Abstract
Reflexes from the larynx induce cessation of breathing in newborn animals. The magnitude of respiratory inhibition is inversely related to the level of central chemical input. Recent studies indicate that selective inhibition of Na+/H+ exchanger type 3 (NHE3) activates CO2/H+-sensitive neurons, resembling the responses evoked by hypercapnic stimuli. Hence, the use of NHE3 inhibitors may reduce reflexly mediated respiratory depression and duration of apnea in the neonatal period. This possibility was examined in decerebrate, vagotomized, ventilated, and paralyzed piglets by testing the effects of i.v. administration of NHE3 blocker S8218 on the response of phrenic nerve amplitude, frequency, and duration of apnea induced by graded electrical stimulation of the superior laryngeal nerve. Superior laryngeal nerve stimulation caused a significant decrease in phrenic nerve amplitude, frequency, minute phrenic activity, and inspiratory time (all p < 0.01) that was proportional to the level of electrical stimulation. Increased levels of stimulation were more likely to induce apnea both during and after cessation of stimulation. NHE3 blocker S8218 reduced the superior laryngeal nerve stimulation–induced decrease in phrenic nerve amplitude, minute phrenic activity, and phrenic nerve frequency (all p < 0.05) and reduced superior laryngeal nerve stimulation-induced apnea and duration of poststimulation apnea (p < 0.05). In six other pigs the brain concentrations of S8218 were measured at different intervals after i.v. administration of the drug and were found to be higher in the brain tissue than plasma at all intervals. These findings suggest that the use of NHE3 blockers may decrease the duration of apnea and possibly reduce the pathophysiologic consequences of potentially life-threatening apnea in infants.
Similar content being viewed by others
Main
Disorders of rhythmic breathing, including apnea, are common in premature infants. If prolonged or frequent, apnea may impair gas exchange and have adverse reflex cardiovascular consequences. It has been shown that infants who exhibited periodic apnea of more than 20 s duration have a higher incidence of intraventricular hemorrhage, hydrocephalus, need for mechanical ventilation, and abnormal neurologic development during the first year of life (1). Apnea has also been associated with impaired cognitive function and poor academic achievement in early school-age children (2). Furthermore, apnea of infancy requiring treatment with theophylline was associated with later development of cerebral palsy, possibly reflecting the effects of repeated hypoxia induced by such apneic episodes (3). However, a cause and effect relationship between poor neurologic outcome and apnea is not established.
Depression and cessation of breathing can be elicited by multiple pathways, including laryngeal reflexes, which are enhanced in premature infants and in newborn animals (4–7). Newborn animals respond to instillation of water or some other liquids to the laryngeal mucosa with a sustained apnea, which may lead to death unless the offending material is removed (8, 9). The apneic response is mediated through SLN afferents, and its magnitude during early postnatal life is inversely related to the level of central chemical input (7, 10, 11).
Recent studies showed that selective inhibition of NHE3 activates CO2/H+-sensitive neurons within the ventrolateral medulla oblongata, resembling the responses evoked by hypercapnic stimuli (12, 13). This raised the hypothesis that NHE3 inhibitors may reduce reflexly mediated respiratory depression and duration of apnea in the neonatal period. This assumption was tested in the current study by examining the effects of NHE3 blockade on the decrease of phrenic nerve amplitude, slowing of breathing frequency, and duration of reflex apnea induced by graded stimulation of the SLN in newborn piglets. The results showed that the extent of respiratory depression and duration of central apnea induced by laryngeal stimulation during early postnatal life could be reduced by blockade of NHE3.
METHODS
Experimental design.
Newborn piglets 5–10 d old were purchased from a local vendor (n = 6). The study protocol was approved by the Institutional Animal Care and Use Committee. The body temperature was maintained at 37–38°C using a warming pad. Animals were initially sedated with a mixture of xylazine 2.8 mg/kg and ketamine 14.4 mg/kg (intramuscular), and then anesthetized with i.v. thiopental (25–30 mg/kg). The trachea was intubated, and the animals were placed on a volume ventilator (Harvard Apparatus, Inc., Holliston, MA, U.S.A.). The volume and frequency of tidal breaths were adjusted to keep a stable eucapnic Paco2 of 35–40 mm Hg confirmed by an end-tidal CO2 analyzer, and inspired O2 concentration was maintained at 100%. A peripheral i.v. line was placed, and a catheter was inserted in the femoral artery for blood pressure monitoring and blood gas measurements.
To avoid the confounding effects of anesthesia on breathing and the laryngeal reflex (5, 7, 14), experiments were performed after midcollicular decerebration, which was performed as we have previously described (15). After the entire surgery was completed anesthesia was discontinued, and the animals were allowed to recover for at least 1 h before any experimental recordings were obtained. Gallamine triethiodide (Flaxedil, 20 mg/kg, Sigma, St. Louis, MO, U.S.A.) was used for paralysis.
The phrenic nerve was dissected, desheathed, and placed on a bipolar recording electrode. The phrenic nerve ENG was amplified (P511, Grass Instruments, Quincy, MA, U.S.A.) and rectified, and the moving average signal was fed to a strip-chart recorder and to a computer analog. Bilateral midcervical vagotomy was performed. The SLN was identified on one side as it exited from the nodose ganglion, dissected, desheathed, and placed on a bipolar electrode connected to a stimulator (S11, Grass Instruments). The SLN stimulation threshold was identified as the amount of electrical current needed to decrease the phrenic amplitude by 20%. Stimulation was performed using impulses of constant voltage and frequency (25 Hz) and variable current applied through a photoelectric isolation unit (PSIU6, Grass Instruments).
The phrenic nerve ENG was measured in response to multiple levels of SLN stimulation (threshold, 1.5 times threshold, 2 times threshold, and 4 times threshold), each of 15-s duration, before and 15 min after i.v. administration of NHE3 blocker S8218. The average cumulative dose of S8218 was 9.7 ± 1.5 mg/kg (range, 3–20 mg/kg per piglet). Five of the six animals received more than one dose, and the sixth animal received only one dose of 3 mg/kg. The effects of the following concentrations were studied: one animal received 1 mg/kg, four animals received 3–4 mg/kg, four animals received 10–11 mg/kg, one animal received 14 mg/kg, and two animals received 20 mg/kg. S8218 was obtained from Aventis Pharma (Frankfurt, Germany).
To estimate the ability of S8218 to cross the blood–brain barrier, the brain and plasma levels of S8218 were studied in six male German Landrace pigs (weight, 26–28 kg; 2–3 mo of age). The levels of S8218 were measured in two pigs each at 15 min, 45 min, and 4 h after completion of a 15-min infusion of S8218 at 3 mg/kg. At the times indicated the brains of the animals were dissected and weighed. Thereafter an identical weight of saline was added, and the mixture was homogenized by means of Ultra-Turrax appliances (Jahne & Kunkel KG IKA-Werk, Staufen, Germany). The concentrations of S8218 in plasma and brain homogenates were determined by HPLC/MS/MS (API 365, Sciex Applied Biosystems). The limit of quantification was 0.01 μg/mL in plasma and blood samples and 0.02 μg/mL in brain (brain homogenate) samples.
Data collection and statistical analysis.
The physiologic data were collected and analyzed using a custom-made computer program. Phrenic nerve discharge at baseline was analyzed for 1 min before SLN stimulation, then for 15 s at each level of stimulation before and after i.v. administration of S8218. Phrenic ENG was analyzed to reflect changes in phrenic nerve amplitude, TI, TE, and frequency during different levels of stimulation, as well as the duration of apnea after cessation of stimulation. Phrenic nerve amplitude was expressed as a percent of the control value, control being 100%.
One-way ANOVA was used to test the effect of increasing levels of SLN stimulation on phrenic nerve activity, both before and after i.v. administration of the drug S8218. Two-way ANOVA was used to test the effect of S8218 on phrenic nerve responses to SLN stimulation, with Newman-Keuls test for post hoc comparisons. We initially used only one dose per animal for this statistical analysis, and this dose was the one repeated in most animals (10–14 mg/kg); however, in one animal we used only one dose (3 mg/kg) and that dose was used for the statistics. We performed another statistical analysis using all doses administered, and the results of that analysis were comparable to that using only one dose. Therefore, in the average data and graphs we used all collected data. Friedman test was used to compare the duration of poststimulation apnea before and after S8218. The results are presented as mean ± SEM, and a p value less than 0.05 was considered to be statistically significant.
RESULTS
SLN stimulation.
Stimulation of the SLN before administration of NHE3 blocker S8218 caused a significant decrease in phrenic nerve amplitude, frequency, TI, and minute phrenic activity (all p < 0.01), which was proportional to the level of stimulation. Maximal electrical stimulation (at 4 times threshold) caused apnea in all piglets. Electrical stimulation at 2 times threshold caused a decrease in phrenic nerve amplitude to 12 ± 12% of control (p < 0.001) and a decrease in phrenic nerve frequency from 35 ± 3/min to3 ± 3/min (p < 0.001). The decrease in frequency was secondary to significant prolongation of TE, from 1.1 ± 0.1 s to 34.1 ± 16.9 s (p < 0.05). SLN stimulation caused apnea (total cessation of phrenic activity throughout stimulation) in a greater number of piglets as the level of stimulation increased: three of six piglets at 1.5 times threshold, five of six piglets at 2 times threshold, and in all piglets at 4 times threshold. The duration of poststimulation apnea was also directly related to the level of electrical stimulation, ranging from 0.9 ± 0.8 s at 1.5 times threshold stimulation to apnea of 27 ± 18 s duration at 4 times threshold.
Intravenous NHE3 blocker (S8218).
Administration of S8218 caused variable and transient changes in the baseline amplitude and frequency of phrenic activity. Overall, there was no significant effect of S8218 on the baseline phrenic amplitude activity (average increase of 15 ± 7%). Amplitude of phrenic discharge activity increased after S8128 administration on seven occasions by a mean of 32 ± 7%, remained unchanged on two occasions, and decreased on three occasions by a mean of 13 ± 2%. Similarly, S8218 had no significant effect on baseline phrenic frequency (35 ± 3/min and 33 ± 2/min before and after S8218 administration, respectively).
There was a significant effect of NHE3 blocker S8218 on the phrenic nerve amplitude and frequency responses to SLN stimulation (p < 0.05; two-way ANOVA). The lower doses of S8218 (3–4 mg/kg) were as effective as higher doses in blocking phrenic nerve inhibition in response to SLN stimulation beyond 1.5 times threshold stimulation (Fig. 1).
Effect of multiple doses of S8218 on phrenic nerve amplitude response to increasing levels of SLN stimulation. The amplitude response is presented as percentage of baseline, baseline being 100%. SLN stimulation caused a significant decrease in phrenic nerve amplitude that was proportional to the level of stimulation (p < 0.01). S8218 attenuated the decrease in phrenic nerve amplitude in response to SLN stimulation. Lower doses of S8218 (3–4 mg/kg) were as effective as higher doses (10–20 mg/kg) in attenuating the phrenic nerve response to SLN stimulation.
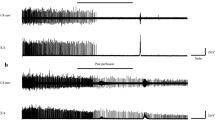
Figure 2 shows the effect of NHE3 blocker S8218 on the phrenic nerve amplitude and duration of apnea induced by SLN stimulation. In this animal, S8218 increased peak phrenic nerve activity and completely blocked apnea induced by SLN stimulation. In addition, in this as in other animals, i.v. S8218 reduced the SLN stimulation-induced decrease in phrenic nerve amplitude and phrenic nerve frequency (Figs. 3 and 4). At maximal SLN stimulation, which elicited apnea before NHE3 blockade, the phrenic nerve amplitude was 29 ± 11% of baseline and the frequency changed from 32 ± 2/min to 11 ± 4/min (p < 0.05). S8218 inhibited the decrease in phrenic nerve frequency with SLN stimulation through inhibition of the prolongation in TE. Maximal electrical stimulation caused TE to decrease from 45.1 ± 17.4 s before to 12.5 ± 2.7 s after S8218 administration. S8218 also attenuated significantly the decrease in TI in response to SLN stimulation (p < 0.01;Fig. 5).
Tracing of the phrenic nerve ENG (Phr ENG) and end-tidal Pco2 (ET CO2) at 1.5 times threshold stimulation before and after S8218 administration. SLN stimulation before S8218 caused apnea that persisted beyond the duration of stimulation. The same level of stimulation after i.v. S8218 failed to inhibit phrenic nerve activity.
Effect of S8218 on phrenic nerve amplitude response to increasing levels of SLN stimulation. The amplitude response is represented as percentage of baseline, baseline being 100%. Control data are presented by an open circle (○ and data after S8218 by a closed circle (•). SLN stimulation caused a significant decrease in phrenic nerve amplitude that was proportional to the level of stimulation (p < 0.01). S8218 attenuated the decrease in phrenic nerve amplitude in response to SLN stimulation (p < 0.05).
Effect of S8218 on phrenic nerve frequency response to multiple levels of SLN stimulation. Control data are presented by an open circle (○, and data after S8218 administration by a closed circle (•). SLN stimulation caused a significant decrease in phrenic nerve frequency that was proportional to the level of stimulation (p < 0.01). S8218 attenuated the decrease in phrenic nerve frequency in response to SLN stimulation (p < 0.05).
Phrenic nerve respiratory timing response to multiple levels of SLN stimulation before and after S8218. Control data are presented by an open circle (○, and data after S8218 by a closed circle (•). SLN stimulation caused a significant shortening of TI (left) and significant prolongation of TE (right; both p < 0.01). S8218 attenuated both the shortening in TI (p < 0.01) and the prolongation in TE in response to SLN stimulation (p < 0.05).
The NHE3 blocker S8218 almost abolished the episodes of apnea in response to SLN stimulation. In the six piglets studied, apnea occurred in no animal at 1.5 times threshold, in one animal at 2 times threshold, and in four animals at 4 times threshold. S8218 also decreased the duration of poststimulation apnea, 17.3 ± 9.2 s versus 0.9 ± 0.7 s, before and after maximal S8218 dose, respectively.
Blood pressure and acid-base balance.
There was a transient but significant decrease in arterial blood pressure during administration of the NHE3 inhibitor S8218 that recovered completely within minutes after stopping the infusion and before SLN stimulation. The mean arterial blood pressures decreased from 60 ± 5 mm Hg to 38 ± 3 mm Hg after S8218 administration (p < 0.05). Administration of S8218 did not affect the acid-base status of the animals. The blood pH was 7.40 ± 0.03 and 7.38 ± 0.03 before and after S8218, respectively (NS). Paco 2 also was not affected by S8218 administration; Paco 2 levels were 39.2 ± 1.2 mm Hg and 38.2 ± 1.7 mm Hg before and after S8218, respectively.
Pharmacokinetics of S8218.
Table 1 summarizes the plasma and brain levels of S8128 at different intervals after infusion. Concentrations of S8218 were higher in the brain tissue than in plasma (brain versus plasma ratio varied between 14 and 60). These data indicate the ability of S8218 to cross the blood–brain barrier adequately and concentrate in the brain tissues long after it disappears from plasma.
DISCUSSION
Reflex-induced apnea elicited through stimulation of the laryngeal mucosa has been well characterized in humans and animals of different species. This reflex apnea is mediated through the SLN and is abolished by bilateral sectioning of the SLN (5, 7, 16). Persistence of an exaggerated reflex-induced apnea in preterm and term infants might predispose to multiple pathologic processes such as gastroesophageal reflux–induced apnea (6), apnea of prematurity (17), and sudden infant death syndrome (18–20). The results of the present study show for the first time that blockade of NHE3 significantly inhibits the decrease in phrenic nerve amplitude, minute phrenic activity, and phrenic nerve frequency elicited by SLN stimulation, in addition to reducing reflex apnea and duration of poststimulation apnea.
Repeated stimulation of the SLN in newborn piglets does not seem to affect the degree of phrenic nerve inhibition; on the contrary it might result in greater inhibition of phrenic activity (16). Therefore, the effect of administering S8218 on the phrenic nerve response to SLN stimulation could not have been secondary to repeated stimulation; if anything, repeated stimulation should have caused more inhibition. Furthermore, repeated stimulation of SLN was not attempted until enough time was allowed for recovery of phrenic nerve activity.
There appears to be similarity between the effects of NHE3 inhibition and breathing an elevated inspired CO2 fraction on the magnitude of respiratory depression induced by laryngeal reflexes. Recently, our group showed that the respiratory responses to SLN stimulation differ under conditions that appear to alter the level of central chemosensitivity in piglets. Hypercapnia increases, whereas hypocapnia decreases, the threshold for SLN stimulation–induced apnea (10, 11). Cooling of the ventromedullary surface, a technique used to decrease central chemosensitivity by inhibiting synaptic transmission at this site, decreased the threshold for laryngeal stimulation–induced apnea (11). Theophylline, which stimulates respiratory neural output, has been shown to block SLN stimulation–induced apnea (7). These data indicate that the magnitude of respiratory inhibition induced by laryngeal stimulation during early postnatal life is inversely related to the level of central chemical input. Hence, immaturity and functional deficiency of central chemosensory elements may contribute to the expression of potentially life-threatening apnea in infants.
Despite increasing knowledge of central chemosensitivity accumulated during the last five decades, there is a growing need to better understand the mechanisms involved in sensing changes in CO2/H+ content of extracellular fluid. From recent studies it is assumed that a decrease in pHi is a substantial part of the CO2/H+ sensor–transducing mechanism of central chemosensory neurons (21). Therefore, alterations in mechanisms involved in appropriate pHi regulation could contribute to neurochemical mechanisms underlying centrally mediated respiratory dysfunction, such as neonatal apnea, central sleep apnea, obstructive sleep apnea syndrome, and even sudden infant death syndrome.
The NHEs are a group of antiporters that are involved in the maintenance of neural steady-state pHi, and help in the recovery from intracellular acidification (22–24). They are probably also involved in the control of excitability via changes of cell volume or pHi (25–27). NHE3 is present in ventrolateral neurons from medullary organotypic cultures, and blockade of this exchanger using high-affinity inhibitors caused a drop in pHi and potentiated the response of these cells to CO2(13). The NHE3 inhibitor S8218 was found to activate CO2/H+-sensitive neurons in vitro(12) and to potentiate the CO2 ventilatory response and decrease the apneic threshold in rabbits (28). NHE3 seems to be the predominant NHE subtype in chemosensitive neurons. The potent NHE1 inhibitor cariporide (HOE 642) was not able to activate CO2/H+-sensitive neurons within the ventrolateral medulla oblongata at 100 μM (13), a dose that would be expected to completely inhibit NHE1 as well as NHE2 (29). Furthermore, amiloride, which is a 30 to 40 times less potent blocker of NHE3 than either NHE1 or NHE2 (29), did not affect ventilatory response to CO2 in rabbits (30). Therefore, the effect of blockade of NHE3 is confined to the medullary chemosensory neurons, as it did not affect non–CO2/H+-responsive medullary neurons or hippocampal CA3 neurons (13, 29).
Unlike CO2, S8218 did not enhance the baseline phrenic activity. The underlying mechanism for the ability of S8218 to attenuate reflex apnea without affecting baseline phrenic activity is not clear. A possible explanation might be that S8218 stimulated both excitatory neurons, which are inhibited by SLN stimulation, and inhibitory chemosensory neurons, the net result of which had no effect on the baseline phrenic activity. Rigatto et al.(31) found that CO2 was able to stimulate pacemaker neurons from rostral medulla that were stimulated by morphine and a placental extract that inhibited fetal breathing, raising the possibility that some of these neurons might be inhibitory. Furthermore, S8218 might have inhibited some chemosensory neurons as CO2 was found to stimulate as well as inhibit some neurons in slices of the fetal rat medulla (32). Further studies are clearly needed to identify the neuronal network involved in SLN stimulation–induced apnea and how NHE3 blockers affect their responses.
The investigational compound S8218 readily enters the brain within minutes. The purpose of the pharmacodynamic study of S8218 was to demonstrate that the compound crosses the blood–brain barrier, which is clearly the case. In the 2-mo-old pigs, concentrations in brain tissue obtained as early as 15 min after i.v. infusion of the drug were significantly higher than in plasma. As brain levels are already very high in juvenile animals, it is unlikely that levels would be much higher in newborn animals because of a possible immaturity of the blood–brain barrier; however, this possibility cannot be excluded. Furthermore, brain concentration lasted as long as 4 h, which extended beyond the duration of the experimental protocol. In piglets, we observed physiologic effects of the compound at a dose between 1 and 10 mg/kg.
CONCLUSION
We conclude that blockade of NHE3 prevents reflex apnea induced by SLN stimulation in piglets. We speculate that this effect of NHE3 inhibitor is secondary to stimulation of chemosensory neural output and resultant increase in the threshold for laryngeal stimulation-induced apnea in early life. It is possible that such inhibition of NHE might help prevent pathophysiologic processes associated with apnea of prematurity or reflex-induced apnea.
Abbreviations
- NHE3:
-
Na+/H+ exchanger type 3
- SLN:
-
superior laryngeal nerve
- Paco2:
-
arterial Pco2
- ENG:
-
electroneurogram
- TI:
-
inspiratory time
- TE:
-
expiratory time
- pHi:
-
intracellular pH
References
Butcher-Puech MC, Henderson-Smart DJ, Holley D, Lacey JL, Edwards DA 1985 Relation between apnoea duration type neurological status of preterm infants. Arch Dis Child 60: 953–958
Taylor HG, Klein N, Schatschneider C, Hack M 1998 Predictors of early school age outcomes in very low birth weight children. J Dev Behav Pediatr 19: 235–243
Kitchen WH, Yu VY, Orgill AA, Ford G, Rickards A, Astbury J, Lissenden JV, Bajuk B 1983 Collaborative study of very-low-birth-weight infants: correlation of handicap with risk factors. Am J Dis Child 137: 555–559
Al-Shway SF, Mortola JP 1982 Respiratory effects of airflow through the upper airways in newborn kittens puppies. J Appl Physiol 53: 805–814
Harding R 1984 Function of the larynx in the fetus newborn. Ann Rev Physiol 46: 645–659
Leape LL, Holder TM, Franklin JD, Amoury RA, Ashcraft KW 1977 Respiratory arrest in infants secondary to gastroesophageal reflux. Pediatrics 60: 924–928
Lee JC, Stoll BG, Downing SE 1977 Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol 233: R30–R36
Boggs DF, Bartlett D 1982 Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol 53: 455–462
Lawson EE 1981 Prolonged central respiratory inhibition following reflex-induced apnea. J Appl Physiol 50: 874–879
Lawson EE 1982 Recovery from central apnea: effect of stimulus duration end-tidal CO2 partial pressure. J Appl Physiol 53: 105–109
Litmanovitz I, Dreshaj IA, Miller MJ, Haxhiu MA, Martin RJ 1994 Central chemosensitivity affects respiratory muscle responses to laryngeal stimulation in the piglet. J Appl Physiol Respir Environ Exerc Physiol 76: 403–408
Wiemann M, Sautmann H, Heinelt U, Wirth KJ, Lang H-J, Kiwull P, Kiwull-Schöne H, Bingmann D 2000 Effects of S8218-a novel inhibitor of the Na+/H+ exchanger subtype 3 on chemosensitive neurons in vitro. Pflugers Arch Suppl 439: R399
Wiemann M, Schwark JR, Bonnet U, Jansen HW, Grinstein S, Baker RE, Lang HJ, Wirth K, Bingmann D 1999 Selective inhibition of the Na+/H+ exchanger type 3 activates CO2/H+-sensitive medullary neurons. Pflugers Arch 438: 255–262
Miller AJ 1976 Characterization of the postnatal development of superior laryngeal nerve fibers in the postnatal kitten. J Neurobiol 7: 483–494
Dreshaj IA, Haxhiu MA, Abu-Shaweesh J, Carey RE, Martin RJ 1999 CO2-induced prolongation of expiratory time during early development. Respir Physiol 116: 125–132
Goding GS, Pernell KJ 1996 Effect of a second laryngeal stimulation during recovery from the laryngeal chemoreflex. Otolaryngol Head Neck Surg 114: 84–90
Pickens DL, Schefft D, Thach BT 1988 Prolonged apnea associated with upper airway protective reflexes in apnea of prematurity. Am Rev Respir Dis 137: 113–118
Downing SE, Lee JE 1975 Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics 55: 640–649
Sasaki CT 1979 Development of laryngeal function: etiologic significance in the sudden infant death syndrome. Laryngoscope 89: 1964–1982
Wetmore RF 1993 Effects of acid on the larynx of the maturing rabbit their possible significance to the sudden infant death syndrome. Laryngoscope 103: 1242–1254
Neubauer JA, Gonsalves SF, Chou W, Geller HM, Edelman NH 1991 Chemosensitivity of medullary neurons in explant tissue cultures. Neuroscience 45: 701–708
Bevensee MO, Boron WF 1998 pH regulation in mammalian neurons. In: Kaila K, Ransom MO (eds) pH and Brain Function. Liss, New York, 211–233
Bonnet U, Gastpar M, Bingmann D 1996 Ethacrynic acid: effects on postsynaptic GABA responses electrical activity of CA3 neurons. Neuroreport 7: 2983–2987
Orlowski J, Grinstein S 1984 Na+/H+ exchangers of mammalian cells. J Biol Chem 272: 22373–22376
Bonnet U, Wiermann M 1999 Ammonium prepulse: effects of intracellular pH bioelectric activity of guinea pig hippocampal CA3 neurones. Brain Res 840: 16–22
Dietzel I, Heinemann U 1986 Dynamic variations of the brain cell microenvironment in relation to neuronal hyperactivity. Ann NY Acad Sci 481: 72–86
Hoffmann EK, Simonsen LO 1989 Membrane mechanisms in volume pH regulation in vertebrate cells. Physiol Rev 69: 315–382
Kiwull-Schöne H, Wiemann M, Frede S, Bingmann D, Wirth KJ, Heinelt U, Lang HJ, Kiwall P 2001 A novel inhibitor of the Na+/H+ exchanger type 3 activates the central respiratory CO2 response lowers the apneic threshold. Am J Respir Crit Care Med 164: 1303–1311
Schwark J-R, Jansen HW, Lang H-J, Krick W, Burckhardt G, Hropot M 1998 S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflugers Arch 436: 797–800
Sullivan MP, Adams JM 1994 Cisternal Na+ transport inhibition the ventilatory responses to CO2 . J Appl Physiol 77: 2572–2577
Rigatto H, Rehan V, Lemke RP, Idiong N, Hussain A, Cates D 2000 Respiratory pacemaker cells responsive to CO2 in the upper medulla: dose response mediators. Pediatr Pulmonol 30: 359–367
Wellner-Kienitz MC, Shams H 1998 CO2-sensitive neurons in organotypic cultures of the fetal rat medulla. Respir Physiol 111: 137–151
Acknowledgements
The authors thank Aventis Pharma for providing the drug S8218. We also thank Cecily Lewis for secretarial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Institutes of Health grants HL-50527 (M.A.H.), NINDS-1U54 NS 39407 (M.A.H.), and HL 62527 (R.J.M.).
Rights and permissions
About this article
Cite this article
Abu-Shaweesh, J., Dreshaj, I., Martin, R. et al. Inhibition of Na+/H+ Exchanger Type 3 Reduces Duration of Apnea Induced by Laryngeal Stimulation in Piglets. Pediatr Res 52, 459–464 (2002). https://doi.org/10.1203/00006450-200209000-00026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200209000-00026