Abstract
Newborn infants are susceptible to a range of problems attributed to excessive production of free radicals. Because of a higher content of antioxidants, above all bilirubin, and a lower content of oxidizable lipids, newborn plasma should be better protected against oxidation than adult plasma. To test this hypothesis, we measured the susceptibility of plasma to in vitro oxidation in microsamples (7 μL) from 57 healthy newborns and 18 adults. Heparin plasma was diluted 150-fold and oxidized by 50 μM Cu2+. Oxidation was monitored as an increase in sample absorbance at 234 nm. Plasma oxidizability was found to be significantly lower in newborns than in adults. Accordingly, the level of bilirubin, an important antioxidant, was significantly higher, and the level of polyunsaturated fatty acids, a major substrate of lipid peroxidation, was significantly lower in newborn plasma. In addition, plasma oxidizability correlated positively with the level of polyunsaturated fatty acids and negatively with that of bilirubin. These data indicate that plasma is better protected against oxidative stress in newborns than in adults, owing to its higher content of antioxidants like bilirubin and its lower content of oxidizable lipids.
Similar content being viewed by others
Main
Newborn infants, especially premature ones, are susceptible to a wide range of problems related to an excessive production of free radicals (1, 2). In utero, fetal organs are exposed to relatively hypoxic tensions, which increase abruptly after birth (3). This transition may cause oxidative injury.
In healthy humans, a balance exists between oxygen-derived free radical production and their removal by antioxidants (4). In preterm infants, inadequate antioxidant defenses may contribute to the pathogenesis of some complications of prematurity. Oxygen radical injury may be a common pathogenic mechanism in several neonatal diseases. For this reason, the term “oxygen radical disease of prematurity” was proposed for intracerebral hemorrhage, bronchopulmonary dysplasia, and retinopathy in premature neonates (1).
Antioxidants are therefore of critical importance in protecting newborns against oxygen toxicity. Low plasma antioxidant activity at birth has been reported to be an independent risk factor for mortality in preterm babies (5). The measured and calculated TRAP was higher in newborns than in adults (6) and declined postnatally with increasing age (7). Accordingly, the susceptibility of cord plasma to in vitro oxidation by Cu2+ was significantly lower in comparison with maternal plasma (8).
Increased TRAP in newborns and the decreased susceptibility of neonatal plasma to oxidation are likely related to its higher content of two potent antioxidants, namely urate and, more importantly, bilirubin, which is highly increased in newborn plasma (7, 9–11). Similarly, higher levels of selenium and other important antioxidants such as sulfhydryl groups have also been reported in newborn plasma (8, 12). This suggests that the levels of some antioxidants are up-regulated in infants to compensate for increased oxidative stress at birth.
On the other hand, some antioxidants can be decreased as a result of their increased consumption by free radicals. It is well known, for example, that plasma levels of vitamin E are lower in newborns than in adults (13, 14). However, this may also be related to lower levels of total lipids in newborn plasma (14–16). Inasmuch as oxidizable lipids are important determinants of oxidative resistance (17), their lower levels may also be responsible for higher resistance to oxidation; however, it is known that lipid peroxidation can be very high at birth irrespective of lipid composition and is closely related to acidosis and hypoxia, two pathologic conditions associated with strongly decreased levels of antioxidants (18). In comparison with other fatty acids, highly oxidizable lipids, such as PUFAs, appear to be selectively decreased after birth (15, 18).
Taken together, these data suggest that newborn plasma may be better protected against oxidation than adult plasma, owing to its higher content of antioxidants, above all bilirubin, and its lower content of oxidizable lipids. To assess this hypothesis, we measured the susceptibility to in vitro oxidation in plasma obtained from 57 infants and 18 adults and correlated it with plasma levels of bilirubin and PUFAs.
METHODS
Chemicals.
Chelex 100 resin (50–100 mesh) was obtained from Bio-Rad (Richmond, VA, U.S.A.). All other chemicals and solvents were from Sigma Chemical Co. (Deisenhofen, Germany) or Merck (Darmstadt, Germany). Reagents used for plasma oxidation were prepared in Chelex-treated, double-distilled deionized water to minimize contamination with transition metal ions.
Subjects.
The study population comprised 57 icteric healthy newborn infants (31 girls, 26 boys; median age, 6 d; range, 1–9 d) in whom measuring blood bilirubin levels and hematocrit were found necessary by the attending pediatrician and 18 adults (8 women, 10 men). Infants were born at term (37–42 postmenstrual wk) in the University Hospital Eppendorf in Hamburg. Their birth weight was 3390 ± 480 g; 49 of the newborn infants were breast-fed, five infants received adapted milk, and for three of the children the kind of feeding is not known. Except for the jaundice, all children appeared healthy. Their jaundice turned out to be physiologic and nonhemolytic, and did not require treatment. Apparently healthy adults were recruited from hospital personnel (median age, 32 y; range, 18–63 y). They did not take any medication or antioxidant supplements.
Blood sampling.
For the clinical purposes mentioned, 55 μL of blood was taken by skin puncture from the infants within the first 9 postnatal d. Blood was collected in heparin-containing hematocrit glass capillaries and immediately centrifuged in a hematocrit centrifuge. The hematocrit value was read, and the bilirubin concentration was determined photometrically in the supernatant plasma. For the purpose of this study, no extra blood was taken, and only the plasma remaining in the capillary was used. Informed parental consent was obtained for the clinically indicated withdrawal of blood, but the further use of minute plasma remainders otherwise to be discarded was not discussed with all parents. Plasma was separated and stored at 4°C for not longer than 2 h to measure its oxidizability. In addition, for a small number of newborn samples (n = 25) aliquots were stored at −80°C under argon for no longer than 2 mo to measure fatty acids. After informed consent, blood was sampled from overnight-fasted adults using the same protocol. The study has been approved by the Board of the Childrens' Hospital of the University of Hamburg.
Analytical measurements.
To characterize plasma oxidizability, samples (7 μL) were diluted 150-fold with PBS, pH 7.4, containing 0.16 M NaCl and oxidized in the presence of 50 μM Cu2+ as described previously (19). Oxidation was performed in a spectrophotometric cuvette at 37°C and monitored as a change in sample absorbance at 234 nm. This measurement is known to reflect the level of lipid hydroperoxides, which are intermediate oxidation products of the lipid compound of lipoprotein particles containing a conjugated diene structure (20). It has been shown to correlate with other indices of lipid peroxidation and provides an indicator of lipoprotein oxidation in human plasma (19, 21). The absorbance was measured every 5 min for a period of 20 h.
Bilirubin, an important antioxidant in plasma samples (22), was measured in infants during regular clinical follow-ups and in adults for comparison. Because of the limited volume of plasma available from infants, other antioxidants were not measured. Bilirubin was measured photometrically using a commercially available analytical bilirubinometer (Bilimeter; Ortho Diagnostic Systems, Neckargemund, Germany).
Fatty acid composition of plasma was characterized by capillary gas chromatography as described elsewhere (23). The concentration of total fatty acids was calculated as a sum of concentrations of linoleic, linolenic, γ-linolenic, eicosadienoic, eicosatrienoic, arachidonic, eicosapentaenoic, docosadienoic, docosatetraenoic, docosapentaenoic, and docosahexaenoic acids (PUFAs); palmitoleic, oleic, vaccenic, eicosaenoic, docosaenoic, and nervonic acids (MUFAs); and myristic, palmitic, stearic, arachidic, behenic, and lignoceric acids (SFAs).
Statistical analysis.
Between-group differences in continuous variables were analyzed by Mann-Whitney U test. Spearman's rank correlation coefficients were calculated to evaluate relationships between variables. The results are presented as mean ± SD.
RESULTS
When plasma samples from infants and adults were diluted 150-fold with PBS and oxidized by 50 μM Cu2+, their absorbance at 234 nm was found to increase consistently (Fig. 1A). In accordance with previously reported data (19, 21, 24), the absorbance increase of adult plasma was characterized by three consecutive phases similar to the lag, propagation, and decomposition phases of LDL oxidation (25, 26). This time course of the absorbance at 234 nm has been shown to reflect the oxidation of plasma lipoproteins (19, 21, 24). The oxidation curves obtained with these samples were therefore described using variables normally applied to characterize LDL oxidation curves, i.e. the lag phase duration, the mean oxidation rate within the lag phase, and the maximal oxidation rate within the propagation phase (19, 25, 26).
Typical time courses of Cu2+-induced oxidation of plasma obtained from adults (A) and newborns (B). Heparinized plasma (7 μL) was diluted 150-fold with PBS containing 0.16 M NaCl and oxidized at 37°C by 50 μM Cu2+. Oxidation was measured as an increase in sample absorbance at 234 nm. Time courses recorded with representative samples of adult plasma (n = 5) and newborn plasma (n = 5) are shown. In adult samples, we always observed a lag phase, a propagation phase, and a decomposition phase. In the newborn samples, the majority of the samples showed a slow monotonic oxidation rate (sample ο, +). Only in nine of 57 samples, a propagation phase could be identified (sample Δ, −), and in some samples (seven of 59) a very fast increase in absorbance, followed by a decomposition phase, (sample ⋄) was shown.
In contrast, the absorbance increase in most plasma samples obtained from newborns was characterized by the absence of any clear lag phase, which was only observed in nine of 57 samples (Fig. 1B). Newborn samples normally exhibited a monotonic increase in their absorbance at 234 nm. In some (seven of 57) samples, a very fast increase in absorbance was observed (Fig. 1B). The oxidation curves obtained with the newborn samples were therefore described using a single variable, i.e. the mean oxidation rate within the first monotonic phase of oxidation. The mean oxidation rate within the lag phase and the mean oxidation rate within the first monotonic phase of oxidation were interpreted as the initial rates of plasma oxidation, because these phases started immediately after addition of Cu2+ to the plasma. When triphasic kinetics were seen in newborn samples, the maximal oxidation rate during the propagation phase and the duration of the lag phase were calculated as for adult samples.
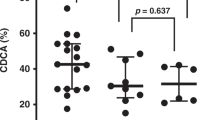
When the initial rate of plasma oxidation was compared between newborns and adults, it was found to be significantly lower in the newborn samples (Fig. 2A). When the maximal oxidation rate within the propagation phase was calculated for those samples which revealed triphasic kinetics (all adult and nine infant samples), it was significantly lower in the newborn samples, too (Fig. 2B). In accordance, the lag phase duration calculated for those samples tended to be higher in the infant group (Fig. 2C).
Oxidation rates in the lag phase (A) and propagation phase (B) and the lag phase duration (C) measured in plasma from newborns and adults. Oxidation rate in the lag phase was calculated as an oxidation rate averaged for the first linear oxidation phase. Oxidation rates in the propagation phase and the lag phase duration were only calculated when a propagation phase was observed. Open circles represent median values for each group. Dotted lines correspond with the median value and interquartile range for the infant group. Significance of the difference between the groups is shown as a p value.
The initially lower oxidation rate measured in the newborn plasma samples was accompanied by a lower plasma content of fatty acids compared with the adult samples (Fig. 3A). The absolute concentration of total fatty acids and the concentrations of all their major groups (SFAs, MUFAs, and PUFAs) were significantly lower in newborns. However, when plasma concentrations of SFAs, MUFAs, and PUFAs were calculated as a percentage of total fatty acids, only PUFAs, the major substrate for lipid peroxidation, were significantly lower in the newborn group (Fig. 3B). In contrast, the relative content of SFAs and MUFAs was significantly higher in newborns than in adults.
Bilirubin was measured as an important antioxidant in plasma samples (22). As expected, the bilirubin levels were significantly higher in newborns (median, 8.3 mg/dL; range, 1.1–16.3 mg/dL) than in adults (median, 0.9 mg/dL; range, 0.5–2.1 mg/dL;p < 0.001), and bilirubin levels of the newborn plasma samples were compared among the three groups of plasma oxidation patterns. The bilirubin levels of the newborns showing a very fast absorbance increase during plasma oxidation were decreased (median, 6.40 mg/dL; range, 2.20–13.20 mg/dL;n = 7;p < 0.05) compared with the infant group with monotonic increase of absorbance (median, 8.80 mg/dL; range, 1.10–16.30 mg/dL;n = 41). No difference was seen between the group showing lag phase (median, 9.20 mg/dL; range, 2.40–14.10 mg/dL;n = 9;p = 0.37) and the group with a very fast absorbance increase during plasma oxidation (see above), which was likely related to the low number of samples in these groups.
Plasma oxidizability, expressed as initial or maximal oxidation rate, revealed several significant correlations with fatty acids and bilirubin. In both the whole study population and in the adult group, the initial oxidation rate correlated positively with total fatty acids and PUFAs (Table 1). However, the correlation with PUFAs, expressed as a percentage of total fatty acids, was only significant in the whole study population. In addition, the initial oxidation rate correlated negatively with bilirubin in the whole population and in the newborn group. Similarly, the maximal oxidation rate correlated positively with total fatty acids and PUFAs and negatively with bilirubin in the whole study population (Table 1).
DISCUSSION
In this study, we compared the susceptibility to in vitro oxidation by Cu2+ in plasma of newborns and adults. We found that plasma oxidizability was significantly lower in newborn than in adult plasma. The level of bilirubin was significantly higher and the level of PUFAs was significantly lower in newborn plasma. In addition, plasma oxidizability correlated positively with the level of PUFAs and negatively with that of bilirubin.
These data indicate that plasma is better protected against oxidative stress in newborns than in adults. This conclusion is in agreement with the results of previous studies. The susceptibility of cord plasma to in vitro oxidation by Cu2+ has been recently reported to be lower in comparison with maternal plasma (8). The measured and calculated TRAP values were also higher in newborns than in adults (6) and decreased postnatally with age (7).
Our data suggest that the better protection of infant plasma against oxidation is related to its higher content of bilirubin and lower content of PUFAs. Bilirubin is an important antioxidant in human plasma (22), which, because of its high plasma levels, is especially important in newborns (8). Analysis of the relationship between the total plasma antioxidant activity and the bilirubin concentration shows a direct, highly significant correlation for the term babies (9). Total antioxidant status of newborn plasma is also directly related to plasma bilirubin levels in preterm infants (27). In addition, in jaundiced infants the antioxidant capacity of neonatal plasma correlated positively with plasma bilirubin concentrations (10).
The importance of bilirubin in protecting plasma against Cu2+-induced oxidation is in agreement with a negative correlation between the maximal plasma oxidation rate by Cu2+ and plasma bilirubin concentration recently reported by us for adult plasma (28). This is in conformity with the observed stronger correlation between bilirubin level and maximal oxidation rate versus initial oxidation rate calculated in the present study (Table 1). The bilirubin levels of the newborns showing a very fast absorbance increase during plasma oxidation were decreased. These data indicate that bilirubin delays late stages of plasma oxidation. Mechanisms of its antioxidative action may involve direct free radical scavenging and recycling of α-tocopherol in plasma lipoproteins (22).
It should be noted that urate is another important hydrophilic antioxidant that may account for the better plasma protection against oxidation in infants. It is higher in infant than in adult plasma (8). Antioxidant status of newborn plasma is directly correlated with plasma urate levels (11, 27). In addition, a postnatal decrease in TRAP appears to be related to the coincident fall in urate concentrations (7). Owing to the limited volume of plasma available, urate was not measured in our study.
Other antioxidants, such as selenium and sulfhydryl groups, can be higher in infant plasma as well and contribute to its better protection against oxidation compared with adult plasma (8, 12). In contrast, low levels of vitamin E (13) and carotenoids (14) observed in newborns seem to have no negative impact on plasma oxidative resistance. This is in agreement with low levels of lipids in newborn plasma (14–16), which need to be protected by vitamin E and other lipophilic antioxidants. When compared with the total lipid plasma concentration, levels of vitamin E are only slightly subnormal (29).
Low levels of PUFAs, too, are indicative of low oxidizability of newborn plasma. PUFAs represent a measure of the amount of substrate available for lipid peroxidation in plasma samples. The fetus obtains fatty acids from a combination of de novo synthesis, passive transplacental passage of nonesterified fatty acids, and selective placental transport of certain fatty acids, such as physiologically important long-chain PUFAs (15). The latter are essential for normal lipid metabolism of the fetus. However, besides their essential physiologic functions, PUFAs also accelerate plasma oxidation. This indicates that supplementation of infants with high amounts of these easily oxidizable lipids can theoretically increase plasma susceptibility to oxidation. This potential drawback must be experimentally addressed in the future.
It is interesting to note that the increased resistance to oxidation measured in newborn plasma is paralleled by an increased content of various oxidation products, such as vitamin E quinone (16) or the oxidized form of coenzyme Q10 (18). This finding additionally emphasizes the increased oxidative stress to which newborn plasma is subjected in vivo.
Antioxidative properties of plasma should be of a great importance in vivo. For example, it has been shown that plasma from adults and from term babies is able to inhibit Fe3+-catalyzed lipid peroxidation in vitro(30). In contrast, plasma from preterm babies often stimulates peroxidation. The ability to stimulate peroxidation is associated with the presence of non–protein-bound iron and has been proposed to contribute to the pathogenesis of the respiratory distress syndrome (30). The diminished capacity of cord blood plasma to prevent Fe3+-induced lipid peroxidation may predispose the newborn baby to the toxic effects of oxygen (31). The differences in the role of iron in the development of oxidative stress in the newborn, particularly the preterm neonate, depend not only on prematurity but also mainly on asphyxia and acidosis (32).
However, the clinical relevance of the measurement of plasma resistance to oxidation remains to be determined. In one study, low plasma antioxidant activity at birth has been reported to be an independent risk factor for mortality in preterm babies (5), although this conclusion was not confirmed by others (11). It should be noted that in both studies (5, 11) the TRAP variable was measured, which is used to assess the overall ability of all plasma constituents to scavenge free radicals. Such an overwhelming oxidation is unlikely to occur in vivo, where much milder oxidative conditions have been proposed [see Stocker (33) for discussion]. Therefore, a variable related to earlier stages of plasma oxidation can be expected to better reflect its oxidation in vivo. This could be the initial rate of plasma oxidation in the presence of a high amount of oxidant, such as measured in our study, or rate of plasma oxidation in the presence of a low amount of oxidant averaged over the whole (long enough) oxidation time, as we have recently proposed (19). Whatever the case, this may provide a basis for the use of our method as a means to assess plasma oxidizability in future research.
It should be mentioned that we used plasma oxidation by Cu2+ to model in vivo lipoprotein oxidation. The increase in absorbance of oxidizing plasma or serum at 234 nm has been found to reflect the oxidation of plasma lipoproteins (20). Furthermore, it has been shown to correlate also with other indices of lipid peroxidation and to represent an indicator of lipoprotein oxidation in human plasma (19, 21).
However, it is currently unclear to what extent the results obtained from such a model are applicable to biologic systems (33), although there is some evidence that transition metal ions may be involved in the pathologic oxidation process in vivo(34). On the other hand, the Cu2+-induced model of lipoprotein oxidation offers practical advantages with respect to simplicity, good reproducibility, and efficient sample processing (19, 35).
The plasma oxidation assay used in our study requires only small sample volumes (5–10 μL), which is particularly useful when only low amounts of blood are available as, for example, with newborn infants. It can therefore be used as a micromethod. Other advantageous features of the assay include fast, simple, and efficient sample processing, avoidance of artifactual oxidation during lipoprotein isolation, and simple photometric registration of the oxidation course. The assay also permits a qualitatively correct estimation of anti- and prooxidative action of major plasma constituents. Another advantage is that, in contrast to TRAP-like assays, lipophilic antioxidants and fatty acids play an important role in the oxidative process. Taken together, this suggests that plasma oxidizability measured as an increase in absorbance at 234 nm may be proposed as a practical measure of oxidizability of plasma lipoproteins in newborns.
Abbreviations
- MUFA:
-
monounsaturated fatty acid
- PUFA:
-
polyunsaturated fatty acid
- SFA:
-
saturated fatty acid
- TRAP:
-
total radical-trapping ability of plasma
References
Sullivan JL 1988 Iron, plasma antioxidants, and the ‘oxygen radical disease of prematurity’. Am J Dis Child 142: 1341–1344
Huertas JR, Palomino N, Carrasco R, Quiles J, Ramirez-Tortosa MC, Ochoa J, Cassinello M, Battino M, Robles R, Mataix J 1997 Lipid peroxidation and antioxidants in newborns. Mol Aspects Med 18( suppl): S229–S232
Evans HE, Rosenfeld W, Jhaveri R, Concepcion L, Zabaleta I 1986 Oxidant-mediated lung disease in newborn infants. J Free Radic Biol Med 2: 369–372
Halliwell B 1994 Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? (see comments). Lancet 344: 721–724
Silvers KM, Gibson AT, Russell JM, Powers HJ 1998 Antioxidant activity, packed cell transfusions, and outcome in premature infants. Arch Dis Child Fetal Neonatal Ed 78: F214–F219
Lindeman J, van Zoeren-Grobben D, Schrijver J, Speek A, Poorthuis B, Berger H 1989 The total free radical trapping ability of cord blood plasma in preterm and term babies. Pediatr Res 26: 20–24
van Zoeren-Grobben D, Lindeman JH, Houdkamp E, Brand R, Schrijver J, Berger HM 1994 Postnatal changes in plasma chain-breaking antioxidants in healthy preterm infants fed formula and/or human milk. Am J Clin Nutr 60: 900–906
Kiely M, Morrissey PA, Cogan PF, Kearney PJ 1999 Low molecular weight plasma antioxidants and lipid peroxidation in maternal and cord blood. Eur J Clin Nutr 53: 861–864
Gopinathan V, Miller NJ, Milner AD, Rice Evans CA 1994 Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett 349: 197–200
Belanger S, Lavoie JC, Chessex P 1997 Influence of bilirubin on the antioxidant capacity of plasma in newborn infants. Biol Neonate 71: 233–238
Luukkainen P, Aejmelaeus R, Alho H, Metsa-Ketela T, Ikonen SR, Salo MK 1999 Plasma chain-breaking antioxidants in preterm infants with good and poor short-term outcome. Free Radic Res 30: 189–197
Araujo V, Ruiz E, Llovera M, Tokashiki N, Abellan C, Dominguez C 1998 Impact of oxygen therapy on antioxidant status in newborns: relationship with infection risk. Biofactors 8: 143–147
Gutcher GR, Raynor WJ, Farrell M 1984 An evaluation of vitamin E status in premature infants. Am J Clin Nutr 40: 1078–1089
Kiely M, Cogan PF, Kearney PJ, Morrissey PA 1999 Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur J Clin Nutr 53: 711–715
Berghaus TM, Demmelmair H, Koletzko B 1998 Fatty acid composition of lipid classes in maternal and cord plasma at birth. Eur J Pediatr 157: 763–768
Jain SK, Wise R, Bocchini JJ 1996 Vitamin E and vitamin E-quinone levels in red blood cells and plasma of newborn infants and their mothers. J Am Coll Nutr 15: 44–48
Kontush A, Hubner C, Finckh B, Kohlschutter A, Beisiegel U 1996 How different constituents of low density lipoprotein determine its oxidizability by copper: a correlational approach. Free Radic Res 24: 135–147
Hara K, Yamashita S, Fujisawa A, Ishiwa S, Ogawa T, Yamamoto Y 1999 Oxidative stress in newborn infants with and without asphyxia as measured by plasma antioxidants and free fatty acids. Biochem Biophys Res Commun 257: 244–248
Kontush A, Beisiegel U 1999 Measurement of oxidizability of blood plasma. Methods Enzymol 299: 35–49
Esterbauer H, Striegl G, Puhl H, Oberreither S, Rotheneder M, el-Saadani M, Jurgens G 1989 The role of vitamin E and carotenoids in preventing oxidation of low density lipoproteins. Ann NY Acad Sci 570: 254–267
Regnstrom J, Strom K, Moldeus P, Nilsson J 1993 Analysis of lipoprotein diene formation in human serum exposed to copper. Free Radic Res Commun 19: 267–278
Neuzil J, Stocker R 1994 Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem 269: 16712–16719
Kontush A, Hubner C, Finckh B, Kohlschutter A, Beisiegel U 1994 Low density lipoprotein oxidizability by copper correlates to its initial ubiquinol-10 and polyunsaturated fatty acid content. FEBS Lett 341: 69–73
Schnitzer E, Pinchuk I, Fainaru M, Schafer Z, Lichtenberg D 1995 Copper-induced lipid oxidation in unfractionated plasma: the lag preceding oxidation as a measure of oxidation-resistance. Biochem Biophys Res Commun 216: 854–861
Esterbauer H, Striegl G, Puhl H, Rotheneder M 1989 Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun 6: 67–75
Kleinveld HA, Hak-Lemmers HL, Stalenhoef AF, Demacker PN 1992 Improved measurement of low-density-lipoprotein susceptibility to copper-induced oxidation: application of a short procedure for isolating low-density lipoprotein. Clin Chem 38: 2066–2072
Drury JA, Nycyk JA, Baines M, Cooke RW 1998 Does total antioxidant status relate to outcome in very preterm infants?. Clin Sci Colch 94: 197–201
Spranger T, Finckh B, Fingerhut R, Kohlschutter A, Beisiegel U, Kontush A 1998 How different constituents of human plasma and low density lipoprotein determine plasma oxidizability by copper. Chem Phys Lipids 91: 39–52
van Zoeren-Grobben D, Jacobs NJ, Houdkamp E, Lindeman JH, Drejer DF, Berger HM 1998 Vitamin E status in preterm infants: assessment by plasma and erythrocyte vitamin E-lipid ratios and hemolysis tests. J Pediatr Gastroenterol Nutr 26: 73–79
Moison RM, Palinckx JJ, Roest M, Houdkamp E, Berger HM 1993 Induction of lipid peroxidation of pulmonary surfactant by plasma of preterm babies. Lancet 341: 79–82
Lindeman JH, Houdkamp E, Lentjes EG, Poorthuis BJ, Berger HM 1992 Limited protection against iron-induced lipid peroxidation by cord blood plasma. Free Radic Res Commun 16: 285–294
Dorrepaal CA, Berger HM, Benders MJNL, van Zoeren-Grobben D, Van de Bor M, Van Bel F 1996 Nonprotein-bound iron in postasphyxial reperfusion injury of the newborn. Pedatrics 98: 883–889
Stocker R 1994 Lipoprotein oxidation: mechanistic aspects, methodological approaches and clinical relevance. Curr Opin Lipidol 5: 422–433
Mukhopadhyay CK, Mazumder B, Lindley PF, Fox PL 1997 Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc Natl Acad Sci USA 94: 11546–11551
Kontush A, Spranger T, Reich A, Djahansouzi S, Karten B, Braesen JH, Finckh B, Kohlschutter A, Beisiegel U 1997 Whole plasma oxidation assay as a measure of lipoprotein oxidizability. Biofactors 6: 99–109
Acknowledgements
We thank Barbara Sehringer-Mansour for careful measurement of plasma fatty acids and Dr. David Evans for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant Klinische Forschergruppe Gr 258/10-1, Deutsche Forschungsgemeinschaft.
This study contains essential data from the thesis work of M.W.
Rights and permissions
About this article
Cite this article
Wiedemann, M., Kontush, A., Finckh, B. et al. Neonatal Blood Plasma Is Less Susceptible to Oxidation Than Adult Plasma Owing to Its Higher Content of Bilirubin and Lower Content of Oxidizable Fatty Acids. Pediatr Res 53, 843–849 (2003). https://doi.org/10.1203/01.PDR.0000057983.95219.0B
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000057983.95219.0B
This article is cited by
-
Is neonatal phototherapy associated with a greater risk of childhood cancers?
BMC Pediatrics (2022)
-
The Effects of Parenteral Iron Administration on Thyroid Hormones, Hematology, Oxidative Stress Characteristics, Performance, and Health in Neonatal Holstein Calves
Biological Trace Element Research (2021)
-
UGT1A1*28 polymorphism and acute lymphoblastic leukemia in children: a Danish case–control study
Pediatric Research (2014)
-
Neonatal blood is more resistant to oxidative stress induced by stable nitroxide radicals than adult blood
Archives of Gynecology and Obstetrics (2008)