Abstract
In mammals, release from growth-inhibiting conditions results in catch-up growth. To explain this phenomenon, we proposed the following model:1) The normal senescent decline in growth plate function depends not on age per se, but on the cumulative number of replications that growth plate chondrocytes have undergone. 2) Conditions that suppress growth plate chondrocyte proliferation therefore slow senescence. 3) After transient growth inhibition, growth plates are thus less senescent and hence show a greater growth rate than expected for age, resulting in catch-up growth. To test this model, we administered dexamethasone to growing rabbits to suppress linear growth. After stopping dexamethasone, catch-up growth occurred. In distal femoral growth plates of untreated controls, we observed a senescent decline in the growth rate and in the heights of the proliferative zone, hypertrophic zone, and total growth plate. During the period of catch-up growth, in the animals previously treated with dexamethasone, the senescent decline in all these variables was delayed. Prior treatment with dexamethasone also delayed epiphyseal fusion. These findings support our model that linear catch-up growth is caused, at least in part, by a delay in growth plate senescence.
Similar content being viewed by others
Main
More than 35 y ago, Prader, Tanner, and von Harnack observed that children undergo supranormal linear growth after release from growth-inhibiting conditions (1). This phenomenon, termed catch-up growth, was attributed to a CNS mechanism that compares the individual's actual body size with an age-appropriate set-point and then adjusts the growth rate accordingly (2). However, this neuroendocrine hypothesis has not been subjected to a definitive experimental test.
More recently, we demonstrated that transient growth inhibition within a single growth plate is followed by local catch-up growth (3). This local catch-up growth cannot be readily explained by a systemic mechanism, which would have affected all the growth plates equally. Instead, it suggests that catch-up growth is due, at least in part, to a mechanism intrinsic to the growth plate.
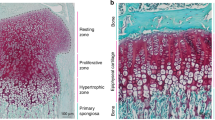
To account for this finding, we speculated that catch-up growth might be caused by a delay in the process of growth plate senescence. The growth plate, a layer of cartilage located between the epiphysis and the metaphysis of mammalian long bones, is composed of three distinct zones: the resting zone, proliferative zone, and hypertrophic zone. With increasing age, the growth plate undergoes a program of structural and functional changes. These senescent changes include a progressive decline in the growth rate accompanied by a decrease in the heights of the proliferative zone (4), the hypertrophic zone (5), and the overall growth plate (6). In some mammals, including humans and rabbits, the senescent growth plates are eventually replaced by bone tissue, a process termed epiphyseal fusion.
Specifically, to explain the mechanism underlying local catch-up growth, we hypothesized that growth plate senescence is not a function of age per se, but rather of the cumulative number of cell divisions that the chondrocytes have undergone. Glucocorticoid excess and other growth-inhibiting conditions slow linear growth (7), in part, by decreasing the proliferation rate of growth plate chondrocytes (5, 8). According to our hypothesis, this decrease in proliferation rate would slow growth plate senescence. If the growth-inhibiting condition resolves, the growth plates would be less senescent and therefore grow more rapidly than expected for age, resulting in catch-up growth.
To test this model, in the current study we transiently inhibited longitudinal bone growth in rabbits by administering glucocorticoid systemically. We then stopped the glucocorticoid treatment and assessed markers of growth plate senescence to determine whether senescence had, in fact, been delayed by the previous growth inhibition.
METHODS
Animals.
Animals were housed and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (9). The experimental procedures were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee.
Five-week-old male New Zealand White rabbits (Covance Research Products, Denver, PA, U.S.A.) were treated with dexamethasone (Phoenix Pharmaceutical, St. Joseph, MO, U.S.A.), 0.5 mg/kg per day s.c. for 5 wk. Male New Zealand White rabbits begin sexual maturation at approximately 2 months of age (10) and undergo epiphyseal fusion by approximately 6 mo of age (11). Treatment was begun at 5 wk of age to allow sufficient time for induction of growth retardation and recovery before epiphyseal fusion occurs. Untreated animals served as concurrent controls. One day after discontinuation of dexamethasone, all rabbits began receiving oxytetracycline (Phoenix Pharmaceutical), 10 mg/kg i.m. weekly, to label newly formed bone. The last dose was given 1 d before sacrifice. Animals were weighed weekly throughout the study. Rabbits were killed by pentobarbital overdose at 2, 9, 16, 30, and 114 d (expressed as 0, 1, 2, 4, and 16 wk for convenience) after the discontinuation of dexamethasone (eight animals in each treatment group per time point), and the femurs were removed. Two of the dexamethasone-treated animals died before scheduled sacrifice, and their femurs were not included in the analysis.
Femoral length measurement.
Left femur length was measured in triplicate by a single, blinded observer using a digital caliper (Mitutoyo Corp, Kawasaki, Japan). Length was measured from the distal femoral articular surface to the greater trochanter.
Quantitative histology.
The right distal femurs were dehydrated in serial solutions of graded ethanol followed by clearing in xylene and infiltration with methyl methacrylate. Serial 5-μm sections were cut near-parallel to the long axis of the femur and stained with Goldner's trichrome. Using a light microscope with a VIA-100 video measurement system (Boeckler, Tuscon, AZ, U.S.A.), the heights of the resting, proliferative, and hypertrophic zones were measured at four sites along the growth plate by a single, blinded observer and an average was taken. The four sites were selected a priori because, at these locations, the growth plate is perpendicular to the long axis of the bone (Fig. 1). Total growth plate height was determined by summing the heights of the three zones.
Diagram of a longitudinal section through the distal femur. The four single-headed arrows indicate the regions of the growth plate where the heights of the resting zone, proliferative zone, and hypertrophic zone were measured. The height of the metaphyseal single-labeled bone was measured beneath the growth plate in the same four regions.
Sections from the 16-wk group were evaluated for growth plate fusion by a blinded observer. Any areas of growth plate with chondrocytes arranged in columns, even if disorganized, were considered unfused. If chondrocyte columns were replaced by blood vessels and bone cells or only scattered lone chondrocytes and cartilage matrix remnants were seen, the area was considered to be fused. Some sections showed fusion beginning in one region but not yet involving the entire growth plate. For these growth plates, the percent fusion was estimated visually.
Growth rate and proliferation rate.
To measure growth rate, the animals were injected weekly with oxytetracycline, which is incorporated into bone and can be visualized by fluorescence microscopy. The final dose, given 1 d before sacrifice, labels essentially all of the metaphyseal trabecular bone. The previous dose, given 8 d before sacrifice, is only incorporated into bone that had been present at the time that dose was given. Therefore, the bone formed beneath the growth plate during the 8 d before sacrifice contains only a single band of fluorescent label whereas the older bone contains more than one band of label. Thus, the linear growth in the week before sacrifice was assessed by measuring the height of the single-labeled metaphyseal bone beneath the growth plate (Fig. 1). To make this measurement, unstained 8-μm longitudinal sections of the distal femoral metaphyses were examined in the same areas used for quantitative histology (Fig. 1) using a fluorescence microscope, a videocamera, a computer, and Oncor Image software (Version 2.0, Oncor Inc., Gaithersburg, MD, U.S.A.). The four measurements were averaged for each specimen. If no clear junction between singly and doubly labeled bone could be identified, that subregion was not included in the average. For this reason, growth velocity was unmeasurable in four animals previously treated with dexamethasone. If the growth plate had fused and there was no evidence of linear growth by fluorescence microscopy, a growth rate of 0 mm/d was recorded. The observer was blinded to experimental group.
Proliferation rate (new cells per chondrocyte column per day) was assessed by dividing the growth rate by the height of the terminal hypertrophic cell according to the method of Kember and Sissons (12). The terminal hypertrophic cell was defined, in Goldner's trichrome-stained sections, as the cell in the last lacuna not invaded by metaphyseal blood vessels. For each specimen, the heights of approximately 20 cells were measured parallel to the chondrocyte columns and the results averaged. Proliferation rate could not be assessed in fused growth plates.
Statistical analysis.
Data are presented as mean ± SEM. Comparisons between treatment groups and time points were made, where appropriate, using a two-way ANOVA test followed by a Tukey test for post hoc pairwise multiple comparisons. For analyses of the recovery period, the 0-wk time point was not included. For analyses of proliferative zone height, hypertrophic zone height, and total growth plate height, the 16-wk time point was excluded because there was a high incidence of epiphyseal fusion. Inclusion of the 16-wk time point would have increased statistical significance. Differences in epiphyseal fusion were analyzed using a Mann-Whitney U test.
RESULTS
Body weight.
During the 5-wk treatment period, dexamethasone-treated animals did not gain weight as rapidly as control animals (p < 0.001;Fig. 2). After dexamethasone treatment was stopped, the treated animals gained weight more rapidly than controls (p < 0.05; Fig. 2). Despite the recovery, differences in weight were still present at the end of the experiment (p < 0.005).
Body eight (mean ± SEM) during and after dexamethasone (Dex) treatment. Young rabbits received dexamethasone (5 mg/kg per day, n = 7) s.c. for 5 wk and were then allowed to recover for 16 wk. Untreated animals served as controls (n = 8). The box below the curves represents the period of dexamethasone treatment. At some time points, SEM bars are smaller than the symbols.
Femoral growth and proliferation.
Femoral length in the dexamethasone-treated animals was 17.4 ± 1.4 mm less than control animals at the end of the treatment period (p < 0.001; Fig. 3A). Progressive catch-up growth occurred (p < 0.001), reducing this deficit to 1.6 ± 1.6 mm (NS) by 16 wk (Fig. 3A). Distal femoral growth rates, measured by fluorescence microscopy, were not significantly different between the two groups during the first week after the dexamethasone treatment; however, there was a marked increase in the growth rate of dexamethasone-treated animals between weeks 1 and 2 of the recovery period (p < 0.001, Fig. 3B). The growth rate then gradually decreased yet remained elevated compared with controls of the same age (Fig. 3B). The proliferation rate of the growth plate chondrocytes (Fig. 3C), assessed by the method of Kember and Sissons (12), showed a pattern similar to that of the femoral growth rate.
Femoral ength (A), distal femoral growth rate (B), and chondrocyte proliferation rate (C) after stopping dexamethasone treatment (mean ± SEM). Five-week-old rabbits received dexamethasone (0.5 mg/kg per day, n = 7–8 per time point) s.c. for 5 wk and were then allowed to recover for 0 to 16 wk. Untreated animals served as controls (n = 8 per time point). After dissection, femoral length was measured with a digital caliper. To measure growth rate, animals received weekly injections of oxytetracycline. Undecalcified longitudinal sections were then obtained from the distal femurs, and the growth rates were determined by fluorescence microscopy. Chondrocyte proliferation rate was assessed by dividing the growth rate by the height of the terminal hypertrophic cell.
Growth plate histologic measurements.
In the control animals, the distal femoral growth plates showed typical senescent changes; over time, there was a gradual decrease in the heights of the total growth plate (Fig. 4A), the proliferative zone (Fig. 4B), and the hypertrophic zone (Fig. 4C). In the dexamethasone-treated animals, at the end of the treatment period, the heights of the total growth plate, the proliferative zone, and the hypertrophic zone were decreased compared with controls (Fig. 4). In the first week after the dexamethasone was stopped, these heights increased then subsequently exhibited a gradual senescent decline (Fig. 4). The senescent curves for the dexamethasone-treated animals appeared right-shifted compared with controls (p < 0.001, growth plate and hypertrophic zone;p < 0.005, proliferative zone; Fig. 4). The magnitude of the rightward shift was approximately 3 wk. Thus, at a given time point, the chondrocyte columns of the treated animals were longer than those of control animals (Fig. 5A).
Heights mean ± SEM) of the total growth plate (A), the proliferative zone (B), and the hypertrophic zone (C) of the distal femur after stopping dexamethasone treatment. Five-week-old rabbits received dexamethasone (0.5 mg/kg per day, n = 7–8 per time point) s.c. for 5 wk and were then allowed to recover for 0 to 16 wk. Untreated animals served as controls (n = 8 per time point). Longitudinal sections were obtained from the distal femurs, and growth plate heights were measured using light microscopy and a video measurement system.
Distal emoral growth plate structure 1 wk (A) and 16 wk (B) after stopping dexamethasone. Five-week-old rabbits received dexamethasone (0.5 mg/kg per day) s.c. for 5 wk and were then allowed to recover. Sections were stained with Goldner's trichrome. Scale bar, 200 μm. E, epiphysis;RZ, resting zone;PZ, proliferative zone;HZ, hypertrophic zone;M, metaphysis. In B, the growth plate in the control animal has been largely replaced with bone tissue, indicating epiphyseal fusion.
Epiphyseal fusion.
At the end of the dexamethasone treatment period, none of the animals had fused distal femoral epiphyses. By 16 wk of recovery, 88% of the control animals had fused their distal femoral growth plates compared with only 14% of the treated animals (p < 0.01; Fig. 5B).
DISCUSSION
In the control animals, the growth plates showed the expected senescent decline in function and size. With age, the rate of longitudinal bone growth progressively decreased. This slowing of growth was accompanied by a gradual decrease in the rate of chondrocyte proliferation as well as the heights of the proliferative zone, hypertrophic zone, and total growth plate. Similar senescent changes have been observed in other mammalian species (4–6). In humans, there is a dramatic decrease in longitudinal bone growth, which begins during intrauterine life and is interrupted only briefly by the pubertal growth spurt (13). The senescent decline in growth rate is caused by a decrease in the proliferation rate of chondrocytes in the proliferative zone (4) and in the hypertrophic cell size in the hypertrophic zone (14). On the basis of growth plate transplantation experiments, senescence appears to be caused by a mechanism intrinsic to the growth plate (15).
Administration of systemic glucocorticoid for 5 wk inhibited longitudinal bone growth. Excess glucocorticoid inhibits linear growth in large part by suppressing chondrocyte proliferation (5, 8, 16). After the glucocorticoid was stopped, the treated animals exhibited catch-up growth. This catch-up growth was rapid in onset and sustained throughout the period of growth. This phenomenon of supranormal linear growth after release from growth-inhibiting conditions has been attributed to a CNS mechanism (2, 17). However, more recently, we demonstrated that catch-up growth is due, at least in part, to a mechanism intrinsic to the growth plate, rather than the CNS (3).
To explain the mechanism underlying local catch-up growth, we proposed the following model:1) The normal process of growth plate senescence depends not on time, per se, but rather on the cumulative number of replications that the growth plate chondrocytes have undergone. Thus, as growth plate chondrocytes replicate, they become more senescent, and therefore subsequent replications occur more slowly. 2) Conditions that inhibit growth plate chondrocyte proliferation therefore slow growth plate senescence. 3) After transient growth inhibition, growth plates are thus less senescent and hence grow more rapidly than expected for age, resulting in catch-up growth.
To test this delayed senescence model, we assessed structural and functional markers of growth plate senescence during the recovery period. The model predicts that the decline in these markers would be delayed in the animals previously treated with dexamethasone. Our data are consistent with these predictions; once the effects of the dexamethasone had worn off, the treated animals showed a delay in the senescent decline in growth plate structure and function. Specifically, the proliferative zone height, hypertrophic zone height, and total growth plate height of the treated animals were increased compared with controls of the same age but similar to control animals approximately 3 wk younger. The same pattern of delay was seen for the growth rate and chondrocyte proliferation rate, functional markers of growth plate senescence.
In addition, epiphyseal fusion was delayed in the animals previously treated with dexamethasone. Epiphyseal fusion denotes the replacement of the growth plate by bone tissue. This process occurs only in senescent growth plates (18). Therefore, the delayed fusion observed in animals previously treated with dexamethasone provides further support for the delayed senescence model.
The catch-up growth observed after systemic glucocorticoid administration in the current study was more complete than that previously observed after local glucocorticoid administration (3). This difference may be related to differences in the nature of the growth inhibition. Local infusion of glucocorticoid into the growth plate probably inhibits growth by acting directly on growth plate chondrocytes (16, 19). Systemic glucocorticoid administration could act on the growth plate both directly and also indirectly through nutritional and hormonal mechanisms (20–22). In the current study, for example, dexamethasone impaired weight gain, which may have indirectly impaired linear growth. These different mechanisms of growth inhibition might delay growth plate senescence to different degrees and thus affect the magnitude of the catch-up growth.
Another possible explanation for the difference in completeness of recovery is that both local and systemic mechanisms might contribute to catch-up growth. It has been suggested, for example, that increased GH levels might play a role (20, 23). Increased GH could also explain the observed increased height of the growth plates. However, increased GH levels would not explain the observed delay in epiphyseal fusion.
In contrast to the complete catch-up in linear growth, recovery of weight was more gradual, occurred later, and was incomplete. We did not study the mechanisms contributing to catch-up in body weight, which probably involve tissues other than the growth plate. Rabbits do not appear to be a good model for the glucocorticoid-induced weight changes in humans; unlike humans, glucocorticoid excess causes weight loss in rabbits.
Although glucocorticoid excess appears to decrease both growth rate and growth plate senescence, it is not known whether other factors that affect growth, including nutritional, mechanical, and other hormonal conditions, also affect senescence. Indeed, we speculate that some conditions may affect growth rate not by altering chondrocyte proliferation rate but rather by altering the amount of cell enlargement in the hypertrophic zone (24). In such cases, the rate of senescence may not be affected.
The delayed senescence model is based on the hypothesis that growth plate senescence depends on the replicative history of the growth plate chondrocytes. Such replicative senescence does occur in other cell types. The limited proliferative capacity of animal cells in culture, the Hayflick phenomenon, appears to result from replicative senescence (25). Replicative senescence also probably occurs in vivo(26). The underlying mechanisms are not fully understood but may include telomere shortening (27) and increased expression of genes that negatively regulate progression through the cell cycle (26).
In summary, our findings are consistent with the hypothesis that the normal decline in the linear growth rate is caused by replicative senescence of growth plate chondrocytes. The results further suggest that catch-up growth is due, at least in part, to a delay in the normal program of growth plate senescence.
References
Prader A, Tanner JM, von Harnack GA 1963 Catch-up growth following illness or starvation. J Pediatr 62: 646–659
Tanner JM 1963 Regulation of growth in size in mammals. Nature 199: 845–850
Baron J, Klein KO, Colli MJ, Yanovski JA, Novosad JA, Bacher JD, Cutler GB 1994 Catch-up growth after glucocorticoid excess: a mechanism intrinsic to the growth plate. Endocrinology 135: 1367–1371
Walker KVR, Kember NF 1972 Cell kinetics of growth cartilage in the rat tibia. II. Measurements during ageing. Cell Tissue Kinet 5: 409–419
Kember NF, Walker KVR 1971 Control of bone growth in rats. Nature 229: 428–429
Masoud I, Shapiro F, Moses A 1986 Tibial epiphyseal development: a cross-sectional histologic and histomorphometric study in the New Zealand white rabbit. J Orthop Res 4: 212–220
Allen DB 1996 Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am 25: 699–717
Annefeld M 1992 Changes in rat epiphyseal cartilage after treatment with dexamethasone and glycosaminoglycan-peptide complex. Pathol Res Pract 188: 649–652
National Research Council 1996 Guide for the care and use of laboratory animals. Washington, DC, National Academy Press
Berger M, Jean-Faucher C, de Turckheim M, Veyssiere G, Blanc MR, Poirier JC, Jean C 1982 Testosterone, luteinizing hormone (LH) and follicle stimulating hormone (FSH) in plasma of rabbit from birth to adulthood: correlation with sexual and behavioural development. Acta Endocrinol Copenh 99: 459–465
Gilsanz V, Roe TF, Gibbens DT, Schulz EE, Carlson ME, Gonzalez O, Boechat MI 1988 Effect of sex steroids on peak bone density of growing rabbits. Am J Physiol 255: E416–E421
Kember NF, Sissons HA 1976 Quantitative histology of the human growth plate. J Bone Joint Surg Br 58-B: 426–435
Tanner JM, Whitehouse RH, Takaishi M 1966 Standards from birth to maturity for height, weight, height velocity and weight velocity: British children, 1965 I. Arch Dis Child 41: 454–471
Kuhn JL, DeLacey JH, Leenellett EE 1996 Relationship between bone growth rate and hypertrophic chondrocyte volume in New Zealand white rabbits of varying ages. J Orthop Res 14: 706–711
Stevens DG, Boyer MI, Bowen CV 1999 Transplantation of epiphyseal plate allografts between animals of different ages. J Pediatr Orthop 19: 398–403
Robson H, Anderson E, Eden OB, Isaksson O, Shalet S 1998 Chemotherapeutic agents used in the treatment of childhood malignancies have direct effects on growth plate chondrocyte proliferation. J Endocrinol 157: 225–235
Mosier HD 1978 Catch-up and proportionate growth. Med Clin North Am 62: 337–350
Sissons HA, Kember NF 1977 Longitudinal bone growth of the human femur. Postgrad Med J 53: 433–437
Jux C, Leiber K, Hugel U, Blum W, Ohlsson C, Klaus G, Mehls O 1998 Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology 139: 3296–3305
Marin G, Malozowski SN, Barnes K, Southers J, Cristiano A, Cassorla F 1993 Endocrine profile of catch-up growth in the cynomolgus monkey. Acta Endocrinol Copenh 129: 371–374
Heinrichs C, Colli M, Yanovski JA, Laue L, Gerstl NA, Kramer AD, Uyeda JA, Baron J 1997 Effects of fasting on the growth plate: systemic and local mechanisms. Endocrinology 138: 5359–5365
Krieg RJ, Niimi K, Chan JC, Santos F, Hanna JD, Poletti LF 1991 Cortisone effects on growth, food efficiency, and in vitro growth hormone release. Kidney Int 39: 1135–1139
Mosier HD, Jansons RA 1976 Growth hormone during catch-up growth and failure of catch-up growth in rats. Endocrinology 98: 214–219
Wang J, Zhou J, Bondy CA 1999 Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J 13: 1985–1990
Hayflick L 1965 The limited in vitro lifetime of human diploid strains. Exp Cell Res 37: 614–636
Campisi J 1996 Replicative senescence: an old lives' tale?. Cell 84: 497–500
Sedivy JM 1998 Can ends justify the means? Telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc Natl Acad Sci USA 95: 9078–9081
Acknowledgements
We thank Edward F. McCarthy, M.D., and Tracy Hatcher for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the Annual Meeting of the Society for Pediatric Research, May 12–16, Boston, MA, 2000.
Rights and permissions
About this article
Cite this article
Gafni, R., Weise, M., Robrecht, D. et al. Catch-Up Growth Is Associated with Delayed Senescence of the Growth Plate in Rabbits. Pediatr Res 50, 618–623 (2001). https://doi.org/10.1203/00006450-200111000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200111000-00014
This article is cited by
-
Inner histopathologic changes and disproportionate zone volumes in foetal growth plates following gestational hypoglycaemia in rats
Scientific Reports (2020)
-
The effect of mechanical stretch stress on the differentiation and apoptosis of human growth plate chondrocytes
In Vitro Cellular & Developmental Biology - Animal (2017)
-
Effects of corticosterone on the metabolic activity of cultured chicken chondrocytes
BMC Veterinary Research (2015)
-
Multi-scale finite element model of growth plate damage during the development of slipped capital femoral epiphysis
Biomechanics and Modeling in Mechanobiology (2015)
-
Bone health in children and adolescents with steroid-sensitive nephrotic syndrome assessed by DXA and QUS
Pediatric Nephrology (2014)