Abstract
We studied the expression of the CD95 receptor (APO-1/Fas) on peripheral blood T cell subpopulations in 37 HIV-1-infected children and adolescents stratified according to disease stage or antiretroviral treatment regimen and compared the results to values obtained in 12 healthy age-matched control subjects. CD95 expression on CD45RA+ CD45RO−/CD62L+ (resting/naive) and CD45RO+ CD45RA− (primed/memory) CD4+ and CD8+ T cells was assessed quantitatively by four-color and three-color flow cytometry. CD4+ T cells contained a population of predominantly CD95− resting/naive cells and a population of CD95high primed/memory cells, whereas CD8+ T cells had a more uniform pattern of CD95 expression. The percentage of CD95+ CD4+ T cells increased with disease progression because of both an augmented median fluorescence intensity on resting/naïve cells and an increased percentage of CD95high cells. Patients with highly active antiretroviral combination therapy who maintained stable CD4 counts in the presence of elevated plasma viral load had nearly normal numbers of CD95− resting/naive CD4+ T cells, whereas CD95 expression in the CD8+ T cell subset was still elevated compared with control subjects. Low CD95 expression on resting/naive CD4+ T cells may therefore indicate a low risk for disease progression in antiretrovirally treated and untreated patients.
Similar content being viewed by others
Main
Increased activation-induced cell death caused by apoptosis of T cells has been observed in individuals infected with HIV type-1 and is thought to contribute to the gradual loss of CD4+ T helper cells during progression toward AIDS (1). The CD95/APO-1/Fas receptor (R)/ligand (L) system is critically involved in induction of apoptosis in mature T cells (2–5). Dysregulation of this system contributes at least in part to increased activation-induced cell death of T cells from HIV-1–infected children (6, 7) and adults (8–12). A strong increase in CD95 expression has been demonstrated on freshly isolated T cells from HIV-1–infected individuals (13). The percentage of CD95+ T cells increases further with disease progression as reflected by decreasing peripheral blood CD4+ T helper cell counts (7–11, 14).
It has been shown that the accelerated loss of peripheral blood CD4+ T cells in advanced stages of pediatric HIV-1 infection is preceded by a decrease in T cells expressing only the long isoform of the membrane-bound hematopoietic cell-specific phosphatase CD45 (CD45RA+ T cells) (15, 16). These cells represent a population of resting (i.e. not previously activated), antigen-naive recent thymic emigrants that allow the immune system to react appropriately to newly encountered nonself antigens (17). Once activated, these cells down-regulate CD45RA and up-regulate the short isoform CD45RO by differential splicing at the mRNA level (17). CD45RO+ CD45RA− T cells represent a population of previously activated, antigen-primed memory cells that rapidly respond upon restimulation with previously encountered antigens.
In a study of isolated CD4+ and CD8+ T cells from asymptomatic HIV-1–infected children, we had identified CD45RO+ primed/memory cells as the major CD95+ T cell subpopulation by three-color flow cytometry (18). The percentage of CD95+ peripheral blood T cells increased with decreasing proportions of circulating resting/naive T cells (18). However, in a cross-sectional study of HIV-1–infected children in different stages of the disease, we detected CD95+ CD45RA+ cells in both CD4+ and CD8+ T cell subsets in a substantial number of patients (6). Thus, the increase in CD95+ T cells with progressive disease (7, 14) may be caused by 1) a relative increase in CD95high CD45RO+ (primed/memory) T cells, reflecting the loss of CD95−, resting/naive T cells, or 2) an absolute increase in CD95 receptor density on all peripheral blood T cells.
To clarify this controversy, we measured CD95 expression on T cell subpopulations in 12 HIV-1–infected children, two healthy control subjects, and one child with acute EBV infection with a newly available test system for quantitative assessment of antigen expression using four-color flow cytometry. The results of these experiments enabled us to develop a simple flow cytometric method for measurement of differential CD95 expression on CD4+ T cell subpopulations. A subsequent cross-sectional study in a cohort of 27 HIV-1–infected patients showed that the increase in CD4+ CD95+ T cells with progressive disease was caused by both an augmented CD95 expression on resting/naive cells and an increase in the percentage of CD95high primed/memory T cells, predominantly in late-stage disease. Most importantly, a nearly normal proportion of CD95− resting/naive CD4+ T cells was seen in patients receiving HAART, including HIV-protease inhibitors, for ≥6 mo even in the presence of elevated HIV-1 plasma viral load levels.
METHODS
Patient and control blood donors.
Between January 1996 and December 1999 a total of 37 HIV-1–infected children and adolescents were studied. Twenty-nine patients had acquired HIV-1 by vertical transmission, four via contaminated blood products, and in four patients the mode of infection was unknown. Patients were seen at least at 3-mo intervals at the University Children's Hospitals in Heidelberg, Ulm, Munich, and Frankfurt, Germany. The study was conducted according to the Declaration of Helsinki and approved by the ethical committee of the University Hospital in Heidelberg. CD4+ T cell counts were determined according to published guidelines (19) and used for immunologic classification according to standardized criteria (20). To avoid the unpredictable effects of immune stimulation on these factors, we excluded from the analysis patients with concomitant opportunistic or other infectious diseases at the time of the immunologic investigation.
Measurement of CD95-expression on T cell subpopulations by four-color flow cytometry was performed in 12 randomly selected HIV-1–infected children aged 1 to 14 y, two healthy adult control subjects, and one 3-y-old boy with short-term EBV-infection. Seven of these HIV-1–infected patients received antiretroviral therapy with combinations of two nucleosidic HIV-1 RTI (zidovudine, lamivudine, didanosine or stavudine), three patients received an additional HIV-1 protease inhibitor (indinavir or ritonavir), and two patients had no specific treatment. Immunologically, patients were classified as follows: eight patients in class 1, three in class 2, and one in class 3. Clinical disease stages were four patients in stage A, five in stage B, and three in stage C.
CD95 expression on T cell subpopulations during disease progression was analyzed by stratification of the patients according to the actual disease stage at the time of the study. Patients receiving antiretroviral therapy were only included if they did not take HIV-1 protease inhibitors and treatment had not been changed during the preceding 6 mo. Clinical data of 27 patients eligible for this part of the study are shown in Table 1. In an additional cross-sectional analysis, we stratified all 37 patients according to their treatment regimen at the time of the immunologic investigation (Table 2): no treatment (group A); >6 mo of combination therapy with two HIV-1 RTI (group B); or >6 mo of two RTI plus at least one HIV-1 protease inhibitor, i.e. HAART (group C). Four patients were studied repeatedly before and after changes in their therapeutic regimen and were included in either patient group. All three patient groups were comparable with regard to CD4 counts and HIV-1 plasma viral load levels at the time of the immunologic study, and the proportion of patients with detectable plasma viral load was not significantly different between the two treatment groups.
However, mean patient age in group C was slightly higher than in the other groups, and eight of 15 patients receiving HAART had recovered from severe immune deficiency (immunologic class 3) during therapy. Only three of 16 patients without treatment (group A) and three of 13 patients with RTI alone (group B) had ever reached such an advanced disease stage. Thus, the lack of major differences in CD4 counts among these groups at the time of the immunologic study as well as the absence of HIV-1–related symptoms indicated a significant treatment-induced restoration of T cell functions in patients receiving HAART at least during the observation period of approximately 12 mo.
Control blood samples were obtained from two healthy male adults as well as from 12 age-matched healthy children and adolescents who were followed in our outpatient clinic and who were repeatedly shown to be HIV-1–negative by Western blot, HIV-p24 antigen assay and viral culture, or HIV-1–specific PCR. Children attended the clinic either because they were born to HIV-1–infected mothers (n = 4) or for follow-up of nonmalignant tumors that had been surgically removed and required no radiation or chemotherapy (n = 8). An additional control blood sample was obtained from an HIV-1–negative boy with short-term EBV infection. Patients and control subjects or their relatives gave informed consent before venipuncture.
Immunophenotyping of peripheral blood T cells.
Heparinized peripheral venous blood was obtained during routine blood sampling from all patients. Circulating peripheral blood T cell subpopulations were identified by flow cytometry on a FACScan (Becton Dickinson, Heidelberg, Germany) using combinations of MAb labeled with fluorescein-isothiocyanate (FITC), phycoerythrin (PE) or peridin-chlorophyll (PerCP) in a standardized whole blood lysis assay as recommended by the manufacturer (FACSLyse, Becton Dickinson): CD45RA-FITC, CD45RO-PE (Coulter-Immunotech, Krefeld, Germany), CD62L-PE (Pharmingen, Hamburg, Germany), CD4-PerCP, CD8-PerCP, and CD95-PE (Becton Dickinson). Analysis of CD95 expression as well as the relative distribution of resting/naive (CD45RA+ CD45RO− CD4+ and CD45RA+ CD62L+ CD8+) and primed/memory (CD45RO+ CD45RA−) T cell subpopulations was performed on 10,000 CD4+ or CD8+ lymphocytes using the CellQuest software (Becton Dickinson). The percentage of circulating CD95+ and CD95high T cells as well as that of resting/naive and primed/memory T cells was calculated as described (7, 18, 21).
Quantitation of CD95 expression on T cell subpopulations.
We performed quantitative assessment of surface expression of the CD95 receptor on resting/naive and primed/memory peripheral blood T cell subpopulations in 12 HIV-1–infected patients, two healthy adults, and one patient with short-term EBV infection by four-color flow cytometry using the Quantum Simply Cellular microbead kit (QSC, Sigma Chemical Co., Deisenhofen, Germany) (22). In this assay, the lymphocyte subsets were identified by conventional three-color flow cytometry as described above. CD95 expression on the respective subset was measured after electronic gating with the use of an allophycocyanine (APC)-labeled monoclonal anti-CD95 antibody (a generous gift from Becton Dickinson) on a FACSCalibur flow cytometer (Becton Dickinson). The Quantum Simply Cellular kit provides a mixture of microbeads with defined numbers of binding sites for fluorochrome-labeled Mab, which were stained with CD95-APC together with every patient sample. Following the instructions of the manufacturer, a calibration plot was created, which was subsequently used to convert the immunofluorescence intensity (peak channel) of a given cell population in the patient sample into a mean antibody-binding capacity of a single cell in that population.
Measurement of CD95 expression on resting/naive CD4+ T cells.
Four-color immunophenotyping and flow cytometry of peripheral blood T cells revealed a striking difference in the CD95 expression pattern between CD4+ and CD8+ T cells (Fig. 1). The CD95 expression histogram of CD4+ T cells (Fig. 1A) showed a bimodal expression curve caused by the presence of two clearly distinguishable subpopulations, whereas CD8+ T cells showed a more uniform CD95 expression pattern that did not allow the separation of different subpopulations (Fig. 1B). As shown in Figure 1A, the first peak (peak 1) of the CD95 histogram of CD4+ T cells represents mainly CD95− CD45RA+ CD45RO− (resting/naive) CD4+ T cells with a median fluorescence intensity of about 12, and the second peak corresponds to CD45RO+ CD45RA− (primed/memory) CD4+ T cells with a high expression level of CD95 cells (CD95high cells; median fluorescence intensity >100). CD95 expression was therefore additionally assessed by calculating the median fluorescence intensity of peak 1 resting/naive CD4+ T cells as well as total CD8+ T cells by three-color flow cytometry together with the percentage of circulating CD95+ and CD95high T cells as described above.
FACS analysis of peripheral blood T cells of a patient with congenital HIV-1 infection (stage B2). A, upper histogram shows CD95 expression of electronically gated CD4+ T cells (gated on region R2 in the upper dot plot), the middle histogram shows CD95-expression of CD45RO+ CD45RA− (primed/memory) CD4+ T cells (gated on region R2 in the upper dot plot combined with region R3 in the lower dot plot), and the lower histogram shows CD95-expression of CD45RA+ CD45RO− (resting/naive) CD4+ T cells (gated on region R2 in the upper dot plot combined with region R4 in the lower dot plot). B, respective histograms show CD95 expression of CD8+ T cells (upper histogram; gated on R2), CD45RA− (primed/memory) CD8+ T cells (middle histogram; gated on R2 and R3), and CD45RA+ CD62L/L-selectin+ (resting/naive) CD8+ T cells (lower histogram; gated on R2 and R4). CD4+ T cells show a biphasic expression pattern of the CD95 receptor (peak 1 corresponds to resting/naive cells, peak 2 to primed/memory cells), whereas the pattern of CD8+ T cells is nearly monophasic (primed/memory and resting/naive cells are less well distinguishable). Median fluorescence intensity (MFI) values reflect the CD95 expression level on these T cell subpopulations.
Plasma levels of interferon-γ.
For the measurement of circulating interferon-γ in HIV-1–infected individuals, plasma was immediately separated from freshly drawn venous blood anticoagulated with sodium heparin by centrifugation (1200 ×g, 10 min) and stored at −80°C. Interferon-γ was measured in thawed plasma specimens using a sensitive sandwich ELISA following the instructions of the manufacturer (CLS, Amsterdam, The Netherlands).
Determination of HIV-1 plasma viremia.
For the measurement of HIV-1 viremia (viral load), plasma was immediately separated from freshly drawn venous blood anticoagulated with EDTA by centrifugation (1200 ×g, 10 min) and stored in aliquots of 1 mL at −80°C. For analysis, the thawed aliquots were centrifuged at 16,000 ×g at 4°C for 1 h. Thereafter, the pellet was treated with guanidine thiocyanate, and RNA was extracted with phenol/chloroform. HIV-1 plasma viral load was quantified using either a sensitive second-generation b-DNA assay (Quantiplex HIV RNA 2.0 Assay, Chiron Diagnostics, Ferning, Germany) or a reverse transcription PCR (Amplicor Monitor, Roche Diagnostics, Grenzach-Wyhlen, Germany) as recommended by the manufacturers (23). The lower limit of detection was 500 RNA copies per milliliter in the b-DNA assay and 80 copies per milliliter by reverse transcription PCR.
Statistical analysis.
Data are given as arithmetic mean and SEM or median and range unless otherwise stated. Correlation analysis was performed by calculating both parametric and nonparametric correlation coefficients (Pearson's r and Spearman's ρ, respectively). Differences between groups of patients in different stages of the disease or with different therapeutic regimens and healthy control subjects were tested by ANOVA. Otherwise, testing for statistical significance was done using Wilcoxon's rank sum test or signed rank test (for paired data), as appropriate. A p < 0.05 was assumed to indicate statistical significance. All calculations were performed on a microcomputer using the InStat statistical software package (GraphPad Software Inc., San Diego, CA, U.S.A.).
RESULTS
Differences in CD95 expression patterns among CD4+ and CD8+ T cells.
Assessment of CD95 receptor density by calculating the antibody-binding capacity of different T cell subpopulations using the Quantum Simply Cellular kit allowed us to quantify differences in CD95 expression on peripheral blood T cell subpopulations in 12 randomly selected HIV-1–infected children and adolescents, two healthy adult control subjects and a child with short-term EBV infection (Fig. 2). In all patients and control subjects, we found a significantly higher CD95 expression in primed/memory T cells (Fig. 2B) compared with their resting/naive counterparts (Fig. 2A). In HIV-1–infected patients, CD95 receptor density on resting/naive (CD45RA+ CD45RO−) CD4+ T cells reached only 5% of the receptor density on the respective primed/memory (CD45RO+ CD45RA−) subpopulation (568 ± 43 versus 11,320 ± 1,019 antibody-binding units; mean ± SEM, p < 0.01). In primed/memory T cells, CD95 expression was significantly higher on CD4+ compared with CD8+ cells (3828 ± 274 antibody-binding units;p < 0.01). CD95 expression on resting/naive CD4+ T cells did not differ significantly between CD45RA+ CD45RO− and CD45RA+ CD62L+ cells (599 ± 86 antibody-binding units;p = NS), whereas CD95 receptor density was significantly lower on CD8+ CD45RA+ CD62L+ cells (660 ± 140 antibody-binding units) compared with CD8+ CD45RA+ CD45RO− cells (1644 ± 290 antibody-binding units;p < 0.01). Similar differences in CD95 expression between T cell subpopulations were seen in samples from two healthy adult control subjects, indicating that aging may alter the representation of resting/naive and primed/memory subsets but does not lead to increased CD95 receptor density on circulating resting/naive T cells.
CD95 expression on resting/naive (A) and primed/memory (B) T cells from 12 HIV-1–infected children and adolescents with (•) or without (○ detectable HIV RNA in plasma was slightly increased compared with T cells from two healthy adult control subjects (⋄) and from a 3-y-old boy with short-term EBV infection (▵). The antibody-binding capacity of CD45RO+/CD45RA− (primed/memory) CD4+ T cells was higher than that of the respective CD8+ T cell subpopulation and was apparently not influenced by the HIV-1 plasma viral load level. CD45RA+ CD45RO− CD4+ and CD45RA+ CD62L+ CD8+ (resting/naive) T cells had a lower CD95 receptor density than the corresponding primed/memory subset. CD95 expression on resting/naive T cells tended to increase in patients with detectable HIV-1 plasma viral load; however, the difference between both groups of patients was statistically not significant (p > 0.05). A pronounced increase in CD95 expression on peripheral blood T cell subpopulations was also seen in short-term EBV infection.
To analyze the effect of viral replication on CD95 expression in T cell subpopulations, we further stratified 12 HIV-1–infected patients into six patients with undetectable HIV-1 RNA (<500 copies/mL in the b-DNA assay) and six patients with detectable HIV-1 RNA in plasma (median, 4.6 log10 copies/mL; range, 3.7–4.8). As shown in Figure 2A, patients with detectable plasma viral load tended to have a higher CD95 expression level on resting/naive T cells than patients with undetectable plasma viral load. However, differences between these two groups were not statistically significant, and no major effect of plasma viral load on CD95 expression was detected in the primed/memory subpopulations (Fig. 2B). The apparent effect of active viral replication on CD95 expression on resting/naive T cells seems not to be specific for HIV-1 because it was also seen in a child with short-term EBV infection.
Increase in CD95+ T cells because of elevated CD95 expression on resting/naive T cells and an increased proportion of CD95high T cells.
When comparing CD95 expression patterns in CD8+ T cells from 27 HIV-1–infected children and adolescents (stratified into immunologic classes 1–3) and 12 healthy age-matched control subjects (Fig. 3), we observed that differences in the percentage of CD95+ cells (71 ± 4%versus 47 ± 4%; mean ± SEM), CD95high cells (15 ± 2%versus 4 ± 1%), and the CD95 median fluorescence intensity (20 ± 3 versus 9 ± 1) were statistically significant already during early stages of the disease (immunologic class 1;p < 0.05 when compared with healthy control subjects, ANOVA). In contrast, the increase in CD95 expression on CD4+ T cells was most pronounced in advanced disease stages (immunologic class 3): the elevated percentage of CD95+ CD4+ T cells from these patients compared with healthy control subjects (93 ± 5%versus 48 ± 5%;p < 0.05) was accompanied by both an increase in the percentage of CD95high CD4+ T cells (59 ± 7%versus 15 ± 4%;p < 0.05) and an increase in CD95 receptor density on resting/naive CD4+ T cells (37 ± 10 versus 7 ± 1;p < 0.05).
CD95 expression on CD4+ (•) and CD8+ (○ T cells from 27 HIV-1–infected patients and 12 healthy control subjects (CTRL). Patients are stratified according to the immunologic classes of a standardized classification system (20) : class 1 = normal CD4 counts, n = 7; class 2 = moderately reduced CD4 counts, n = 14; class 3 = severely reduced CD4 counts, n = 6). Horizontal bars indicate the arithmetic mean of the respective patient population. The increased proportion of CD95+ T cells in HIV-1–infected children (A) was caused by both an altered representation of CD95high primed/memory T cells (B) and an increased CD95 expression (median fluorescence intensity) on resting/naive CD4+ and total CD8+ T cells (C) compared with healthy control subjects.
Increased CD95 expression reflects loss of resting/naive T cells.
As shown in Figure 4A, HIV-1–infected children and adolescents showed a decreased percentage of resting/naive CD8+ T cells already in immunologic class 2 (23 ± 2%versus 62 ± 4%;p < 0.05 when compared with healthy age-matched control subjects, ANOVA), whereas primed/memory CD8+ T cells were increased (35 ± 5%versus 9 ± 2%, p < 0.05; Fig. 4B). A significant loss of resting/naive CD4+ T cells was observed only in patients in immunologic class 3 (25 ± 7%versus 60 ± 4%;p < 0.05 compared with healthy age-matched control subjects, ANOVA; Fig. 4A). This loss was accompanied by a relative increase in primed/memory CD4+ T cells (68 ± 7%versus 24 ± 3%, p < 0.05; Fig. 4B). The percentage of resting/naive CD4+ T cells in healthy control subjects decreased significantly with increasing age (r = −0.66;p < 0.05), thus potentially confounding this analysis. However, the change in the distribution of resting/naive and primed/memory T cell subpopulations induced by advanced HIV-1 infection seems to be more pronounced than an effect of aging on the percentage of resting/naive CD4+ T cells alone.
Resting/naive (A) and primed/memory (B) CD4+ (•) and CD8+ (○ T cell subpopulations in 27 HIV-1–infected patients and 12 healthy control subjects (CTRL). Patients are stratified as described in Figure 3. Horizontal bars indicate the arithmetic mean of the respective patient population. The reduced percentage of resting/naive T cells in HIV-1–infected children during late stages of the disease was accompanied by an increased proportion of primed/memory T cells compared with healthy control subjects.
In the CD4+ T cell subpopulation, the percentage of CD95high cells was highly correlated with the percentage of CD45RO+ CD45RA− primed/memory cells (r = 0.88;p < 0.001). However, the slightly higher percentage of CD95+ CD4+ T cells in class 1 patients compared with healthy control subjects (mean difference, 19%; 95% confidence interval, 2 to 36%) was mainly caused by an increased CD95 expression on peak 1 resting/naive CD4+ cells (mean difference, 4.0; 95% confidence interval, 0.8 to 7.2). Neither the percentage of CD95high CD4+ T cells nor the proportion of primed/memory and resting/naive CD4+ T cells of patients in immunologic class 1 was significantly different from healthy control subjects. Thus, two mechanisms contribute to the rise in CD95+ T cells during HIV disease:1) a significant increase in the percentage of primed/memory and CD95high T cells with disease progression, occurring early in the CD8+ T cell compartment and late in CD4+ T cells; and 2) a simultaneous increase in CD95 receptor density on resting/naive T cells, which is already apparent during early stages of the disease (immunologic class 1), at least in CD8+ and, to a lesser extent, also in CD4+ T cells.
Effect of circulating interferon-γ and HIV-1 plasma viral load on CD95 expression.
In CD4+ T cells of HIV-1–infected individuals, the up-regulation of CD95 expression may be caused by an indirect mechanism involving cross-linking of the CD4 receptor by the HIV-1 envelope glycoprotein gp120 (24). Isolated CD4+ T cells treated in vitro with recombinant gp120 and anti-gp120 antibodies showed an up-regulation of CD95, which was shown to depend on secretion of interferon-γ. We therefore determined plasma levels of circulating interferon-γ in 14 patients by means of a sensitive ELISA. Statistical analysis of these data showed that neither the median fluorescence intensity of peak 1 resting/naive CD4+ T cells nor the percentage of CD4+ CD95+ or CD4+ CD95high T cells correlated with plasma levels of interferon-γ (Fig. 5). In the CD8+ T cell compartment, changes in CD95 expression and distribution of resting/naive and primed/memory subsets may be caused by ongoing antigen-specific T cell activation in vivo, which leads to an accelerated naive-to-memory shift, especially of HIV-1–specific cytotoxic T cells. However, CD95 expression on peripheral blood T cells from HIV-1–infected children and adolescents was not correlated with HIV-1 plasma viral load levels (Fig. 6).
No statistically significant correlation was observed between plasma levels of interferon-γ and CD95 expression on CD4+ T cells in 15 HIV-1–infected children and adolescents. A, percentage of CD4+ CD95+ T cells;B, percentage of CD4+ CD95high T cells; and C, CD95 median fluorescence intensity (MFI) on resting/naive (peak 1) CD4+ T cells.
CD95 median fluorescence intensity (MFI) on resting/naive (peak 1) CD4+ T cells (•) and total CD8+ T cells (○ was not related to HIV-1 plasma viral load levels in 27 HIV-1–infected children and adolescents. Note that one data point of CD8+ T cells is hidden because of overlap with data points of CD4+ T cells.
CD95low resting/naive CD4+ T cells and antiretroviral treatment.
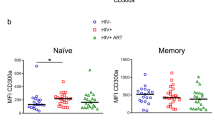
Determination of the percentage of unprimed, antigen-naive T cells (i.e. recent thymic emigrants) and CD95 expression on circulating T cell subpopulations may be used clinically for monitoring of immunologic changes during antiretroviral therapy (25). We therefore analyzed these variables in HIV-1–infected children and adolescents without therapy or with different antiretroviral treatment regimens and compared these values with data obtained from 12 healthy control subjects (Fig. 7). HIV-1–infected individuals showed signs of cellular activation (increase in CD95 expression and decrease in resting/naive cells) in the CD8+ T cell compartment (p < 0.001 compared with control subjects, ANOVA) irrespective of the therapeutic regimen. The mean percentages of resting/naive CD4+ T cells in the patient groups were not significantly different from the value in the control group (p = NS).
CD95 expression and circulating resting/naive CD4+ (•) and CD8+ (○ T cells in HIV-1–infected children and adolescents before antiretroviral therapy (group A;n = 16) and after ≥6 mo of treatment with either two HIV-1 RTI (group B, n = 13) or two RTI plus at least one inhibitor of HIV-1 protease (HAART; group C, n = 15) compared with 12 age-matched healthy control subjects (CTRL). A, percentage of circulating CD4+ CD95+ T cells;B, percentage of circulating resting/naive CD4+ T cells; and C, expression of the CD95 receptor measured as peak 1 median fluorescence intensity (MFI) of resting/naive CD4+ and total CD8+ T cells. Horizontal bars indicate the arithmetic mean of the respective patient population.
In the group without treatment (group A) nine of 16 patients had an increased percentage of CD95+ CD4+ T cells and an increase in median CD95 fluorescence intensity of resting/naive CD4+ T cells (Fig. 7, A and C). These patients were symptomatic at presentation and had already developed laboratory signs of progressive disease. The other patients in group A were clinical and immunologic nonprogressors requiring no antiretroviral therapy (26). These patients had a normal CD95 expression pattern of CD4+ but not of CD8+ T cells. Most importantly, however, 12 of 15 patients treated with HIV-1 protease inhibitors (group C) had a normal number of CD4+ CD95+ T cells as well as a normal proportion of resting/naive CD4+ T cells displaying low CD95 expression (median CD95 fluorescence intensity of peak 1 cells <12) despite high HIV-1 plasma viral load levels.
DISCUSSION
In HIV-1–infected individuals, the gradual loss of CD4+ and CD8+ T cells during progression toward AIDS may in part be caused by the profound dysregulation of the CD95/APO-1/Fas system, which is involved in apoptosis of mature T cells (2–5), hematopoietic stem cells (27), and thymocytes (28). In the study presented here we show that the increase in the percentage of CD95+ T cells with disease progression in HIV-1–infected children and adolescents is related to both an augmented CD95 expression on resting/naive T cells and an increase in the percentage of CD95high primed/memory T cells. In advanced disease stages (immunologic class 3), nearly 100% of the circulating peripheral blood T cells ultimately express the CD95 receptor; this is a consequence of a diminished proportion of CD95− resting/naive T cells and a concomitant increase in CD95high primed/memory T cells. Most patients in this stage of the disease who had been treated with HIV-1 protease inhibitors (HAART) for >6 mo had normal proportions of circulating CD95− resting/naive CD4+ T cells despite the persistence of signs of CD8+ T cell activation and high HIV-1 plasma viral load levels.
CD95 expression patterns clearly differ between CD4+ and CD8+ T cells: although a single, broad peak in CD95 fluorescence intensity is seen in CD8+ T cells, analysis of CD4+ peripheral blood T cells reveals the presence of two different populations:1) CD95low or CD95− cells (peak 1) corresponding to resting/naive cells; and 2) CD95high primed/memory cells. The dynamic process of antigen-induced CD4+ T cell maturation from recent thymic emigrants to antigen-primed effector and memory T cells (naive-to-memory shift) is accompanied by surface expression of specific T cell differentiation markers, and CD4+ T cells may only be regarded as functionally resting and antigen-naive as long as they are CD45RA+ and do not express either CD45RO or CD95 (29, 30).
At least three potential mechanisms may lead to the increased CD95 expression on T cells of HIV-1–infected individuals. First, there is evidence that viral infection of T cells in vitro up-regulates the CD95 receptor on the infected cell (31). Direct infection of target cells, however, is an unlikely cause of increased CD95 expression on peripheral blood T cells in HIV-1–infected patients, as HIV-1 RNA or DNA is detected only in a minority of circulating T cells (32). Second, antigen-specific activation has been shown to induce up-regulation of the CD95 receptor on T cells, e.g. during short-term infection with EBV [ Fig. 2, see also Uehara et al.(33)]. Because HIV-1–infected individuals harbor a large percentage of circulating HIV-1–specific CD8+ cytotoxic T cells (34), CD95 expression on these cells may be a marker of antigen-specific cellular activation. A high percentage of CD95+ CD8+ T cells in such a patient may not necessarily implicate a disturbed function of these cells in vivo.
The frequency of circulating HIV-1–specific CD4+ T helper cells in HIV-1–infected individuals seems to be much lower than that of CD8+ cytotoxic T cells (35), and antigen-specific T cell activation does not completely explain the expression pattern of the CD95 receptor in the CD4+ T cell subset in these patients. Therefore, a third, indirect mechanism of aberrant cellular activation involving cross-linking of CD4 receptors by soluble HIV-1 envelope proteins (gp120) and autologous anti-gp120 antibodies has been invoked in HIV-1–induced up-regulation of CD95 expression. Binding of soluble HIV gp120 protein to the CD4 receptor and subsequent cross-linking by antibodies directed against gp120 has been shown to cause multiple immunologic changes (36, 37), including an increase in autocrine or paracrine secretion of interferon-γ, which may increase CD95 expression specifically on CD4+ T helper cells (24). The lack of correlation between circulating levels of interferon-γ and CD95 expression on CD4+ T cells observed in our study does not contradict this hypothesis. Increased CD95 expression on resting/naive CD4+ T cells (recent thymic emigrants) may occur if cellular activation takes place intrathymically or immediately before the cells leave the thymus. Thus, gp120-induced interferon-γ secretion may induce CD95 expression already on T cells in the thymic medulla, and systemic levels of interferon-γ may not reflect this localized activity.
It is not known whether regulation of CD95 expression at the molecular level is different in CD4+ and CD8+ T cells. In HIV-1–infected children and adolescents, both the decrease in resting/naive CD8+ T cells and the increase in CD95 expression on these cells (compared with healthy control subjects) appears earlier during disease progression than the respective changes in the CD4+ T cell compartment. This may be a consequence of the early expansion of large numbers of activated, antigen-specific CD8+ cytotoxic T cells during an immune response against HIV-1 (34). Wills and coworkers (38) have recently shown that human virus-specific CD8+ cytotoxic T cells expressing high levels of CD45RA may be memory T cells that reverted from a CD45RO+ phenotype. Resting/naive CD8+ T cells are thought to retain the expression of the lymphocyte homing receptor L-selectin (CD62L), and loss of CD62L expression indicates recent cellular activation (15, 16, 18). CD95 expression on CD45RA+ CD62L− CD8+ T cells is slightly higher than on CD45RA+ CD62L+ CD8+ T cells. However, it has not been formally proven that expression of CD62L and CD95 differs between CD45RA+ resting/naive and CD45RA+ primed/memory CD8+ cytotoxic T cells in vivo.
T cell activation in vitro leads to coexpression of both CD45RA and CD45RO (transitional phenotype). These cells always express the CD95 receptor, albeit at a somewhat lower level than cells with a mature primed/memory phenotype. Thus, if naive CD4+ T cells are identified by the expression of CD45RA alone, recently activated cells coexpressing CD45RA, CD45RO, and CD95 are falsely classified as being naive. As quantification of circulating naive T cells (extended T cell phenotyping) becomes increasingly available as a clinical routine procedure in the care of pediatric HIV-1 infection, we suggest including assessment of CD95 expression into these assays. Combining extended T cell phenotyping with a direct measurement of thymic output [e.g. quantification of T cell receptor excision circles in peripheral blood T cells; see Douek et al.(39)] would allow testing of the hypothesis that resting/naive CD4+ T cells with low CD95expression are true recent thymic emigrants. It could then be studied whether the ability of HIV-1–infected children and adolescents to maintain nearly normal numbers of these cells really indicates a low risk for disease progression.
Although CD95 expression is an absolute prerequisite for sensitivity toward CD95-mediated cell death in human lymphocytes (40, 41), up-regulation of the CD95 receptor and sensitivity toward CD95-induced proapoptotic signals are clearly distinct events at the molecular level (42, 43). This is demonstrated by the fact that freshly isolated CD45RO+ primed/memory T cells of healthy donors are completely resistant toward anti-CD95–induced apoptosis despite the elevated expression of CD95 (42, 44). Thus, our observation of an increased CD95 expression on resting/naive T cells does not per se imply a direct relationship between accelerated programmed cell death induced via the CD95 receptor and the disappearance of resting/naive T cells during disease progression. Indeed, in asymptomatic HIV-1–infected children, we found a complete resistance of these cells toward anti-CD95 treatment in vitro(18).
In summary, our data suggest that the presence of a high percentage of circulating resting/naive CD4+ T cells with low CD95 expression is associated with slow or absent disease progression in antiretrovirally treated and untreated HIV-1–infected children and adolescents. These cells probably represent antigen-naive recent thymic emigrants that are able to react specifically to newly presented nonself antigens. The quantification of this CD4+ T cell subpopulation by a simple flow cytometric assay might be used for clinical decision making, e.g. when to change antiretroviral drugs in a patient treated with a protease inhibitor exhibiting a rising HIV-1 plasma viral load. This patient may have developed viral rebound caused by resistance against antiretroviral drugs. Under these circumstances, HIV-1 plasma viral load may not be a reliable indicator of disease progression (45). To clarify whether therapeutic decision making in such patients could be guided by the measurement of CD95 expression on resting/naive CD4+ T cells, the predictive value of extended T cell phenotyping for the risk of disease progression toward AIDS should be assessed prospectively in a larger cohort of HIV-infected children.
Abbreviations
- b-DNA:
-
branched-chain DNA
- CD:
-
cluster of differentiation
- EBV:
-
Epstein-Barr virus
- HAART:
-
highly active antiretroviral therapy
- RTI:
-
reverse transcriptase inhibitor
References
Gougeon M-L, Lecoeur H, Dulioust A, Enouf M-G, Crouvoisier M, Goujard C, Debord T, Montagnier L 1996 Programmed cell death in peripheral lymphocytes from HIV-infected persons. Increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol 156: 3509–3520
Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH 1995 Autocrine T cell suicide mediated by APO-1 (Fas/CD95). Nature 373: 438–441
Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboul A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR 1995 Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T cell hybridomas. Nature 373: 441–444
Ju ST, Panka DJ, Cul H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rohstein A 1995 Fas (CD95)/FasL interactions required for programmed cell death after T cell activation. Nature 373: 444–448
Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH 1995 Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 181: 71–76
Bäumler CB, Böhler T, Herr I, Benner A, Krammer PH, Debatin K-M 1996 Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood 88: 1741–1746
Böhler T, Bäumler C, Herr I, Groll A, Kurz M, Debatin K-M 1997 Activation of the CD95 system increases with disease progression in human immunodeficiency virus type 1-infected children and adolescents. Pediatr Infect Dis J 16: 754–759
Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA 1995 Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med 181: 2029–2036
Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC 1996 Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87: 4959–4966
Gehri R, Hahn S, Rothen M, Steuerwald M, Nuesch R, Erb P 1996 The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS 10: 9–16
Silvestris F, Cafforio P, Frassanito MA, Tucci M, Romito A, Nagata S, Dammacco F 1996 Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS 10: 131–141
Sloand EM, Young NS, Kumar P, Weichold FF, Sato T, Maciejewski JP 1997 Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood 89: 1357–1363
Debatin K-M, Fahrig-Faissner A, Enenkel-Stoodt S, Kreuz W, Benner A, Krammer PH 1994 High expression of APO-1 (CD95) on T-lymphocytes from human immunodeficiency virus infected children. Blood 83: 3101–3103
Stricker K, Knipping E, Böhler T, Benner A, Krammer PH, Debatin K-M 1998 Anti-CD95 (APO-1/Fas) autoantibodies and T cell depletion in human immunodeficiency virus Type 1 (HIV-1)-infected children. Cell Death Differ 5: 222–230
Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA 1995 CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest 95: 2061–2066
Rabin RL, Roederer M, Maldonado Y, Petru A, Herzenberg LA, Herzenberg LA 1995 Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Invest 95: 2054–2060
Cerottini J-C, MacDonald HR 1989 The cellular basis of T-cell memory. Annu Rev Immunol 7: 77–89
Böhler T, Nedel S, Debatin K-M 1997 CD95-induced apoptosis contributes to loss of primed/memory but not resting/naive T cells in children infected with human immunodeficiency virus type 1. Pediatr Res 41: 878–885
Centers for Disease Control (CDC) 1992 Guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus infection. MMWR Morb Mortal Wkly Rep 41( RR-8): 1–17
Centers for Disease Control (CDC) 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep 43( RR-12): 1–10
Böhler T, Walcher J, Hölzl-Wenig G, Geiss M, Buchholz B, Linde R, Debatin K-M 1999 Early effects of antiretroviral combination therapy on T-cell activation and homeostasis in human immunodeficiency virus type-1-infected children and adolescents. AIDS 13: 779–789
Zagursky RJ, Sharp D, Solomon KA, Schwartz A 1995 Quantitation of cellular receptors by a new immunocytochemical flow cytometry technique. Biotechniques 18: 504–509
Böhler T, Debatin KM, Geiss M, Schnitzler P, Geiss HK, Haas J 1998 Molecular diagnosis of human immunodeficiency virus type 1 infection. Pädiatr Grenzgeb 37: 151–163
Oyaizu N, McCloskey TW, Than Soe Hu R, Kalyanaraman VS, Pahwa S 1994 Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood 84: 2622–2631
Böhler T 1998 Emerging issues in the care of HIV-infected infants, children and adolescents. Pädiatr Grenzgeb 37: 333–340
Wahn V, Bialek R, Böhler T, Funk M, Grosch-Wörner I, Horneff G, Notheis G, Wintergerst U 1998 Aktuelle Empfehlungen zur antiretroviralen Therapie bei HIV-infizierten Kindern. Monatsschr Kinderheilkd 146: 1081–1091
Stahnke K, Hecker S, Kohne E, Debatin K-M 1998 CD95 (APO-1/FAS)-mediated apoptosis in cytokine-activated hematopoietic cells. Exp Hematol 26: 844–850
Debatin K-M, Süss D, Krammer PH 1994 Differential expression of APO-1 on human thymocytes: implications for negative selection?. Eur J Immunol 24: 753
Carter LL, Zhang X, Dubey C, Rogers P, Tsui L, Swain SL 1998 Regulation of T cell subsets from naive to memory. J Immunother 21: 181–187
Opferman JT, Ober BT, Ashton-Rickardt PG 1999 Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283: 1745–1748
Sieg S, Huang Y, Kaplan D 1997 Viral regulation of CD95 expression and apoptosis in T lymphocytes. J Immunol 159: 1192–1199
Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD 1996 HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271: 1582–1586
Uehara T, Miyawaki T, Ohta K, Tamaru Y, Yokoi T, Nakamura S, Tanaguchi N 1992 Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood 80: 452–458
McMichael AJ 1996 HIV—the immune response. Curr Opin Immunol 8: 537–539
Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD 1997 Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278: 1447–1450
Finkel TH, Banda NK 1994 Indirect mechanisms of HIV pathogenesis: how does HIV kill T cells?. Curr Opin Immunol 6: 605
Berndt C, Möpps B, Angermüller S, Gierschik P, Krammer PH 1998 CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc Natl Acad Sci USA 95: 12556–12561
Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JG 1999 Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhighin vivo : CD45RAhighCD8+ T cells comprise both naive and memory cells. J Immunol 162: 7080–7087
Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA 1998 Changes in thymic function with age and during the treatment of HIV infection. Nature 396: 690–695
Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fischer A, de Villartay JP 1995 Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268: 1347–1349
Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middelton LA, Lin AY, Strober W, Lenardo MJ, Puck JM 1995 Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81: 935–946
Klas C, Debatin K-M, Jonker RR, Krammer PH 1993 Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol 5: 625–630
Peter ME, Kischkel FC, Scheuerpflug CG, Medema JP, Debatin K-M, Krammer PH 1997 Resistance of cultured peripheral T cells towards activation-induced cell death involves a lack of recruitment of FLICE (MACH/caspase 8) to the CD95 death-inducing signaling complex. Eur J Immunol 27: 1207–1212
Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Tanguchi N 1992 Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol 149: 3753–3758
Kaufmann D, Pantaleo G, Sudre P, Telenti A, for the Swiss HIV Cohort Study 1998 CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Lancet 351: 723–724
Acknowledgements
The authors thank Gaby Hölzl-Wenig and Magdalena Geiss for e-cellent technical assistance, the nurses at the Children's Hospitals in Heidelberg, Ulm, Munich, and Frankfurt for their continuous support in patient care, and our patients and their families for their willingness to participate in the study.
Author information
Authors and Affiliations
Additional information
Dr. Thomas Böhle, University Children's Hospital, Prittwitzstraβe 43, 89075 Ulm, Germany.
Rights and permissions
About this article
Cite this article
Böhler, T., Wintergerst, U., Linde, R. et al. CD95 (APO-1/Fas) Expression on Naive CD4+ T Cells Increases with Disease Progression in HIV-Infected Children and Adolescents: Effect of Highly Active Antiretroviral Therapy (HAART). Pediatr Res 49, 101–110 (2001). https://doi.org/10.1203/00006450-200101000-00021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200101000-00021
This article is cited by
-
Activation and maturation of peripheral blood T cells in HIV‐1‐infected and HIV‐1‐uninfected adults in Burkina Faso: a cross‐sectional study
Journal of the International AIDS Society (2011)
-
Constitutive activation and accelerated maturation of peripheral blood t cells in healthy adults in burkina faso compared to Germany: The case of malaria?
European Journal of Medical Research (2011)