Abstract
Inhaled nitric oxide (iNO) improves oxygenation in premature infants, but concern has been raised about its potential oxidative toxicity. We designed this study to assess the oxidative balance in premature infants who were exposed to low dose iNO and the relationship with their clinical outcome on day 28 of life. A total of 274 infants who were <32 wk gestation were randomized at birth to receive 5 ppm of iNO if they presented with hypoxemic respiratory failure. Nonhypoxemic infants were studied as the reference group. Blood samples were withdrawn 24 h apart, within the first 4 d of life, to assess malondialdehyde (MDA) concentration as oxidative stress marker and total plasmatic glutathione (GSH), intraerythrocyte GSH peroxidase, and GSH reductase activities as antioxidant defenses. After 24 h, the rise in MDA was blunted in the iNO group compared with controls and was close to the reference infants. Conversely, GSH was more stable in the iNO group, when there was no difference for the GSH peroxidase and GSH reductase activities. On day 28, Oxygen dependence was linked with a higher increase in MDA as was the risk for death, whereas intraventricular hemorrhage was associated with a higher initial drop in GSH. Early low-dose iNO in hypoxemic preterm infants improves oxidative balance and seems to be clinically beneficial up to day 28 of life.
Similar content being viewed by others
Main
Since 1992, several randomized trials have shown that inhaled nitric oxide (iNO) significantly improved oxygenation in term or near-term infants, with a significant reduction in the use of extracorporeal membrane oxygenation (1–6). In 1997, Skimming et al. (7) were the first to report a positive response of 23 premature infants with respiratory distress syndrome to low-dose iNO (5 ppm). Since then, a few clinical trials involving a small number of premature infants have shown that the initial improvement in oxygenation seemed to be transient (8–10) because no improvement in mortality or in morbidity had been demonstrated (11). However, most of these studies were considering iNO therapy in premature infants who had severe hypoxemic respiratory failure or refractory hypoxemia (8,10,11) or were at an advanced stage of respiratory insufficiency (12). Most of them used a high dosage of iNO (10–20 ppm).
Finally, concerns have been raised about specific side effects of this new molecule that could worsen long-term outcome after an initial improvement in this high-risk population (13–15). Besides the risk for increased brain hemorrhage as a result of a prolonged bleeding time (16), neonatologists remain cautious in the use of this treatment because NO behaves as a “chameleon” molecule for the oxidative balance. Endogenous NO is incriminated in the cascade leading to cerebral lesions in perinatal experimental models of hypoxo-ischemic injuries (17). In addition, iNO may interact with oxygen to form nitrogen dioxide and peroxynitrites, which would enhance pulmonary toxicity. This is a main concern when dealing with premature infants, who already are unable to face oxidant stress (18,19). However, at low dosage and in experimental models, NO is described as antioxidant at the lung level, able to counteract oxidative stress and enhance surfactant function (20,21). Low doses of iNO also seem to improve oxygenation in premature infants with chronic lung disease (11,12) without alteration of their inflammatory markers. Thus, a policy of treatment aiming to get a beneficial and sustained protective effect of iNO in preterm infants should take into account their fragile oxidative balance. Therefore, we designed this study, as part of a large multicenter trial studying the safety and efficacy of early low-dose iNO (22), to assess the oxidative balance of very premature infants according to their respiratory status and the effect of iNO on this balance.
METHODS
Study population.
This prospective, randomized, controlled trial was conducted in an academic level III neonatal unit and approved by the “Comite Consultatif de Protection des Personnes soumises à la Recherche Biomedicale” and by our Institutional Review Board. Inclusion criteria were gestational age (GA) <32 wk at birth and <48 h of life, with written informed consent of the parents. Exclusion criteria were initial refractory hypoxemia, thrombocytopenia <50,000/mm3, or the presence of major fetal abnormality.
Study design.
Randomization was stratified by GA (<28 wk and 28–31 wk GA) and kept blind until 6 h of age with optimal care performed according to standardized written protocols. Subsequently, up to 48 h after birth, when the infants presented with hypoxemic respiratory failure (HRF) defined as the need for mechanical ventilation with a fraction of inspired oxygen (Fio2) >0.4 and had an arterio-alveolar O2 ratio [aAO2; aAO2 = arterial oxygen pressure (Pao2)/713 × Fio2 − arterial partial pressure of CO2] <0.22 but no refractory hypoxemia (defined as Po2 < 50 and Pco2< 50 mm Hg for Fio2 = 1.0), the allocation was disclosed as “control” or “iNO” group. For both HRF groups, one blood sample (1 mL) was withdrawn at the time of randomization (T1) through a central line, and a second sample (T2) was drawn 24 h later for biologic analysis. iNO was delivered from a certified 225-ppm cylinder (Air Liquide Sante International, Paris, France) with a compact unit including a pressure regulator and a low-flow meter (0.1–1.5 L/min). NO and nitrogen dioxide were continuously monitored at the T-piece with electrochemical cells (Polytron, DrägerAG, Lübeck, Germany). A dose of 5 ppm of iNO was first used for 1 h in the iNO group, and then subsequent dosage was determined according to aAO2 response. As soon as the response was positive (defined as an aAO2 increase >0.22), iNO was decreased to 2 ppm for 2 h and then weaned according to blood gas examination; when the response was intermediate (aAO2 remaining <0.22 but increasing by at least 25%), iNO was left at 5 ppm for 2 h and the response was re-evaluated every 2 h thereafter. Finally, when the infants showed no response, iNO was increased up to 10 ppm for 2 h and then re-evaluated. In case of treatment failure, iNO was weaned after 4 h. If during these 48 h the infants did not present with HRF (“reference group”), then randomization was kept blind until analysis, usual optimal care was performed, and two blood samples (T1 and T2) were drawn 24 h apart during the first 72 h of life.
Biologic analysis.
All blood samples were processed immediately and frozen at −20°C and −80°C until final biochemical analysis, blinded for the allocation group.
Malondialdehyde (MDA) plasmatic concentration, the marker of oxidative stress, was assessed by colorimetric method (Kit MDA Sobioda). This measurement was based on the reaction of MDA with two molecules of thiobarbituric acid, after heating at 95°C, to form a pink fluorescent complex at 553 nm (23,24). Results were expressed in nmol/g of serum protein.
Total plasmatic glutathione (GSH) was assessed by enzymatic assay by a method based on the spectrophotometric monitoring of GSH-mediated reduction of 5,5′-dithio-bis (2-nitrobenzoic acid) at 412 nm (25). Plasmatic GSH, chosen as antioxidant substrate, was expressed in nmol/L.
Intraerythrocyte glutathione peroxidase (GPX) activity was determined by enzymatic assay following the oxidation of reduced GSH, in the presence of glutathione reductase (GR), by measuring the decrease of NADPH concentration at 340 nm. Results were expressed in μmol of oxidized NADPH per min/g Hb (26).
Intraerythrocyte GR activity acts as a GSH regenerator by catalyzing the reduction of GSH disulfite in the presence of NADPH (27), which is oxidized to NADP+. The decrease in absorbance at 340 nm was measured (Kit RANDOX), and results were expressed in μmol · min−1 · g Hb−1.
Data collection and clinical outcome measures.
History of toxemia, gestational diabetes, premature rupture of membranes, chorioamnionitis, maternal-fetal infection, and the use of antenatal steroids was recorded carefully following a standardized review of the maternal medical records. We prospectively recorded placental abruption; mode of delivery; newborn characteristics; episodes of illness; all events that occurred at birth and within the first 28 d of life, such as the incidence and severity of intraventricular hemorrhage (IVH) and periventricular leukomalacia thanks to weekly head ultrasound examination; and the prevalence of oxygen dependence on day 28. The primary measure of outcome of the clinical trial was defined as survival on day 28 without any respiratory support, oxygen supplementation, or IVH greater than grade I (28).
Statistical analysis.
Categorical variables were compared among the three groups by either χ2 or Fischer exact test. Continuous variables were compared by two-way ANOVA for one or two parameters and completed by Tukey pair-wise comparisons test when global test was positive. We considered p < 0.05 as significant.
The relationship between MDA or GSH and the clinical outcome on day 28 was studied by ANOVA when normality of the data was achieved. Otherwise, we used Kruskal-Wallis test to measure differences of a single variable across two or more independent groups of cases. When linearity of the data was achieved, MDA and GSH were categorized using 5 nmol/g protein and 0.1-nmol/L intervals, respectively, and odds ratio (OR) were calculated. Data are presented as mean ± SEM or median (25th–75th quartiles). Statistical analysis was performed with SAS 8.2 software (SAS Institute, Cary, NC).
RESULTS
Population.
From July 1999 to February 2001, 274 premature infants received care in our unit. After exclusion for parental refusal or exclusion criteria (n = 22), lack of biologic sampling (n = 6), or protocol violation (n = 6), 240 infants were studied and distributed within three subgroups: reference (n = 164), HRF-control (n = 39) and HRF-iNO (n = 37; Fig. 1). Demographic and baseline characteristics before randomization are presented in Table 1. Mean GA was significantly higher in the reference group in comparison with both HRF groups. There was no statistical difference among the three groups for birth weight, sex distribution, incidence of maternal toxemia, chorioamnionitis, severe intrauterine growth retardation, or the incidence of cesarean section. We noticed a higher rate of antenatal steroid use for the reference group versus the control and the iNO groups, whereas there was no difference within these two HRF groups (Table 1). The incidence of hyaline membrane disease was almost twice as much in both HRF groups than in the reference group. The incidence of poor postnatal adaptation assessed by the neonatal CRIB score (29) was higher in both HRF groups than in the reference group (Table 1). At the time of randomization, Fio2 requirement was significantly higher to start with in the iNO group than in the control group (Table 2).
Protocol.
In our study, median duration of iNO administered according to the protocol was 35.1 h (21.7–53.9). There was no significant difference for the postnatal age at T1 between the two hypoxemic groups (14.1 ± 1.4 versus 15.9 ± 1.8 h in controls, respectively). It was significantly higher, however, in the reference group (29.4 ± 0.8 h; p < 0.01). We verified within this group that there was no effect of time for the studied variables except a minimal effect on MDA (between 1 and 2% over 96 h). Twenty-four hours after entering the randomization (T2 for biologic sampling), there was a significant drop in the Fio2 requirement in the iNO group (−20.4 ± 4.4 versus −7.4 ± 3.7% for the controls; p < 0.01).
Biologic results. MDA.
At the time of randomization (T1), MDA levels were not significantly different among the three groups (Table 2). At T2, MDA increased moderately in the reference population (1.63 ± 0.79; median, 1.4 [−3.7 to 6.5]) as in the iNO HRF group [2.20 ± 2.57; 4.6 (−2.6 to 9.7)], whereas during this 24-h period, HRF controls increased significantly their MDA level [12.11 ± 2.67; 8.4 (4.4–20.6); p < 0.05; Fig. 2A].
Total plasmatic GSH.
At the time of randomization (T1), GSH levels were not significantly different among the three groups (Table 2). At T2, GSH level decreased moderately in the reference population [−0.025 ± 0.014; −0.020 (−0.080 to 0.060)] in comparison with the iNO group [−0.080 ± 0.027; −0.055 (−0.120 to −0.010)] and the HRF control [−0.105 ± 0.029; −0.070 (−0.210 to −0.000)], where the drop reached statistical significance versus the reference group (p < 0.05; Fig. 2B).
Intraerythrocyte GPX activity. At the time of randomization (T1), the GPX activity was not significantly different among the three groups. At T2, there was a significant increase (p < 0.05) in GPX activity for the iNO group [0.90 ± 0.25; 0.65 (−0.10 to 1.40)] in comparison with the reference group [0.06 ± 0.12; 0.10 (−0.40 to 0.70)], when the rise in GPX was not significantly different for the HRF controls [0.36 ± 0.21; 0.00 (−0.30 to 1.00)].
Intraerythrocyte GR activity. At the time of randomization (T1), the GR activity was not significantly different among the three groups. At T2, the moderate rise in GR activity (Table 2) remained statistically not different among the three groups.
Clinical outcome on day 28.
On day 28 of life, survival rate was 92% in the reference group versus 69% in the control group and 59% in the iNO group. Fifty-five percent of the infants in the reference group versus 23% in the control group and 19% in the iNO group reached the primary measure of outcome (p < 0.0001 for the reference group versus the control and iNO groups, respectively), a composite value to describe “intact survival.” The incidence of O2 dependence on day 28 was different among the three groups, with a significantly higher incidence for the HRF controls, when this incidence was not different between the reference population and the HRF infants who were treated with iNO (Table 3). The incidence of patent ductus arteriosus was significantly higher in both HRF groups in comparison with the reference group (Table 3). There was no statistical difference among the three groups for the incidence of IVH of any grade or the incidence of significant brain lesion (greater than IVH grade 1 and/or periventricular leukomalacia and/or hydrocephaly; Table 3).
Correlations between Fio2 changes and early oxidative stress.
For MDA, the oxidative stress marker, we found a positive correlation with Fio2 requirements (r = 0.158, p = 0.02). Moreover, MDA changes over 24 h (T2 − T1 values) were also correlated with Fio2 changes within the same period of time (r = 0.148, p = 0.03). For GSH, the antioxidant substrate, we found that the drop in GSH level over 24 h was correlated with the initial Fio2 requirements (r = 0.182, p = 0.01).
Relationship between early oxidative stress and clinical outcome on day 28.
We found no significant relationship between the early oxidative stress marker evaluated at T1 and the postnatal clinical outcome. However, MDA evolution, over a 24-h period (T2 − T1) was significantly associated with the risk for death, respiratory, and/or neurologic outcome on day 28.
For MDA, we found by univariate analysis that the magnitude of the early increase (T2 − T1) was significantly associated with the risk for death within the first 28 d of life (p = 0.009), with the combined risk for death or having worrisome brain lesion (p = 0.004) or developing an oxygen dependence on D28 (p = 0.003; Table 4). For GSH, we found by univariate analysis that the magnitude of the early drop (T2 − T1) was significantly associated with the risk for developing a significant IVH (>grade I) during the first month of life (p = 0.03).
DISCUSSION
In our study, iNO was not associated with an increased oxidative stress in very preterm infants. On the contrary, we observed a significant improvement in the oxidative balance of very premature infants within the first days of life. Premature infants are highly susceptible to injury from reactive oxygen intermediates (ROI), and tissue injury occurs when ROI from endogenous and exogenous sources exceed the already limited antioxidant capacities (17,30). Therefore, it seemed necessary to study the impact of iNO on both sides of the oxidative balance. Plasmatic MDA, a byproduct of cell membrane lipid peroxidation as a result of ROI, has been accepted as a valid indicator of oxidative stress in the neonate (31,32). In our study, we were able to demonstrate a direct link between the levels of oxygen exposure and the magnitude of MDA rise in very premature infants. iNO led to a significant decrease in early Fio2 requirements, and the MDA levels of the HRF infants who were treated with iNO were not significantly different from the reference population after 24 h, when those of the HRF controls were significantly rising. However, the number of HRF infants was too small to show a direct impact of iNO on MDA levels, and we can only speculate that MDA level stabilization was obtained thanks to the important drop in Fio2 requirement (−20% over 24 h). A rapid improvement in oxygenation after iNO in premature infants is described in several studies (7,14), but the originality of our prospective controlled study was to obtain this response with very low doses (≤5 ppm) (33) over a short period of time, when others were using from 5 (11), to 10 (6), and often 20 ppm (7,8,10).
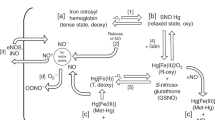
On the other side of the oxidative stress balance, we chose total plasmatic GSH as a biologic marker of the antioxidant defense pool, able to detoxify hydrogen peroxide and other peroxides in a reaction catalyzed by GPX. GR is the main enzyme able to restore intracellular GSH levels. Several clinical data have shown that plasmatic GSH concentrations are low in premature infants and inversely proportional to GA (34,35). A further early depletion as a result of the adjunction of a new oxidant molecule would be worrisome. With the clinical settings of our study, the sick infants who were treated with low-dose iNO had a trend toward a less severe depletion in GSH levels than the sick control subjects and were not significantly different from the reference group. Because NO does not have any direct impact on the GSH metabolism, we can speculate that iNO, by reducing early Fio2 requirements and ROI, indirectly decreases the need for scavenger use. Thus, by reducing the magnitude of both oxidant production and antioxidant depletion, iNO that is administered early in the postnatal course (14–16 h of life) and at a small dosage (≤5 ppm) in hypoxemic premature infants seems to be able to blunt the oxidative cascade triggered by high initial oxygen concentrations.
Because this study was an ancillary part of a larger, prospective, safety-efficacy controlled trial of early iNO in hypoxemic very premature infants (22), we were able to benefit from the collection of all postnatal data of these infants. This gave us clues to answer the yet unsolved question (30,35): does the biologic stabilization of the early oxidative stress have any sustained clinical benefits in this high-risk population? On day 28 of life, when most of these infants have reached a stable clinical condition and the inflammatory processes triggered by oxidative stress has been identified, we found a significant relationship between clinical outcome and early oxidative balance. The early increase of MDA plasmatic levels was significantly associated with the risk for death during the first month of life (OR = 1.23) as well as the risk for death and/or severe neurologic abnormalities (OR = 1.21). The significant relationship between early MDA increase and the risk for having an oxygen dependence on day 28 (OR = 1.24) is consistent with previous publications (30,31,36–39). Data indicating that early lipid peroxidation occurs in infants who subsequently develop bronchopulmonary dysplasia (BPD) came from Pitkanen et al. (37) in 1990. They also demonstrated that increased concentration of exhaled pentane and ethane occurred early and at higher levels in preterm than in term infants, indicating a higher oxidative stress in the former group (37,38). Inder et al. (31) found a positive correlation between lipid peroxidation measured by MDA-thiobarbituric acid in 22 very low birth weight infants and the number of days with high Fio2 levels. MDA-thiobarbituric acid values were ∼50% higher in the infants who developed BPD (31). Ogihara et al. (32) also found a positive correlation between an elevated level of several aldehydes, products of lipid peroxidation in 13 preterm infants, on the first day of life, and the risk for BPD. At 4–6 d of life, no more significant correlation was found, suggesting that early oxidative stress has a stronger impact on subsequent clinical outcome (32). Finally, Weinberger et al. (40), in a study on 25 premature infants, confirmed that elevated urinary MDA in the first days of life was correlated with a risk for oxygen radical disease.
For the antioxidant defenses, we found a link between the magnitude of early depletion in plasmatic GSH and the risk for developing IVH within the first 28 d of life (OR = 0.045). To our knowledge, this is the first time such a relation has been established in very premature infants. Most of the studies focused on the impact of low antioxidant defenses on the incidence of BPD. Neonates are short in antioxidant defenses, on both their extracellular (41,42) and intracellular part (42,43). Moreover, in preterm animals, in contrast with term, induction of antioxidative enzymes does not occur after oxidative stress (41,44). Randomized trials of antioxidant intervention in premature infants, with strong rationale and good study design, failed to demonstrate any improvement on BPD incidence (36,45–47). In our study, we speculate that low-dose iNO probably did not act as an antioxidant by itself but by reducing the oxidative stress as a result of high oxygen concentration allowed a less dramatic consumption of GSH stores.
NO is a free radical that may be oxidized or reduced depending on its concentration and the presence of other oxidants such as oxygen. Hyperoxic exposure of rat pups up-regulates both inducible and endothelial NO synthase and therefore increases the concentration of NO and subsequently peroxynitrite as well (48). This compound is highly toxic for preterm infants. Reports regarding the effect of NO in oxidative stress, however, are conflicting (43). In experimental studies, several authors found a worse clinical outcome or surfactant dysfunction as a result of lipid peroxidation or damage to surfactant protein, but they often used very high doses of NO (40–100 ppm) combined with high and stable Fio2 levels (80–100%) (19,43,49). Because of these data, wide use of iNO therapy in preterm infants is unwarranted by most neonatologists (13–15,50).
In our study, we continuously monitored by transcutaneous Pao2 the infants' response to iNO, and we aimed to wean both iNO and oxygen as quickly as possible keeping Pao2 between 50 and 65 mm Hg. Because of the continuous monitoring of iNO (to ensure constant dosage) and of NO2 (to verify safety), it was difficult to blind the administration of the gas with the design of this study. However, the duration of treatment was short (35 h on average), the infants were treated following standardized written protocol, and the clinicians in charge of the infants on day 28 were not aware of the allocation. In addition, randomization remained blinded for biologic analysis as well as for head ultrasound examination. Therefore, a bias related to unmasked administration of NO on biologic data or neurologic outcome is unlikely. Our strategy of treatment of lowering Fio2 as soon as possible by continuous follow-up of Pao2 may explain the positive results on the oxidative stress balance as well as on clinical outcome, in comparison with experimental models using the combined and toxic combination of high iNO and Fio2 levels without any decrease according to the animal response (48,49). In addition, experimental data suggest that iNO might have direct antioxidant properties reducing the inflammatory reaction in the lungs (51). Kinsella et al. (11) were the first to use 5 ppm of iNO in a prospective trial on 80 very sick premature infants who were ≤32 wk for at least 7 d. They showed, besides an early positive response on oxygenation, no change in the mortality rate but a significant decrease in the need for mechanical ventilation and the incidence of chronic lung disease. Many studies that have evaluated the effect of iNO in premature infants at an advanced stage of respiratory failure (12) failed to demonstrate an effect on mortality and/or outcome (8–11). In those studies, all infants did not respond to iNO, whereas the outcome was improved in the responders (22). Noteworthy, the first study to demonstrate a positive effect of iNO has treated infants early, with a low severity of respiratory disease (52). In this study, Schreiber et al. (52) used 10 ppm of iNO in 105 infants who were <34 wk and compared the incidence of chronic lung disease or death with 102 control subjects. They found a positive effect of iNO despite the low severity of initial HRF disease assessed by a mean oxygenation index of 6.8. In our study, we considered iNO therapy only in infants who required >40% Fio2 and enrolled 76 HRF infants among whom the infants randomly assigned to receive iNO (n = 37) were slightly sicker to start with than control subjects (Table 1). Despite these worse initial conditions, this subgroup was able to respond to low-dose iNO, to decrease its early oxidative stress, and to have a relatively fair clinical outcome in comparison with most of the other studies (14,52). Therefore, one could speculate that the lack of significant results on the outcome in most of the studies with iNO might be related to the policy of treatment used in these studies.
CONCLUSION
Our study demonstrates that low doses of iNO administered soon after birth in very preterm infants who have moderate hypoxemic respiratory failure is associated with a decrease in their oxidative stress. We speculate that this impact of iNO occurs thanks to an early, rapid, and sustained decrease in Fio2 requirements, at a postnatal age when the deleterious cascade of oxidative/inflammatory injuries can be reversed.
Abbreviations
- aAO2:
-
arterio-alveolar ratio in oxygen
- BPD:
-
bronchopulmonary dysplasia
- Fio2:
-
fraction of inspired oxygen
- GA:
-
gestational age
- GPX:
-
glutathione peroxidase
- GR:
-
glutathione reductase
- GSH:
-
glutathione
- HRF:
-
hypoxemic respiratory failure
- iNO:
-
inhaled nitric oxide
- IUGR:
-
intrauterine growth retardation
- IVH:
-
intraventricular hemorrhage
- MDA:
-
malondialdehyde
- OR:
-
odds ratio
- Pao2:
-
arterial oxygen pressure
- ROI:
-
reactive oxygen intermediates
References
Kinsella JP, Neish S, Shaffer E, Abman SH 1992 Low dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340: 819–820
NINOS (The Neonatal Inhaled Nitric Oxide Study Group) 1997 Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336: 597–604
Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, deLemos RA, Sardesai S, McCurnin D, Moreland SG, Cutter GR, Abman SH 1997 Randomized, muticenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 131: 55–62
Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, Rhines J, Chang CT 1998 Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-NO/PPHN Study Group. Pediatrics 101: 325–334
Finer NN, Barrington KJ 2000 Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev ( 2): CD000399
Franco-Belgium Collaborative NO Trial Group 1999 Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. Lancet 354: 1066–1071
Skimming JW, Bender KA, Hutchinson AA, Drummond WH 1997 Nitric oxide inhalation in infants with respiratory distress syndrome. J Pediatr 130: 225–230
Peliowski A, Finer NN, Etches PC, Tierney AJ, Ryan CA 1995 Inhaled nitric oxide for premature infants after prolonged rupture of the membranes. J Pediatr 126: 450–453
Subhedar NV, Ryan SW, Shaw NJ 1997 Open randomised controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Arch Dis Child Fetal Neonatal Ed 77: F185–F190
Meurs KP, Rhine J, Asselin JM, Durand DJ 1997 Response of premature infants with severe respiratory failure to inhaled nitric oxide. Preemie NO Collaborative Group. Pediatr Pulmonol 24: 319–323
Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, Walsh-Sukys MC, Mc Caffey MJ, Cornfield DN, Bhutani VK, Cutter GR, Baier M, Abman SH 1999 Inhaled nitric oxide in premature neonates with severe hypoxemic respiratory failure: a randomised controlled trial. Lancet 354: 1061–1065
Banks BA, Seri I, Ischiropoulos H, Merrill J, Rychick J, Ballard RA 1999 Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics 103: 610–618
Bland RD 1999 Inhaled nitric oxide: a premature remedy for chronic lung disease. Pediatrics 103: 667–670
Hoehn T, Krause MF 2001 Response to inhaled nitric oxide in premature and term neonates. Drugs 61: 27–39
Mercier JC, Franco-Belgium Neonatal Study Group on Inhaled NO 2001 Uncertainties about the use of nitric oxide in preterm infants. Acta Paediatr Suppl 90: 15–18
Cheung PY, Salas E, Etches PC, Phillipos E, Shultz R, Randomski MW 1998 Inhaled nitric oxide and inhibition of platelet aggregation in critically ill neonates. Lancet 351: 1181–1182
Saugstad OD 1996 Mechanisms of tissue injury by oxygen radicals; implications for neonatal disease. Acta Paediatr 85: 1–4
Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S 1993 Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol 265: L555–L564
Hallman M, Waffarn F, Bry K, Turbow R, Kleinman MT, Montz WJ, Rasmussen RE, Bhalla DK, Phalen RF 1996 Surfactant dysfunction after inhalation of nitric oxide. J Appl Physiol 80: 2026–2034
Issa A, Lappalainen U, Kleinman M, Bry K, Hallman M 1999 Inhaled nitric oxide decreases hyperoxia-induced surfactant abnormality in preterm rabbits. Pediatr Res 45: 247–254
Hallman M 1997 Molecular interactions between nitric oxide and lung surfactant. Biol Neonate 71: 44–48
Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A, Cantagrel S, Al Hosri J, Thiriez G, Valdes V, Vittu G, Egreteau L, Henrot A, Buchweiller M-C, Onody P 2005 The safety and efficacy of nitric oxide therapy in very preterm infants. J Pediatr 146: 318–323
Yagi K 1976 A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Res 15: 212–216
Wong SH, Knight JA, Hopfer SM, Zaharia O, Leach CN Jr, Sunderman FW Jr 1987 Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem 33: 214–220
Coutelle C, Iron A, Higueret D, Cassaigne A 1992 Optimization of a spectrophotometry assay of total and oxidized blood glutathione: comparison with a fluorimetric method. Ann Biol Clin (Paris) 50: 71–76
Paglia DE, Valantine WN 1967 Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169
Goldberg DM, Spooner RJ ( 1983) Dosage of erythrocyte glutathione reductase, in Methods of Enzymatic Analysis (Bergmeyer J, Grassl M eds), 3rd ed, pp 258–265. Verlag Chemie, Deerfield Beach, FL.
Papile LA, Burstein J, Burstein R, Koffler H 1978 Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweight less than 1,500 gm. J Pediatr 92: 529–534
Egreteau L, Pauchard JY, Semama DS, Matis J, Liska A, Romeo B, Cneude F, Hamon I, Truffert P 2001 Chronic oxygen dependency in infants born at less than 32 weeks' gestation: incidence and risk factors. Pediatrics 108: E26
Saugstad OD 2001 Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol 13: 147–153
Inder TE, Graham P, Sanderson K, Taylor BJ 1994 Lipid peroxidation as a measure of oxygen free radical damage in the very low birth weight infant. Arch Dis Child Fetal Neonatal Ed 70: F107–F111
Ogihara T, Hirano K, Morinobu T, Kim HS, Hiroi M, Ogihara H, Tamai H 1999 Raised concentrations of aldehyde lipid peroxidation products in premature infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed 80: F21–F25
Desandes R, Desandes E, Droullé P, Didier F, Longrois D, Hascoet JM 2004 Inhaled nitric oxide improves oxygenation in very premature infants with low pulmonary blood flow. Acta Paediatr 93: 66–69
Abman SH 2002 Monitoring cardiovascular function in infants with chronic lung disease of the prematurity. Arch Dis Child Fetal Neonatal Ed 87: F15–F18
Jain A, Mehta T, Auld PA, Rodrigues J, Ward RF, Schwartz MK, Martensson J 1995 Glutathione metabolism in newborns: evidence for glutathione deficiency in plasma, bronchoalveolar lavage fluid, and lymphocytes in prematures. Pediatr Pulmonol 20: 160–166
Welty SE 2003 Antioxidants and oxidations in bronchopulmonary dysplasia: there are no easy answers. J Pediatr 143: 697–698
Pitkanen OM, Hallman M, Andersson S 1990 Correlation of free oxygen radical-induced lipid peroxidation with outcome in the very low birth weight infants. J Pediatr 116: 760–764
Varsila E, Hallman M, Andersson S 1994 Free-radical-induced lipid peroxidation during the early neonatal period. Acta Paediatr 83: 692–695
Varsila E, Pitkanen OM, Hallman M, Andersson S 1994 Immaturity-dependent free radical activity in premature infants. Pediatr Res 36: 55–59
Weinberger B, Anwar M, Henien S, Sosnovsky A, Hiatt M, Jochnowitz N, Witz G, Hegyi T 2004 Association of lipid peroxidation with antenatal betamethasone and oxygen radical disorders in preterm infants. Biol Neonate 85: 121–127
Berger HM, Molicki JS, Moison RM, Van Zoeren-Grobben D 1998 Extracellular defence against oxidative stress in the newborn. Semin Neonatol 3: 183–190
Frank L 1998 Development of the antioxidant defences in fetal life. Semin Neonatol 3: 173–182
Saugstad OD 2003 Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49
Frank L, Sosenko IR 1991 Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared to term rabbits. Pediatr Res 29: 292–296
Ahola T, Lapatto R, Raivio KL, Selander B, Stigson L, Jonsson B, Jonsbo F, Esberg G, Stovring S, Kjartansson S, Stiris T, Lossius K, Virkola K, Fellman V 2003 N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr 143: 713–719
Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, North American Recombinant Human CuZnSOD Study Group 2003 Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant CuZn superoxide dismutase. Pediatrics 111: 469–476
Russell GA, Cooke RW 1995 Randomised controlled trial of allopurinol prophylaxis in very preterm infants. Arch Dis Child Fetal Neonatal Ed 73: F27–F31
Potter CF, Kuo NT, Farver CF, McMahon JT, Chang CH, Agani FH, Haxhiu MA, Martin RJ 1999 Effects of hyperoxia on nitric oxide synthase expression, nitric oxide activity, and lung injury in rat pups. Pediatr Res 45: 8–13
Robbins CG, Davis JM, Merritt TA, Amirkhanian JD, Sahgal N, Morin FC 3rd, Horowitz S 1995 Combined effects of nitric oxide and hyperoxia on surfactant function and pulmonary inflammation. Am J Physiol 269: L545–L550
Saugstad OD 1999 Inhaled nitric oxide for preterm infants—still en experimental therapy. Lancet 354: 1047–1048
Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH 1997 Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 41: 457–463
Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P 2003 Inhaled nitric oxide in premature infants with respiratory distress syndrome. N Engl J Med 349: 2099–2107
Acknowledgements
We are indebted to Prof. Pierre Nabet for providing us with the skills and generous access to his laboratory, and we also thank Dr. Brigitte Dousset and Dr. Henri Schroeder for precious advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Maternite Regionale Universitaire de Nancy.
Rights and permissions
About this article
Cite this article
Hamon, I., Fresson, J., Nicolas, MB. et al. Early Inhaled Nitric Oxide Improves Oxidative Balance in Very Preterm Infants. Pediatr Res 57, 637–643 (2005). https://doi.org/10.1203/01.PDR.0000156507.03879.19
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000156507.03879.19
This article is cited by
-
Exercise-induced bronchoconstriction in school-age children born extremely preterm
Pediatric Research (2013)
-
Inhaled Nitric Oxide Prevents 3-Nitrotyrosine Formation in the Lungs of Neonatal Mice Exposed to >95% Oxygen
Lung (2010)
-
Inhaled nitric oxide in term and preterm infants
Journal of Perinatology (2006)