decrease in oxygen tension up-regulates both of these factors in adolescent rats, whereas only the level of HIF-2α expression is maintained during perinatal transition is not presently clear. The stage of maturation is the major difference between these experimental protocols and represents an obvious possible answer. We can also speculate that expression of HIF-1α may be more responsive to hormones (see also above) and moderate changes in Po2, whereas HIF-2α may be up-regulated in the CB only by severe hypoxia. Therefore, we hypothesize that adaptation of the CB to prolonged hypoxia may be mediated in part either by HIF-1α and/or HIF-2α. Indeed, it has been established that HIF-1α and HIF-2α activate the transcription of genes whose products mediate adaptive responses to hypoxia. To date, more than 30 such HIF-1α-responsive genes have been identified (38).
The perinatal decrease in the level of HIF-1α in the CB during the critical period of resetting, suggests a possible coupling between HIF and dopaminergic mechanisms. An HIF-1/O2-responsive element has been detected in the promoter of the TH gene. Indeed, hypoxia in vitro can regulate TH promoter activity through the binding of HIF-1α or HIF-2α (18). Furthermore, prolonged exposure of rats to hypoxia results in adaptation and up-regulation of the level of TH mRNA and dopamine content of the CB (11,39). Earlier studies have also demonstrated an inverse relationship between the decrease in dopamine metabolism (as reflected in the dopamine content and level of TH mRNA) and the increase in chemoreceptor activity during the first week of postnatal life (7–10).
In our investigation, the level of TH immunoreactivity tended to decrease during this critical period. However, we also observed a fall in the level of immunostaining for HIF-1α in glomus cells during the first postnatal week, i.e. during the same period as the decreases in dopamine content and TH mRNA reported by others (8,10). These results provide evidence that HIF-1α and, possibly, HIF-2α as well participate in activation of the catecholaminergic pathway.
Our present findings are also consistent with reports demonstrating that chronic hypoxia results in up-regulation of the HIF-1α protein in catecholaminergic neurons of the brainstem (40). Moreover, carotid body function in adult HIF-1α −/+ heterozygous mice is abnormal (19). However, several other putative targets for HIF-1α and HIF-2α could also be involved in the adaptation of the CB to hypoxia. For instance, in the CB of adult rats exposed to 4 wk of hypoxia, HIF-1α up-regulates the expression of VEGF and VEGFR-1 (41). Furthermore, HIF-1α might also up-regulate the expression of the inducible nitric oxide synthase and thus increase the local level of NO, which has been described as a key factor in the chemosensitive process (42,43). Finally, HIF-1α plays a key role(s) in the regulation of glucose and overall cell metabolism (44) and metabolic adaptation is a necessary part of the response of any tissue to chronic hypoxia. Thus, HIF-1α could play an important role in the metabolic adaptation of both glomus and sustentacular cells in the CB to chronic hypoxia.
In conclusion, the level of the HIF-1α protein in the CB is selectively regulated during the perinatal transition period. In contrast, sustained hypoxia induces both HIF-1α and HIF-2α in the CB of the adolescent rat. These processes might be associated with trophic adjustments and remodeling of the CB, which occur during acclimatization to hypoxia.
Abbreviations
- CB:
-
carotid bodies
- HIF-1α or HIF-2α:
-
hypoxia-inducible factor 1α or 2α
- TH:
-
tyrosine hydroxylase
References
Gonzalez C, Almaraz L, Obeso A, Rigual R 1994 Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898
Blanco CE, Dawes GS, Hanson MA, McCooke HB 1984 The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol 351: 25–37
Eden GJ, Hanson MA 1987 Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol 392: 1–9
Blanco CE, Hanson MA, McCooke HB 1988 Studies of chemoreceptor resetting after hyperoxic ventilation of the fetus in utero. In: Riberio JA, Pallot DJ (eds) Chemoreceptors in Respiratory Control. Croom Helm, London, pp 221–227
Erickson JT, Mayer C, Jawa A, Ling L, Olson EB Jr, Vidruk EH, Mitchell GS, Katz DM 1998 Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol 509: 519–526
Donnelly DF 2000 Developmental aspects of oxygen sensing by the carotid body. J Appl Physiol 88: 2296–2301
Hertzberg T, Hellström S, Lagercrantz H, Pequignot JM 1990 Development of the arterial chemoreflex and turnover of carotid body catecholamines in the newborn rat. J Physiol 425: 211–225
Holgert H, Hökfelt T, Hertzberg T, Lagercrantz H 1995 Functional and developmental studies of the peripheral arterial chemoreceptors in rat: effects of nicotine and possible relation to sudden infant death syndrome. Proc Natl Acad Sci USA 92: 7575–7579
Hertzberg T, Hellström S, Holgert H, Lagercrantz H, Pequignot JM 1992 Ventilatory response to hyperoxia in newborn rats born in hypoxia—possible relationship to carotid body dopamine. J Physiol 456: 645–654
Gauda EB, Bamford O, Gerfen CR 1996 Developmental expression of tyrosine hydroxylase, D2-dopamine receptor and substance P genes in the carotid body of the rat. Neuroscience 75: 969–977
Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE 1992 Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem 58: 1538–1546
Holgert H, Pequignot JM, Lagercrantz H, Hökfelt T 1995 Birth-related up-regulation of mRNA encoding tyrosine hydroxylase, dopamine beta-hydroxylase, neuropeptide tyrosine, and prepro-enkephalin in rat adrenal medulla is dependent on postnatal oxygenation. Pediatr Res 37: 701–706
Semenza GL, Wang GL 1992 A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454
Wang GL, Jiang BH, Rue EA, Semenza GL 1995 Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92: 5510–5514
Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W 1997 HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev 63: 51–60
Czyzyk-Krzeska MF 1997 Molecular aspects of oxygen sensing in physiological adaptation to hypoxia. Respir Physiol 110: 99–111
Mishra RR, Adhikary G, Simonson MS, Cherniack NS, Prabhakar NR 1998 Role of c-fos in hypoxia-induced AP-1 cis-element activity and tyrosine hydroxylase gene expression. Brain Res Mol Brain Res 59: 74–83
Schnell PO, Ignacak ML, Bauer AL, Striet JB, Paulding WR, Czyzyk-Krzeska MF 2003 Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem 85: 483–491
Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR 2002 Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A 99: 821–826
Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL 1998 The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 12: 3320–3324
Favier J, Kempf H, Corvol P, Gasc JM 1999 Cloning and expression pattern of EPAS1 in the chicken embryo. Colocalization with tyrosine hydroxylase. FEBS Lett 462: 19–24
Macdonald DM 1981 Peripheral chemoreceptors. Structure-function relationships of the carotid bodies. In: Hornbein TF (eds) Regulation of Breathing. Marcel Dekker, New York, pp 105–319
Chavez JC, Agani F, Pichiule P, LaManna JC 2000 Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol 89: 1937–1942
Tian H, McKnight SL, Russell DW 1997 Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82
Lewis DA, Melchitzky DS, Haycock JW 1993 Four isoforms of tyrosine hydroxylase are expressed in human brain. Neuroscience 54: 477–492
Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D 2001 HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 15: 2445–2453
Kietzmann T, Cornesse Y, Brechtel K, Modaressi S, Jungermann K 2001 Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1α, HIF2α and HIF3α, in rat liver. Biochem J 354: 531–537
Freeburg PB, Robert B, St John PL, Abrahamson DR 2003 Podocyte expression of hypoxia-inducible factor (HIF)-1 and HIF-2 during glomerular development. J Am Soc Nephrol 14: 927–938
Schroedl C, McClintock DS, Budinger GR, Chandel NS 2002 Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 283: L922–L931
Böck P, Stockinger L, Vyslonzil E 1970 The fine structure of the human carotid body. Z Zellforsch Mikrosk Anat 105: 543–568
Kummer W, Yamamoto Y 2002 Cellular distribution of oxygen sensor candidates—oxidases, cytochromes, K+ channels—in the carotid body. Microsc Res Tech 59: 234–242
Chavez JC, LaManna JC 2002 Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci 22: 8922–8931
Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B 1998 Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17: 5085–5094
Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL 1999 Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res 59: 3915–3918
Richard DE, Berra E, Pouyssegur J 2000 Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 275: 26765–26771
Jelinek J, Hackenthal R, Hilgenfeldt U, Schaechtelin G, Hackenthal E 1986 The renin-angiotensin system in the perinatal period in rats. J Dev Physiol 8: 33–41
Wollmann HA 2000 Growth hormone and growth factors during perinatal life. Horm Res 53( suppl 1): 50–54
Semenza G 2002 Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64: 993–998
Millhorn DE, Czyzyk-Krzeska M, Bayliss DA, Lawson EE 1993 Regulation of gene expression by hypoxia. Sleep 16: S44–S48
Pascual O, Denavit-Saubie M, Dumas S, Kietzmann T, Ghilini G, Mallet J, Pequignot JM 2001 Selective cardiorespiratory and catecholaminergic areas express the hypoxia-inducible factor-1alpha (HIF-1alpha) under in vivo hypoxia in rat brainstem. Eur J Neurosci 14: 1981–1991
Tipoe GL, Fung ML 2003 Expression of HIF-1alpha, VEGF and VEGF receptors in the carotid body of chronically hypoxic rat. Respir Physiol Neurobiol 138: 143–154
Prabhakar NR 1999 NO and CO as second messengers in oxygen sensing in the carotid body. Respir Physiol 115: 161–168
Ye JS, Tipoe GL, Fung PC, Fung ML 2002 Augmentation of hypoxia-induced nitric oxide generation in the rat carotid body adapted to chronic hypoxia: an involvement of constitutive and inducible nitric oxide synthases. Pflugers Arch 444: 178–185
Semenza GL 2001 HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13: 167–171
Acknowledgements
The authors thank G. Cohen and A. Olsson for critical reading of this manuscript and valuable suggestions for revision and Sara Jonmarker for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Support for this study was provided by grants from the Märta and Gunnar V. Philipson's (H.B.) and Jeanssonska Foundations, Sällskapet Barnavård and Gösta Fraenckels.
Rights and permissions
About this article
Cite this article
Roux, JC., Brismar, H., Aperia, A. et al. Developmental Changes in HIF Transcription Factor in Carotid Body: Relevance for O2 Sensing by Chemoreceptors. Pediatr Res 58, 53–57 (2005). https://doi.org/10.1203/01.PDR.0000163390.78239.EA
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000163390.78239.EA
This article is cited by
-
A narrative review of the mechanisms and consequences of intermittent hypoxia and the role of advanced analytic techniques in pediatric autonomic disorders
Clinical Autonomic Research (2023)
-
Comparative morphological and molecular studies on the oxygen-chemoreceptive cells in the carotid body and fish gills
Cell and Tissue Research (2021)
-
Regulation of carotid body oxygen sensing by hypoxia-inducible factors
Pflügers Archiv - European Journal of Physiology (2016)
-
Hypoxia-inducible factors and hypertension: lessons from sleep apnea syndrome
Journal of Molecular Medicine (2015)