Abstract
Oxygen constriction causes functional closure of the ductus arteriosus (DA) at birth. Although DA closure is crucial for postnatal adaptation, patency of the DA is critical for survival of newborns with duct-dependent cardiac malformations. In these cases, DA patency is achieved by i.v. infusion of prostaglandin E1, which, though effective, is often associated with complications. We hypothesized that sildenafil, a specific phosphodiesterase type 5 inhibitor, is an effective DA vasodilator. In isolated DA rings from term (d 30) fetal rabbits, sildenafil (10−6–10−4 M) and diethylamine NONOate (10−7–10−5 M) induced dose-dependent relaxation of oxygen-constricted DA (−52 ± 4% and −51 ± 6%, respectively) that was inhibited by the soluble guanylyl-cyclase inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (5 × 10−5 M). Sildenafil increased cyclic GMP levels. Iberiotoxin (200 nM), an inhibitor of calcium-sensitive potassium channels, decreased the vasodilatory effect of sildenafil and diethylamine NONOate (−30 ± 2% and −27 ± 4%, respectively). Oxygen inhibition of whole-cell K+ current and membrane depolarization were partially restored by sildenafil, and this was inhibited by iberiotoxin. Immunohistochemistry and immunoblotting confirmed the presence of phosphodiesterase type 5 and calcium-sensitive potassium channels in the DA smooth muscle cells. This is the first study to demonstrate that sildenafil dilates the DA by increasing soluble guanylyl-cyclase–derived cGMP levels and thereby activating calcium-sensitive potassium channels, causing membrane hyperpolarization. Sildenafil, already approved for human usage, might be an alternative or a useful adjunct to prostaglandin E1 as a bridge to cardiac surgery.
Similar content being viewed by others
Main
The DA is a vital fetal structure that connects the pulmonary artery to the descending aorta. During fetal life, the DA shunts over half of the blood flow away from the lung into the umbilico-placental circulation, where gas exchange takes place (1). At birth, closure of the DA, essential for postnatal adaptation, is initiated by an increase in O2. In full-term newborns, the DA closes 24–48 h after delivery (1). However, in newborns with duct-dependent cyanotic cardiac malformations (e.g. transposition of the great vessels) or duct-dependent aortic obstructions (including hypoplastic left heart syndrome and coarctation of the aorta), patency of the DA is critical for survival until surgery. The paramount importance of PG in the regulation of ductal tone is well established [reviewed in (2)]. This has resulted in successful pharmacological manipulation of the DA (3). To date, PGE1 is the only available therapy to maintain DA patency (4). Although effective, numerous side effects are associated with PGE1 infusions (respiratory depression, fever, lethargy, irritability, myoclonic jerks, flushing, edema, pyloric stenosis, hyperostosis, necrotizing enterocolitis, and structural remodeling of the DA and the pulmonary vessels) (5, 6). The reported incidence of this host of complications ranges from 10 to 40%.
Although PGs are major endogenous regulators of DA tone, endothelium-derived NO also modulates DA tone (7, 8). NO activates smooth muscle sGC, which increases intracellular cGMP levels, causing vasodilatation. cGMP in turn, is rapidly degraded by the cGMP-specific PDE-5. Basal stimulation of sGC and increased smooth muscle cGMP levels contribute to DA relaxation (9, 10). Consequently, we hypothesized that inhibition of cGMP breakdown might be efficacious in achieving dilatation of the DA. Sildenafil, readily available orally and widely used in the treatment of erectile dysfunction (11), is a potent vasodilator that enhances and prolongs the action of cGMP by selectively inhibiting PDE-5. Furthermore, cGMP induces vasodilatation, in large part, by activating protein kinase G and thereby opening BKCa, causing membrane hyperpolarization (12, 13). Therefore, we assessed the efficacy and mechanism of action of sildenafil in O2-constricted, term rabbit DA.
METHODS
All procedures were approved by the Animal Health Care Committee of the University of Alberta. Fetal New Zealand White rabbits were delivered by cesarean section at 30 d of gestation (term = 31 d) and killed with an i.p. overdose of pentobarbital. The DA was excised under a dissecting microscope and used within 5 min in the following studies.
Isolated DA rings.
The isolated DA was placed in an organ bath and studied in Krebs solution containing meclofenamate (10−5 M), as previously described (14). After 1 h equilibration in hypoxia (Po2 = 31 ± 1 mm Hg, a level similar to that in utero), the DA was exposed to a normoxic solution (Po2 = 127 ± 1 mm Hg) and the vasodilatory effect of sildenafil (10−6–10−4 M), the NO donor DEANO (10−7–10−5 M), and PGE1 (10−12–10−9 M) were compared by administering each agent at the peak of O2 constriction. To further assess the mechanism of sildenafil-induced DA dilatation, we compared the vasodilatory effect of these drugs after preincubation of the DA with the sGC inhibitor, ODQ (5 × 10−5 M), or the specific BKCa channel blocker IBTX (200 nM). In additional experiments, we studied the effects of preincubation with vehicle, sildenafil (10−4 M), ODQ (5 × 10−5 M), or sildenafil + ODQ on O2 constriction. Relaxation was expressed as percentage relaxation of the active tone.
cGMP levels.
To assess whether sildenafil causes relaxation by increasing cGMP levels in the DA, we determined cGMP levels in the Krebs solution taken from the ring bath under hypoxia and normoxia in the following conditions: after preincubation of the DA with vehicle, sildenafil (10−4 M), or sildenafil + ODQ (5 × 10−5 M). cGMP was measured using a cGMP EIA Kit (Biomedical Technologies, Stoughton, MA, U.S.A.) and normalized to milligrams of tissue.
Whole-cell patch-clamp.
Conventional whole-cell patch-clamp recordings were performed on freshly dispersed DASMC using solution and recording data as previously described (15). The cells were voltage clamped at a holding potential of −70 mV and currents were evoked by steps of 200 ms duration from −70 to +70 mV.
To determine the effect of sildenafil on IK and Em, hypoxic DASMC were successively exposed to O2 and sildenafil (10−5 M). To characterize the type of current activated by sildenafil, the DASMC were exposed to 4-AP (5 mM) and then IBTX (100 nM). In additional experiments, the effects of sildenafil on Em in normoxic DASMC were recorded after exposure to IBTX or 4-AP.
Immunoblotting.
Immunoblots were performed on fresh hypoxic DA using a panel of protease inhibitors, as previously described (15). Antibodies were obtained from Calbiochem (San Diego, CA, U.S.A.; PDE-5) and Alomone Lab (Jerusalem, Israel; BKCa).
Immunohistochemistry.
Immunohistochemistry was performed on paraffin-embedded, formaldehyde-fixed DA, as previously described (15). After overnight incubation with primary antibody (4°C), slides were incubated for 20 min with the secondary antibody, either biotinylated anti-Rabbit IgG (for PDE-5 and BKCa protein) or anti-Mouse IgG [for endothelial nitric-oxide synthase (eNOS) protein] (Vector Laboratories, Burlingame, CA, U.S.A.) each at 1/200 dilution. The signal was revealed using a Chromagen (DAB) kit (Biogenex, San Ramon, CA, U.S.A.). In all cases, the secondary antibody created reddish-brown staining. Antibodies for PDE-5 (1/1000) and BKCa (1/100) were the same as for the immunoblotting. Antibodies for eNOS were obtained from BD Transduction Lab (Oakville, ON, Canada).
Statistics and drugs.
Values are expressed as mean ± SEM. Intergroup comparisons were performed with a t test or a factorial, repeated-measures ANOVA, as appropriate. Fisher's probable least significant differences test was used for post hoc comparisons. A p value of < 0.05 was considered to be statistically significant. All drugs, except sildenafil, a generous gift from Pfizer Pharmaceuticals (Sandwich, England), were purchased from Sigma Chemical (St. Louis, MO, U.S.A.). Sildenafil, ODQ, and IBTX were dissolved in DMSO. PGE1 was dissolved in ethanol. DEANO was dissolved in water immediately before use and kept refrigerated.
RESULTS
Sildenafil relaxes O2-constricted DA.
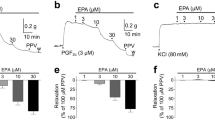
In O2-constricted DA, sildenafil, DEANO, and PGE1 induce cumulative, dose-dependent, relaxations (−52 ± 4%, −51 ± 6%, and −73 ± 4%, respectively, Fig. 1, A–C). Treatment of DA rings with sildenafil (10−4 M) before exposure to O2 also attenuates O2-induced constriction (Fig. 1D).
Sildenafil dilates the DA. (A–C) Representative tracings and mean data showing the percentage relaxation of O2-preconstricted rabbit DA to sildenafil (n = 11), DEANO (n = 8), and PGE1 (n = 8) in the absence and presence of ODQ. ODQ inhibited sildenafil (n = 7) and DEANO-induced relaxation (n = 7), but had no effect on PGE1-induced vasodilatation (n = 6). *p < 0.05. (D) Mean data showing O2-induced constriction of DA rings pretreated with vehicle (DMSO, n = 9) and sildenafil (10−4 M, n = 11). Sildenafil significantly decreased the magnitude of O2-induced constriction of the DA compared with the vehicle. **p < 0.01 vs vehicle; †p < 0.01 vs ODQ; #p < 0.01 vs ODQ + sildenafil
sGC inhibition reduces sildenafil-induced relaxation and cGMP accumulation.
ODQ (5 × 10−5 M) significantly reduces sildenafil- and DEANO-induced relaxation but not PGE1 relaxation (Fig. 1, A–C). Sildenafil-induced increases in cGMP levels were inhibited by ODQ (Fig. 2A).
Sildenafil-induced dilatation occurs through a sGC/PDE-5–dependent pathway. (A) Mean data showing cGMP levels measured from the organ bath (n = 9 in each group). Sildenafil compared with the vehicle (DMSO) increased the production of cGMP. This increase was attenuated by ODQ. p < 0.01 vs vehicle. (B) Immunoblot and immunohistochemistry showing the expression of PDE-5 in rabbit DASMC and endothelium. (C and D) Immunohistochemistry showing the presence of eNOS in the endothelium (endo) and DASMC of the DA. Note the lack of staining in the negative control (no primary antibody).
BKCa channel inhibition attenuates sildenafil-induced relaxation.
IBTX decreases relaxation caused by sildenafil, but not by PGE1 (Fig. 3A). In whole-cell patch-clamp recording of DASMC, normoxia decreases IK (Fig. 3B) and depolarizes the Em (Fig. 3C). Sildenafil increases normoxic IK to levels similar to those seen in hypoxia (Fig. 3B) and repolarizes the Em (Fig. 3C). Sildenafil's effects on IK and Em are completely inhibited by IBTX but not by 4-AP (Fig. 3, B and C).
Sildenafil opens BKCa channels in DASMC. (A) IBTX inhibited sildenafil-induced relaxation (n = 11), but had no effect on PGE1-induced vasodilatation (n = 7). *p < 0.05.(B) Representative tracings and mean data showing the effects of sildenafil on whole-cell IK. The normoxic-induced decrease in IK is partially restored by sildenafil and subsequently inhibited by IBTX;n = 5. *p < 0.05 vs normoxia; **p < 0.01 vs sildenafil. (C) Normoxia depolarizes the DASMC, and sildenafil repolarizes the DASMC. Sildenafil-induced repolarization is inhibited by IBTX;n = 5. *p < 0.05 vs hypoxia; **p < 0.01 vs normoxia. (D) Immunoblot and immunohistochemistry showing BKCa expression in DASMC and endothelium.
PDE-5 and BKCa channels are expressed in the rabbit DA.
PDE-5 (Fig. 2B) and BKCa channels (Fig. 3D) are expressed in DASMC. Immunohistochemistry shows eNOS and BKCa channels in both the endothelium and DASMC (Fig. 2D and Fig. 3D). PDE-5 was strongly expressed in DASMC and the endothelium (Fig. 2B).
DISCUSSION
The primary finding of this study is that sildenafil reverses and prevents O2 constriction in the DA by increasing cGMP levels and activating DASMC BKCa channels. Through its ability to open K+ channels, sildenafil hyperpolarizes DASMC, thereby causing vasodilatation.
Sildenafil relaxes the DA.
Cyclooxygenase inhibition causes a striking increase in DA tone in contrast to the subtle increase in DA resistance induced by the selective NOS antagonist NG-nitro-l-arginine (10). However, there is basal NO production in the DA that activates sGC and thereby contributes to maintaining the DA in a relaxed state (9, 10). cGMP is important in maintaining DA patency. It appears that PDE-5 inhibition with sildenafil causes maximal elevation of cGMP in the DA, because there is no difference in cGMP levels between normoxic and hypoxic DA, in the presence of sildenafil (Fig. 2A). However, when guanylate cyclase is partially inhibited by ODQ, the enhanced NO synthesis caused by normoxia is evident (Fig. 2A) (8). Nitrovasodilators cause a marked dilatation of the DA in vitro(16) and in vivo(17), which is associated with increased DA cGMP content (16). Methylene blue, a nonspecific GC inhibitor, causes a more marked constriction of the DA in vitro(9) and in vivo(10) than does NOS inhibition. This suggests that mediators other than NO may stimulate sGC, increasing cGMP in the DA. Indeed, carbon monoxide has been shown to stimulate sGC and increase cGMP in various vascular beds, including the DA (18, 19).
Synthesis of cGMP also occurs after activation of particulate guanylate cyclase in pulmonary vascular smooth muscle cells by natriuretic peptides (20). Unlike NO, which stimulates sGC, the natriuretic peptides cause vasodilatation by stimulating a membrane-bound, ODQ-resistant, particulate GC. The role of particulate GC in the DA has, to our knowledge, not yet been investigated, although it is important in the fetal pulmonary circulation (20). We speculate that particulate GC may play an important role in cGMP production in the DA during normoxia, thereby attenuating the effect of inhibiting soluble GC, although further study is required to assess this concept.
Regardless of the source of cGMP, inhibition of its degradation is efficacious in elevating cGMP levels (Fig. 2A) and thereby achieving dilatation of the DA (Fig. 1A). We also show that sildenafil dilates the DA via a mechanism distinct from that used by PG. ODQ attenuates sildenafil-mediated cGMP accumulation (Fig. 2A), and decreases relaxation of the DA to sildenafil (Fig. 1A) but not PGE1 (Fig. 1B). The selected dose of ODQ also provides an effective block to DEANO relaxation (Fig. 1C).
Mechanism of sildenafil-induced DA relaxation.
One major mechanism by which cGMP causes vasodilatation is by opening BKCa channels (12, 13). Our functional and electrophysiological data demonstrate that most sildenafil-induced DA relaxation occurs through the opening of BKCa channels and membrane hyperpolarization. The BKCa channel blocker IBTX decreases the vasodilator effect of sildenafil, whereas PGE1-induced vasodilatation remained unaffected (Fig. 3A). Likewise, sildenafil increases IK and repolarizes the Em in normoxic DASMC by activating BKCa channels (Fig. 3, B and C). Immunoblotting and immunohistochemistry confirm the presence of BKCa channels in the DASMC (Fig. 3D). Although not directly measured, membrane hyperpolarization causes relaxation by inactivating voltage-gated L-type Ca2+ channels in smooth muscle cells, lowering cytosolic Ca2+ and inactivating the contractile apparatus (12). This pathway is widely conserved among various vascular beds and in some nerves (21).
Sildenafil improves erectile dysfunction by the same cGMP-BKCa pathway that causes DA relaxation. In an interesting proof of concept experiment, it was shown that age-associated erectile dysfunction in rats can be reversed by overexpression of BKCa channels in the corpus cavernosum using gene transfer (22). Sildenafil is a weak vasodilator in systemic and coronary beds (23), probably reflecting tissue-specific differences in PDE-5 expression. Conversely, the penis, the lung, and the DA (Fig. 2B) are each rich in PDE-5, and sildenafil is a very effective vasodilator in each case (11, 24). Recently, it has been shown that PDE-5 is expressed in both the smooth muscle and endothelial cells of rat mesenteric arteries, and that this enzyme can significantly influence NO-induced relaxation (25). In the rabbit DA, PDE-5 is also found in both the smooth muscle cells and endothelium, although the relative role of each compartment to the vasodilatory effects of sildenafil is unknown.
In summary, sildenafil prevents and reverses O2 constriction in the DA. The functional, biochemical, and electrophysiolgical data are concordant and support the conclusion that sildenafil is acting as a BKCa channel opener via a cGMP-dependent mechanism. The fact that sildenafil is also a pulmonary vasodilator and has a good post-marketing safety record suggests it may be ideal for maintaining DA patency. Sildenafil dilates the DA through a mechanism that is distinct from PGE1's mechanism. Clinical trials are indicated to determine whether sildenafil might be a useful oral alternative or adjunct treatment to PGE1 to maintain DA patency.
Abbreviations
- 4-AP:
-
4-aminopyridine
- BKCa:
-
large conductance calcium-sensitive potassium channels
- cGMP:
-
3′ 5′-cyclic GMP
- DA:
-
ductus arteriosus
- DASMC:
-
DA smooth muscle cells
- DEANO:
-
diethylamine NONOate
- Em:
-
membrane potential
- IBTX:
-
iberiotoxin
- IK:
-
whole-cell potassium current
- NO:
-
nitric oxide
- O2:
-
oxygen
- ODQ:
-
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PDE-5:
-
type 5 phosphodiesterase
- PG:
-
prostaglandin
- sGC:
-
soluble guanylate cyclase
References
Heymann MA, Rudolph AM 1975 Control of the ductus arteriosus. Physiol Rev 55: 62–78
Smith GC 1998 The pharmacology of the ductus arteriosus. Pharmacol Rev 50: 35–58
Olley PM, Coceani F, Bodach E 1976 E-type prostaglandins: a new emergency therapy for certain cyanotic congenital heart malformations. Circulation 53: 728–731
Freedom RM, Lock J, Bricker JT 2000 Pediatric cardiology cardiovascular surgery: 1950–2000. Circulation 102 ( Suppl 4): IV58–IV68
Singh GK, Fong LV, Salmon AP, Keeton BR 1994 Study of low dosage prostaglandin—usages complications. Eur Heart J 15: 377–381
Lewis AB, Freed MD, Heymann MA, Roehl SL, Kensey RC 1981 Side effects of therapy with prostaglandin E1 in infants with critical congenital heart disease. Circulation 64: 893–898
Seidner SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI 2001 Combined prostaglandin nitric oxide inhibition produces anatomic remodeling closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res 50: 365–373
Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, Chen YQ 1998 Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, vasa vasorum. Pediatr Res 43: 633–644
Coceani F, Kelsey L, Seidlitz E 1994 Occurrence of endothelium-derived relaxing factor—nitric oxide in the lamb ductus arteriosus. Can J Physiol Pharmacol 72: 82–88
Fox JJ, Ziegler JW, Ivy DD, Halbower AC, Kinsella JP, Abman SH 1996 Role of nitric oxide cGMP system in regulation of ductus arteriosus tone in ovine fetus. Am J Physiol 271: H2638–H2645
Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA 1998 Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 338: 1397–1404
Robertson BE, Schubert R, Hescheler J, Nelson M 1993 cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol 265: C299–C303
Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK 1994 Nitric oxide cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA 91: 7583–7587
Tristani-Firouzi M, Reeve HL, Tolarova S, Weir EK, Archer SL 1996 Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest 98: 1959–1965
Michelakis ED, Weir EK, Wu X, Nsair A, Waite R, Hashimoto K, Puttagunta L, Knaus HG, Archer SL 2001 Potassium channels regulate tone in rat pulmonary veins. Am J Physiol 280: L1138–L1147
Walsh RS, Mentzer RM 1987 Role of cyclic nucleotides in relaxation of fetal lamb ductus arteriosus. Surgery 102: 313–318
Walsh RS, Ely SW, Mentzer RM Jr 1988 Response of lamb ductus arteriosus to nitroglycerin nitroprusside. J Surg Res 44: 8–13
Christodoulides N, Durante W, Kroll MH, Schafer AI 1995 Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation 91: 2306–2309
Coceani F, Breen CA, Lees JG, Falck JR, Olley PM 1988 Further evidence implicating a cytochrome P-450-mediated reaction in the contractile tension of the lamb ductus arteriosus. Circ Res 62: 471–477
Lakshminrusimha S, d'Angelis CA, Russell JA, Nielsen LC, Gugino SF, Nickerson PA, Steinhorn RH 2001 C-type natriuretic peptide system in fetal ovine pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 281: L361–L368
Medina P, Segarra G, Torondel B, Chuan P, Domenech C, Vila JM, Lluch S 2000 Inhibition of neuroeffector transmission in human vas deferens by sildenafil. Br J Pharmacol 131: 871–874
Christ GJ, Rehman J, Day N, Salkoff L, Valcic M, Melman A, Geliebter J 1998 Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol 275: H600–H608
Herrmann HC, Chang G, Klugherz BD, Mahoney PD 2000 Hemodynamic effects of sildenafil in men with severe coronary artery disease. N Engl J Med 342: 1622–1626
Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR 2001 Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 104: 424–428
Sampson LJ, Hinton JM, Garland CJ 2001 Evidence for expression function of phosphodiesterase type 5 (PDE-V) in rat resistance arteries. Br J Pharmacol 132: 13–17
Acknowledgements
The authors thank Alex Bolivar, Steve Joy, and Dr. Lakshmi Puttagunta for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
B.T. is supported by the Canadian Institutes for Health Research, and by the Alberta Heritage Foundation for Medical Research, a grant from the French Ministry for Foreign Affairs, the Fondation pour la Recherche Médicale and the Société Française de Médecine Périnatale. E.M. and S.L.A. are both supported by the Alberta Heritage Foundation for Medical Research, the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Canada, and the Canadian Foundation for Innovation.
Rights and permissions
About this article
Cite this article
Thébaud, B., Michelakis, E., Wu, XC. et al. Sildenafil Reverses O2 Constriction of the Rabbit Ductus Arteriosus by Inhibiting Type 5 Phosphodiesterase and Activating BKCa Channels. Pediatr Res 52, 19–24 (2002). https://doi.org/10.1203/00006450-200207000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200207000-00006