Abstract
Intrauterine programming of hypertension is associated with evidence of increased renin-angiotensin system (RAS) activity. The current study was undertaken to investigate whether arterial baroreflex and blood pressure variability are altered in a model of in utero programming of hypertension secondary to isocaloric protein deprivation and whether activation of the RAS plays a role in this alteration. Pregnant Wistar rats were fed a normal-protein (18%) or low-protein (9%) diet during gestation, which had no effect on litter size, birth weight, or pup survival. Mean arterial blood pressure (MABP; 126 ± 3 mm Hg 9%versus 108 ± 4 mm Hg 18%; p < 0.05) and blood pressure variability were significantly greater in the adult offspring of the 9% protein–fed mothers. Arterial baroreflex control of heart rate, generated by graded i.v. infusion of phenylephrine and nitroprusside, was significantly shifted toward higher pressure; i.v. angiotensin-converting enzyme inhibitor normalized MABP and shifted the arterial baroreflex curve of the 9% offspring toward lower pressure without affecting the 18% offspring. For examining whether brain RAS is also involved in programming of hypertension, angiotensin-converting enzyme inhibitor and losartan (specific AT1 receptor antagonist) were administered intracerebroventricularly; both significantly reduced MABP of the 9% but not the 18% offspring. Autoradiographic receptor binding studies demonstrated an increase in brain AT1 expression in the subfornical organ and the vascular organ of the lamina terminalis in the 9% offspring. These data demonstrate a major tonic role of brain and peripheral RAS on hypertension associated with antenatal nutrient deprivation.
Similar content being viewed by others
Main
Chronic cardiovascular diseases of adults can have their origins in fetal life. Epidemiologic studies reveal that hypertension, stroke, and coronary heart disease are inversely related to birth weight (1) and that this relationship is independent of genetic factors (2) and lifestyle (3). It has been suggested that a poor nutrient supply at a critical period of early development leads to permanent alterations in the programming of the developing cardiovascular structures or functions (4). This concept has been supported by animal studies demonstrating an association between nutritional deficit during fetal life and increased blood pressure in adulthood (5, 6).
Experimental evidence suggests that activation of the renin-angiotensin system (RAS) (7, 8) is an important element of hypertension programmed during fetal life. Infants who are born with intrauterine growth restriction have increased plasma renin activity and renal renin content (9, 10). In an animal model of in utero programming of hypertension, increased levels of pulmonary and plasma angiotensin converting enzyme (ACE) were found, whereas ACE inhibition (ACE-I) has been shown to normalize blood pressure (7, 8). Angiotensin II (AngII) can increase blood pressure and alter the arterial baroreflex through peripheral (vascular and renal) and central effects. Arterial baroreflex control of heart rate (HR) normally allows tight maintenance of blood pressure around a set point and has a key role in buffering blood pressure variability (BPV). There is indirect evidence suggesting abnormal control of HR in chronically growth impaired fetuses and in hypertensive adults who were nutrient deprived during fetal life (11–13). Whether arterial baroreflex and BPV are impaired and whether peripheral and/or brain RAS could participate in hypertension and resetting of arterial baroreflex are unknown.
The current study was undertaken to test the hypothesis that in utero programmed hypertension is associated with an alteration of the arterial regulation (baroreflex and BPV) secondary to excess activation of the RAS. For this purpose, we used an animal model in which mildly restricting the protein intake of pregnant rats leads to hypertensive offspring (7). The first series of experiments were designed to explore whether arterial baroreflex control of HR was altered in this model and whether this alteration depended on endogenous AngII. The second series of experiments were developed to examine more specifically the role of endogenous central AngII and the AngII receptor subtype AT1 on arterial pressure; the AT1 receptor subtype mediates most, if not all, of the central cardiovascular effects of AngII (14, 15). Last, the expression of AT1 in the brain was studied using autoradiography. Our results reveal a role for both brain and peripheral RAS in maintaining elevated blood pressure in hypertension programmed during early life.
METHODS
Animals
Animals were used according to a protocol approved by the Animal Care Committee of the Hôpital Sainte-Justine in accordance with the principles of the Guide for the Care and Use of Experimental Animals of the Canadian Council on Animal Care. Virgin Wistar rats (initial weight 225–250 g) were mated overnight and on the day of conception (determined by the presence of a vaginal plug) were allocated to feed ad libidum on a diet containing either 18% (control) or 9% (low) protein (casein) diet (5, 7). All diets contained 5 g/kg methionine to avoid sulfur deficiency and were made isocaloric with starch and sucrose supplement. Dams were weighed weekly. Within 12 h of delivery, the dams were returned to regular rat diet. Pups were weaned at 4 wk of age to regular rat diet.
Surgical Preparation
Male offspring (9–12 wk old) were anesthetized with intra-peritoneal ketamine (65 mg/kg) and xylazine (7 mg/kg). Under sterile conditions, polyethylene catheters (PE50; Plastics One, Roanoke, VA, U.S.A.) were inserted into a femoral artery and vein, tunneled subcutaneously to the back of the neck, threaded through a flexible metal spring, and connected to a dual-channel swivel mounted directly above the cage (Lomir Biomedical, Notre-Dame-de-L'Ile-Perrot, QC, Canada). This set-up allowed the rat freedom of movement within the cage.
In a second group of animals, an intracerebroventricular (ICV) cannula was implanted in addition to the femoral catheters. The anesthetized rat was placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, U.S.A.), and a 26-gauge stainless steel guide cannula was implanted immediately above the roof of the right lateral ventricle (stereotaxic coordinates with respect to bregma: 1 mm caudal and 1.5 mm lateral) and lowered 4 mm below the surface of the skull (16). The guide cannula was anchored to the skull with acrylic dental cement and sealed with a dummy cannula (Plastics One). Drugs were injected by inserting a 33-gauge stainless steel internal cannula into the guide cannula, which was connected to a 10-μL Hamilton syringe. The correct ICV placement of the cannula was verified by post mortem injection of 5 μL of black ink and examination of cryostat brain sections. At the end of the surgical procedures, each rat was given a dose of i.v. Cefazolin (25 mg/kg) and allowed to recover for 24 h before the experiment. To verify that this delay after surgery did not have an impact on the results obtained, four 9% and three 18% additional animals were studied 4 d after surgery; as the results obtained are similar, only the rats that were studied 24 h after surgery are presented.
Experimental Procedures
On the day of the experiment, the rats were brought in their cage to the laboratory and allowed to adapt for 30 min. During each experiment, arterial blood pressure and HR were monitored continuously using a pressure transducer (The Perceptor, Namic, Glen Falls, NY, U.S.A.) aligned to the level of the heart, and a Grass recorder (Astro-Med, Grass, RI, U.S.A.) displayed and recorded on-line to a computer via a Grass PVA-1A 8-channel analog-to-digital conversion board using the software Polyview (version 2.3; Astro-Med).
In the first series of studies, baseline mean arterial blood pressure (MABP) and HR were recorded for 15 min. Baroreflex curves were then determined by producing ramp changes in MABP with continuous i.v. infusion of phenylephrine (5–80 μg kg−1 min−1) or nitroprusside (5–80 μg kg−1 min−1) over a 10-min period, using a Harvard infusion pump. A 30-min recovery period was allowed before the alternative drug was administered. The same protocol was repeated 90 min later, 20 min after i.v. ACE-I enalaprilat (150 μg/kg).
The second series of experiments were designed to evaluate the role of endogenous brain AngII on maintaining elevated blood pressure. Resting values for MABP and HR were obtained before and 20 min after ICV enalaprilat [20 μg/kg in 10 μL of saline (pH 7.4)]. In the third series of experiments, the role of brain AngII AT1 receptors on resting MABP, HR, and resetting of arterial baroreflex control of HR was evaluated. For this purpose, baseline recordings and baroreflex curves were generated as described for the first series of experiments, before and after ICV injection of losartan [specific AT1 receptor antagonist, 30 μg/kg in 10 μL of saline (pH 7.4)].
Autoradiographic Expression of AngII-AT1 Receptor in the Brain
Consecutive coronal brain sections (20 μm) from 18% and 9% 9- to 12-wk-old male offspring were cut on a cryostat (Microm Cryostat, Waldorf, Germany) at −25°C and thaw-mounted on microscope slides (Superfrost; VWR Scientific, Ville Mont-Royal, QC, Canada). Sections were preincubated for 15 min at room temperature with binding buffer [10 mM phosphate buffer (pH 7.4), 150 mM NaCl, 5 mM Na2 EDTA, 0.4 mM bacitracin, 0.2% BSA], then for 1 h in the same buffer containing 2.5 × 10−10 M 125I-[Sar1, Ile8]AngII with 2.103 Ci/mmol (Amersham) with or without 10−6 M losartan. Non-specific binding was determined in the presence of 10−6 M unlabeled [Sar1, Ile8]AngII. Subsequently, the tissue sections were transferred through four 1-min washes in 4°C incubation buffer without BSA, dipped in 4°C deionized water, and rapidly dried under a stream of cold air. Slides were exposed to Kodak Biomax MS films for 6 d at −80°C.
Data Analysis
BPV.
Three-minute periods were extracted from baseline recordings when the quality of the arterial blood pressure signal was visually considered to be satisfactory (steady MABP, no agitation, no dampening, and no artifacts). Before spectral analysis, a least-squares linear procedure was used to remove any linear offset and trend from the data. Spectral analysis of the data was performed using the Fast Fourier Transform algorithm with a Hamming window (Polyview 2.3, Astro-Med). The total power spectral density (variance), which is an index of global variability, was calculated by integrating the power spectra over the frequency range (0.1–2.5 Hz) and expressed in mm Hg2. The spectral densities in the very low frequency range (0.1–0.2 Hz), in the low frequency range (0.2 to 0.7 Hz), and in the high frequency range (0.7–2.5 Hz) were also calculated (17). The frequency variations of <0.1 Hz were not considered to avoid possible artifacts as a result of long-term slow oscillations. Normalization procedure was performed by dividing the power of low frequency and high frequency by the total power spectral density and by multiplying the result by 100.
Arterial baroreflex control of heart rate.
The HR response to changes in MABP was used to generate the baroreflex curve. The different baroreflex curves, expressed as the relationship between MABP and HR, were analyzed with a logistic sigmoid function (Graph Pad Inplot version 4.03; GraphPad Software, San Diego, CA, U.S.A.) according to the following equation: HR = P4 + P1/{1 + exp[P2 (MABP − P3)], where P1 is the range between the upper and lower plateaus, P2 is a coefficient used to calculate the gain as a function of pressure, P3 is the MABP at midrange of the curve, and P4 is the lower plateau (18). The gain (slope) was calculated from the first derivative of the above equation. Threshold pressure (lowest pressure that produces a significant decline in HR) and saturation pressure (pressure necessary to achieve maximal inhibition of HR) were calculated from the third derivative of the equation (18).
Quantitative ex vivo receptor autoradiography.
Total 125I-[Sar1, Ile8]AngII receptor binding and radioligand binding in the presence of losartan were quantified in the brain areas that are involved in cardiovascular regulation and that expressed AT1 binding sites (14). For quantifying receptor-ligand binding in the examined brain regions, calibrated OD measurements (transmitted light) of digitized autoradiograms were carried out after normalizing for background tone. Losartan-sensitive binding was used to estimate AT1 binding and was obtained by subtracting binding in the presence of losartan from total binding (ImagePro software; Media Cybernetics, San Diego, CA, U.S.A.). In each experiment (i.e. radiographic film), the binding densities were obtained for one 9% and one 18% brain. Seven brains from each group of rats were studied, with a median of 110 sections per brain. The maximum AT1 binding densities within each brain structure were calculated and expressed as a percentage of the AT1 binding in the corresponding control brain region (set as 100%) and in fmol/mg protein using calibrated autoradiographic 125I microscale (Amersham, Baie D'Urfé, QC, Canada).
Chemicals
The following agents were purchased: sodium nitroprussiate, phosphate buffer, EDTA, AngII, and BSA (Sigma Chemical Co., St. Louis, MO, U.S.A.); bacitracin and (3-[125I]iodotyrosyl4 Sar1 Ile8 AngII (125I-[Sar1, Ile8]AngII; Amersham Biosciences); enalaprilat (Vasotec; Merck Frosst, Kirkland, QC, Canada); Ketamine (Ayerst, Montreal, QC, Canada); Xylazine (Bayer, Etobicoke, Ont, Canada); and Cefazolin (Novopharm, Scarborough, Ont, Canada). Losartan was a gift of Merck Frosst Canada and Du Pont (Kirkland, QC, Canada).
Statistical Analysis
All results are expressed as mean ± SEM. Analysis of differences within and between groups was performed using multivariate ANOVA, t test for paired or unpaired observations, and Wilcoxon or Mann-Whitney tests as appropriate. A simple linear regression analysis was used to determine the correlation between BPV parameters and MABP. Statistical significance was set at p < 0.05.
RESULTS
Net weight gain during pregnancy of dams fed the low-protein (9%) and control (18%) diets was similar (9% 144 ± 7 g, n = 18; 18% 142 ± 5 g, n = 19). Dietary protein affected neither the litter size (9% 14.9 ± 0.9, n = 7; 18% 14.8 ± 0.7, n = 8) nor the survival rate of the offspring during the 12-wk study period. Birth weight did not differ between groups (9% 5.3 ± 0.1 g, n = 14; 18% 5.6 ± 0.2 g, n = 14).
Effects of systemic ACE-I on baseline HR, MABP, BPV, and arterial baroreflex.
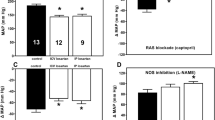
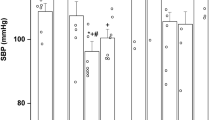
The baseline MABP was significantly higher in the 9% offspring (Fig. 1A), whereas HR was similar (see operating point in Table 1). The BPV, measured by total power spectral density, was also higher in the 9% offspring; this increase was observed in both low- and high-frequency bands (Table 2, Fig. 2).
Effects of maternal diets that contain 18% (control;□) and 9% (low;▪) protein on MABP of the 9- to 12-wk-old offspring in three sets of experiments: before (baseline) and after i.v. administration of ACE-I enalaprilat (n = 7 for each group; A) before (baseline after ICV) and after ICV administration of enalaprilat (n = 3 for each group; B), and before and after ICV losartan (AngII AT1 receptor antagonist, n = 5 for each group; C). Data are means ± SEM. *p < 0.05 vs 18% baseline; **p < 0.05 vs 9% baseline.
Representative tracing of the increase in BPV in the low-protein-exposed offspring group (9%; C and D) when compared with the control group (18%; A and B). (A and C) The blood pressure traces (20-s samples, in gray) are presented together with the evolution of the MABP (low-pass filter, in black). (B and D) Results of the spectral analysis (3-min periods). The higher BPV in the 9% group involves an increase in spectral density in both low-frequency (0.2–0.7 Hz) and high-frequency (0.7–2.5 Hz) ranges (see Table 1).
I.v. enalaprilat significantly reduced MABP of the 9% offspring rats without changing HR; baseline MABP and HR were unaffected in the 18% control animals (Fig. 1A, Table 1). Enalaprilat partially normalized BPV of the 9% group as it abolished the statistically significant difference observed between the groups in baseline conditions (9% post–ACE-I not significantly different from 18% baseline and post–ACE-I values), although the 9% post–ACE-I BPV values remained not significantly different from baseline (Table 2). The BPV parameters were not correlated with resting MABP either before or after enalaprilat. HR baroreflex response curve threshold, saturation, and midpoint pressures of the 9% offspring were elevated, indicating a significant shift of the baroreflex toward higher pressures; i.v. enalaprilat reset the arterial baroreflex curve of 9% toward lower pressure without modifying the baroreflex curve of the 18% offspring (Table 1, Fig. 3).
(A) Representative segment of a recording of blood pressure (top) and HR signal (middle) response to phenylephrine infusion. (Middle) The frequency at which the signal crosses the “0” line correlates with the HR (bpm); i.e. as HR slows with the increase in blood pressure, the amplitude of the signal increases as more time elapses between two beats. (Bottom) Data from the top two panels transposed as MABP (⋄) and HR (♦) as a function of time. (B) Representative illustration of the effects of maternal diets containing 18% (control; left) and 9% (right) protein on arterial baroreflex control of HR of the 9- to 12-wk-old offspring, before (baseline) and after i.v. enalaprilat. An example of the number of points used to generate the baroreflex curve is presented as an inset on the left graph. HR baroreflex response curve of 9% offspring is shifted toward higher pressures; after ACE-I, HR arterial baroreflex curve is reset toward lower pressure in 9% and not modified in 18% (see Table 1).
Effects of brain AngII on baseline MABP and on arterial baroreflex.
ICV enalaprilat, as well as losartan, significantly reduced the MABP of the 9% offspring without changing the resting MABP of the 18% control group (Fig. 1B and C). When compared with baseline, ICV losartan did not significantly change the baroreflex curve of the 9% offspring despite a tendency to shift the curve toward lower pressure (Table 1).
Effects of low-protein diet on brain AT1 receptor expression.
Considering the effects of inhibiting brain AngII on MABP of the 9% but not the 18% offspring, we sought to determine whether brain AT1 receptor expression was increased in the 9% offspring. Nonspecific binding determined with an excess of unlabeled AngII was negligible (<5% of total binding). Because we used a single subsaturating concentration of 125I-[Sar1, Ile8]AngII, the binding results presented below may reflect the affinity as well as the quantity of receptors. Autoradiographic analysis of the brain sections from both groups revealed specific AngII AT1 receptor binding localized in the vascular organ of the lamina terminalis (OVLT), median preoptic nucleus (MEPO), subfornical organ (SFO), paraventricular nucleus of the hypothalamus (PVH), nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus, and area postrema (AP). In all of these areas, losartan displaced 125I-[Sar1, Ile8]AngII binding. The binding analysis did not include the AP, which was not visible in all of the experiments, or the dorsal motor nucleus of the vagus, which was difficult to define from the NTS. All other brain regions demonstrated a higher binding density in the 9% offspring, and the difference reached statistical significance in the OVLT (116 ± 30%) and in the SFO (100 3 38%;Fig. 4).
(Left) Representative autoradiographic distribution of 125I-[Sar1, Ile8]AngII binding sites in coronal sections from 9- to 12-wk-old offspring of dams fed diets containing 18% (control; left) and 9% (right) protein during gestation. Note higher binding intensity in SFO and OVLT of 9% brain. Compiled losartan-sensitive binding in the different brain areas analyzed are presented on the right (18%,⋄; 9%,♦). PIR, piriform cortex; PVH, paraventricular nucleus hypothalamus; Ox, optic chiasma; VL, lateral ventricle; CP, choroid plexus. Data are means ± SEM; n = 7 for each group. *p < 0.05 vs 18%.
DISCUSSION
Epidemiologic studies indicate that antenatal nutrient depletion is associated with hypertension and increased risk of cardiovascular-related morbidity and mortality in adult life (4, 19–22). We have reproduced a rat model of hypertension, induced during early life through isocaloric 50% protein restriction of pregnant dams. The diet provides 75% of the basic protein required during gestation (5, 7) and is not associated with significant intrauterine growth restriction (23). Our results confirm that peripheral blockade of AngII decreases blood pressure of low-protein-diet-exposed offspring and demonstrate that in utero programmed hypertension is associated with increased spontaneous BPV and impaired arterial baroreflex control of HR. In addition, the decrease in blood pressure induced by central blockade of AngII AT1 receptor, as well as the increase in AT1 receptor expression in brain cardiovascular regulating areas of the 9% offspring, indicates an important role for the brain RAS. Taken together, these data demonstrate a major tonic role of both peripheral and brain RAS in maintaining in utero programmed chronic hypertension.
BPV.
Spontaneous BPV of the 9% offspring is increased in both high- and low-frequency ranges, which indicates impaired blood pressure regulation. It is interesting that it was recently shown that children with lower birth weight tend to have higher BPV independent of their blood pressure (24). Whereas hypertension is a primary cardiovascular risk factor, fluctuations in arterial blood pressure may also be of considerable importance and has been shown, independent of blood pressure, to lead to end organ damage classically associated with hypertension (25). The most intensively studied and probably most effective mechanism to maintain arterial blood pressure within narrow boundaries is the arterial baroreceptor reflex. The mechanisms involved in this increased BPV could comprise, at least for the low-frequency oscillations, a change in arterial baroreflex, which we also demonstrate in this series of studies. Activation of RAS has been proposed as a mechanism that contributes to end organ damage induced by increased BPV (25). However, considering that blockade of endogenous AngII formation significantly reset arterial baroreflex control of HR toward values comparable to control animals but did not completely normalize BPV, other factors are likely implicated in this increased BPV. Nitric oxide participates in the buffering of blood pressure variations (17, 26), and a defect in nitric oxide–mediated effects, which we recently demonstrated in brain microvessels of 9% offspring (27), could lead to increased BPV. Reduced arterial distensibility, as reported in those who are born with intrauterine growth restriction (28, 29), is also associated with increased low-frequency BPV in other forms of chronic hypertension (30). The combination of increased BPV and RAS activity observed in in utero programming of hypertension could account for the excess cardiovascular morbidity observed in adults with low birth weight, despite the relatively mild increase in blood pressure reported in this population (4).
Alteration of arterial baroreflex control of HR.
The arterial baroreflex is critical in the development of hypertension. Intrauterine growth restricted animals and humans, as well as hypertensive adult animals that were deprived during fetal life, show evidence of increased blood pressure along with a paradoxic normal or even slightly increased HR (12, 13). This observation was verified in the current study with the absence of a difference in resting HR between the two groups despite the higher MABP in the 9% offspring, suggesting an alteration in the arterial baroreflex. The latter is confirmed by the shift of the arterial baroreflex curve toward a higher pressure in the 9% offspring. As enalaprilat does not cross the blood-brain barrier (31), the effects observed after systemic administration of this ACE-I represent the role of peripherally derived AngII on cardiovascular function. Studies in mature animals have shown that circulating AngII alters baroreflex control of HR primarily by shifting the baroreflex curve to a higher blood pressure without modulating the gain (32, 33). Conversely, systemic administration of ACE-I or AT1 blocker decreases the blood pressure of hypertensive individuals without changing the sensitivity of the HR baroreflex curve (34). As baroreflex is not completely reset to control group values after ACE-I, this indicates that AngII is not the sole factor involved in modulating baroreflex control of HR in 9% animals. Changes in the relationship between arterial pressure and heart rate can result from changes in the baroreceptor itself and/or within the CNS. Baroreceptor endings are located in the walls of the carotid arteries and the aorta and sense the changes in blood pressure through changes in their stretch. Accordingly, vascular compliance, which can be decreased in individuals with low birth weight (28, 29), is an important determinant of the activity of the baroreceptors (35). Also, whether membrane characteristics of baroreceptor endings and changes in ion channels function within the baroreceptor membrane are altered by antenatal diet exposure is unknown (36). Centrally, changes in neurohormones that influence baroreflex efferent autonomic pathway as well as activation of other neural reflex pathways are potential factors that may be modified in fetal programming of high blood pressure and have an impact on arterial baroreflex (36).
The mechanism by which circulating AngII is implicated in HR baroreflex resetting is nonetheless dependent on the CNS. Indeed, circulating AngII does not influence carotid and aortic baroreceptor firing (33) (afferent loop) but can bind to AT1 receptors expressed by some brain circumventricular organs (e.g. AP, OVLT, SFO) (37, 38), which in turn modulate the efferent branch of the arterial baroreflex (39). We observed that blockade of central AngII by losartan in 9% offspring decreases blood pressure without significantly altering arterial baroreflex control of HR, although there was a tendency for the curve to be reset to lower pressure. Losartan can cross the blood-brain barrier (37), but the dose administered centrally in our studies is ∼100 times lower than a peripherally effective dose; we therefore consider that the effect of ICV losartan on arterial pressure reflects solely central blockade of AT1 receptors. Consequently, these data indicate that brain AngII participates in maintaining in utero programmed hypertension. Such a role for brain RAS has been shown in other forms of chronic hypertension such as renovascular hypertension (40) and chronic stress–induced hypertension (41). Endogenous brain AngII can elevate blood pressure through increases in efferent sympathetic activity, release of vasopressin from the hypothalamus, and synaptic inhibition of the baroreflex at the level of the nucleus tractus solitarius (42, 43).
Expression of AngII AT1 receptor in brain.
Our findings of significant increased expression of the AngII AT1 receptor in specific brain areas involved in cardiovascular regulation further support a role for central AngII in the hypertension of 9% offspring rats. The brain structures that are involved in cardiovascular modulation by AngII and that express the AT1 receptor are located in the forebrain (OVLT, MEPO, SFO, and PVH) and in the lower brainstem (NTS, AP, dorsal motor nucleus of the vagus, and nucleus ambiguus) (14). In the current studies, AT1 binding sites were detected in all of these areas, and binding intensity was found to be more strong in 9% offspring, markedly more so in the OVLT and the SFO. Both circulating and central AngII can activate AT1 receptors from the SFO and OVLT (44, 45). AngII microinjected into the SFO causes a pressor effect that is mediated by vasopressin release and increased sympathetic activity (46, 15). Elevated brain AngII binding sites have also been reported in spontaneously hypertensive fetal and newborn rats (47, 48), and blockade of central RAS in adult spontaneously hypertensive rat can reverse their chronic hypertension (49). The underlying mechanisms and regulatory processes that lead to increased AT1 receptor brain expression in 9% offspring are unknown but may involve a defect in nitric oxide pathway (27, 50, 51), positive feedback regulation by elevated brain AngII (52, 53), and glucocorticoids (12, 54, 55).
Abbreviations
- ACE-I:
-
angiotensin-converting enzyme inhibitor
- AngII:
-
angiotensin II
- AP:
-
area postrema
- BPV:
-
blood pressure variability
- HR:
-
heart rate
- ICV:
-
intracerebroventricular
- MABP:
-
mean arterial blood pressure
- MEPO:
-
median preoptic nucleus
- NTS:
-
nucleus of the solitary tract
- OVLT:
-
vascular organ of the lamina terminalis
- PVH:
-
paraventricular nucleus of the hypothalamus
- RAS:
-
renin-angiotensin system
- SFO:
-
subfornical organ
References
Law CM, Shiell AW 1996 Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens 14: 935–941.
Camussi A, Bianchi G 1988 Genetics of essential hypertension. From the unimodalbimodal controversy to molecular technology. Hypertension 12: 620–628.
Beilin LJ 1990 Diet and lifestyle in hypertension: changing perspectives. J Cardiovasc Pharmacol 16: 62–66.
Barker DJP 1994 Mothers, Babies and Disease in Later Life. BMJ Publishing Group, London
Langley-Evans SC, Gardner DG, Jackson AA 1996 Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil 106: 307–312.
Woodall SM, Johnston BM, Breier BH, Gluckman PD 1996 Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res 40: 438–443.
Langley-Evans SC, Jackson AA 1995 Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol 110: 223–228.
Sherman RC, Langley-Evans SC 2000 Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 98: 269–275.
Kingdom JP, McQueen J, Connell JM, Whittle MJ 1993 Fetal angiotensin II levels and vascular (type I) angiotensin receptors in pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol 100: 476–482.
Kingdom JC, Hayes M, McQueen J, Howatson AG, Lindop GB 1999 Intrauterine growth restriction is associated with persistent juxtamedullary expression of renin in the fetal kidney. Kidney Int 55: 424–429.
Su DF, Miao CY 2002 Arterial baroreflex function in conscious rats. Acta Pharmacol Sin 23: 673–679.
Langley-Evans SC 1997 Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci (Lond) 93: 423–429.
Murotsuki J, Challis JR, Han VK, Fraher LJ, Gagnon R 1997 Chronic fetal placental embolization and hypoxemia cause hypertension and myocardial hypertrophy in fetal sheep. Am J Physiol 272:R201–R207.
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C 1997 Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18: 383–439.
Wright JW, Harding JW 1995 Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept 59: 269–295.
Paxinos G, Watson C 1986 The Rat Brain in Stereotaxic Coordinates, 2nd Ed. Academic Press, Sydney
Nafz B, Wagner CD, Persson PB 1997 Endogenous nitric oxide buffers blood pressure variability between 0.2 and 0.6 Hz in the conscious rat. Am J Physiol 272:H632–H637.
Kent BB, Drane JW, Blumenstein B, Manning JW 1972 A mathematical model to assess changes in the baroreceptor reflex. Cardiology 57: 295–310.
Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ 1996 Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94: 3246–3250.
Koupilova I, Leon DA, Lithell HO, Berglund L 1997 Size at birth and hypertension in longitudinally followed 50-70-year-old men. Blood Press 6: 223–228.
Poulter NR, Chang CL, MacGregor AJ, Snieder H, Spector D 1999 Association between birth weight and adult blood pressure in twins: historical cohort study. BMJ 319: 1330–1333.
Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJP 1996 Fetal growth and coronary heart disease in south India. Lancet 348: 1269–1273.
Langley-Evans SC 2000 Critical differences between two low protein diet protocols in the programming of hypertension in the rat. Int J Food Sci Nutr 51: 11–17.
Lurbe E, Torro I, Rodriguez C, Alvarez V, Redon J 2001 Birth weight influences blood pressure values and variability in children and adolescents. Hypertension 38: 389–393.
Su D-F, Miao C-Y 2001 Blood pressure variability and organ damage. Clin Exp Pharmacol Physiol 28: 709–715.
Stauss HM, Nafz B, Mrowka R, Persson PB 2000 Blood pressure control in eNOS knock-out mice: comparison with other species under NO blockade. Acta Physiol Scand 168: 155–160.
Lamireau D, Nuyt AM, Hou X, Bernier S, Beauchamp M, Gobeil F, Lahaie I, Varma DR, Chemtob S 2002 Altered vascular function in fetal programming of hypertension. Stroke 33: 2992–2998.
Martin H, Hu J, Gennser G, Norman M 2000 Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation 102: 2739–2744.
Martyn CN, Barker DJ, Jespersen S, Greenwald S, Osmond C, Berry C 1995 Growth in utero, adult blood pressure, and arterial compliance. Br Heart J 73: 116–121.
Dabire H, Lacolley P, Chaouche-Teyara K, Fournier B, Safar ME 2002 Relationship between arterial distensibility and low-frequency power spectrum of blood pressure in spontaneously hypertensive rats. J Cardiovasc Pharmacol 39: 98–106.
Jouquey S, Mathieu M-N, Hamon G, Chevillard C 1995 Effect of chronic treatment with trandolapril or enalapril on brain ACE activity in spontaneously hypertensive rats. Neuropharmacology 34: 1689–1692.
Brooks VL 1995 Chronic infusion of angiotensin II resets baroreflex control of heart rate by an arterial pressure-independent mechanism. Hypertension 26: 420–424.
Guo GB, Abboud FM 1984 Angiotensin II attenuates baroreflex control of heart rate and sympathetic activity. Am J Physiol 246:H80–H89.
Vesalainen RK, Kantola IM, Airaksinen KE, Tahvanainen KU, Kaila TJ 1998 Vagal cardiac activity in essential hypertension: the effects of metoprolol and ramipril. Am J Hypertens 11: 649–658.
Lanfranchi PA, Somers VK 2002 Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol 283:R815–R826.
Chapleau MW, Hajduczok G, Abboud FM 1988 Mechanisms of resetting of arterial baroreceptors: an overview. Am J Med Sci 295: 327–334.
Li Z, Bains JS, Ferguson AV 1993 Functional evidence that the angiotensin antagonist losartan crosses the blood-brain barrier in the rat. Brain Res Bull 30: 33–39.
Song K, Allen AM, Paxinos G, Mendelsohn FAO 1992 Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol 316: 467–484.
Bishop VS, Sanderford MG 2000 Angiotensin II modulation of the arterial baroreflex Role of the area postrema. Clin Exp Pharmacol Physiol 27: 428–431.
Faber JE, Brody MJ 1984 Central nervous system action of angiotensin during onset of renal hypertension in awake rats. Am J Physiol 247:H349–H360.
Qian ZM, Xiao DS, Huang WQ, Tang PL, Xu B 1997 Central Ang II receptor involved in carotid sinus reflex resetting in chronically stressed rats. Physiol Behav 62: 241–247.
Head GA, Mayorov DN 2001 Central angiotensin and baroreceptor control of circulation. Ann N Y Acad Sci 940: 361–379.
Matsumura K, Averill DB, Ferrario CM 1998 Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol 275:R1611–R1619.
Mangiapane ML, Simpson JB 1980 Subfornical organ lesions reduce the pressor effect of systemic angiotensin II. Neuroendocrinology 31: 380–384.
Van Houten M, Mangiapane ML, Reid IA, Ganong WF 1983 Sar,Alaangiotensin II in cerebrospinal fluid blocks the binding of blood-borne 125Iangiotensin II to the circumventricular organs. Neuroscience 10: 1421–1426.
Mangiapane ML, Brody MJ 1986 Mechanisms of hemodynamic responses to electrical stimulation of subfornical organ. Am J Physiol 250:R1117–R1122.
Cook VI, Grove KL, Speth RC, McMenamin KM, Harding JW 1993 Differences between perinatal angiotensin binding in the brains of SHR and WKY rats. Regul Pept 45: 395–405.
Raizada MK, Lu D, Tang W, Kurian P, Summers C 1993 Increased angiotensin II type-1 receptor gene expression in neuronal cultures from spontaneously hypertensive rats. Endocrinology 132: 1715–1722.
Wu JN, Berecek KH 1993 Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension 22: 139–146.
Kadekaro M, Summy-Long JY 2000 Centrally produced nitric oxide and the regulation of body fluid and blood pressure homeostasis. Clin Exp Pharmacol Physiol 27: 450–459.
Nakata T, Takeda K, Harada S, Oguni A, Hatta T, Kawa T, Itoh H, Sasaki S, Nakagawa M 2001 Role of the central nervous system in the development of hypertension produced by chronic nitric oxide blockade in rats. Hypertens Res 24: 39–45.
Porter JP 1999 Chronic intracerebroventricular infusion of angiotensin II increases brain AT1 receptor expression in young rats. Brain Res Dev Brain Res 112: 293–295.
Jo H, Yang EK, Lee WJ, Park KY, Kim HJ, Park JS 1996 Gene expression of central and peripheral renin-angiotensin system components upon dietary sodium intake in rats. Regul Pept 67: 115–121.
Aguilera G, Kiss A, Luo X 1995 Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol 7: 775–783.
Dodic M, Abouantoun T, OConnor A, Wintour EM, Moritz KM 2002 Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension 40: 729–734.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Canadian Institutes of Health Research and the Banting Research Foundation with matching funds from the Medical Research Council of Canada. P.P. was supported by a Institut National de Santé et Recherche Médicale (France) and the Canadian Institutes of Health Research (INSERM-CIHR) research fellowship and by the Conseil Régional de Bretagne.
Rights and permissions
About this article
Cite this article
Pladys, P., Lahaie, I., Cambonie, G. et al. Role of Brain and Peripheral Angiotensin II in Hypertension and Altered Arterial Baroreflex Programmed during Fetal Life in Rat. Pediatr Res 55, 1042–1049 (2004). https://doi.org/10.1203/01.PDR.0000127012.37315.36
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000127012.37315.36
This article is cited by
-
Interaction of the pre- and postnatal environment in the maternal immune activation model
Discover Mental Health (2023)
-
Kidney and epigenetic mechanisms of salt-sensitive hypertension
Nature Reviews Nephrology (2021)
-
Nebivolol is more effective than atenolol for blood pressure variability attenuation and target organ damage prevention in L-NAME hypertensive rats
Hypertension Research (2021)
-
Anomalous AMPK-regulated angiotensin AT1R expression and SIRT1-mediated mitochondrial biogenesis at RVLM in hypertension programming of offspring to maternal high fructose exposure
Journal of Biomedical Science (2020)
-
Central nervous system neuroplasticity and the sensitization of hypertension
Nature Reviews Nephrology (2018)