Abstract
Maintenance of adequate perfusion is essential for health of the intestinal mucosa. Methods available to assess intestinal perfusion provide information on mesenteric blood flow, which may differ from mucosal flow. Intramucosal pH (pHi) is influenced by tissue oxygenation and perfusion. Gastric pHi can be measured using the technique of tonometry. A prospective observational clinical study was performed to examine relationships between measured gastric pHi and mucosal CO2 (mCO2), and acid-base balance, gastrointestinal complications (necrotizing enterocolitis and perforation), and death in infants <1500 g birth weight. A nasogastric tonometry catheter (size 5F) was inserted into the stomach of infants, and pHi was calculated from mCO2 levels measured using saline tonometry. Measurements were performed at 3, 12, 24, and 48 h, then daily until arterial access was unavailable. Two hundred eleven sets of measurements were performed on 38 infants [birth weight (mean ±SD), 863 ± 241 g; gestation, 26.5 ± 1.8 wk; and median Clinical Risk Index for Babies score, 8.0 (interquartile range, 5.0–10.75)]. Mean pHi was 7.27 (95% confidence interval, 7.26–7.28) and mean mCO2 was 47.0 mm Hg (95% confidence interval, 45.7–48.3 mm Hg). pHi and mCO2 correlated significantly with arterial pH (pHa), arterial Pco2 (Paco2), and arterial base excess. There were no significant relationships between pHa and pH gap (pHa−pHi) or CO2 gap (mCO2−Paco2). Recurrent low pHi (<7.2 on more than one occasion) and an mCO2/Paco2 ratio of ≥1.29 were significantly associated with an increase in gastrointestinal complications. There were no statistically significant associations with death. In conclusion, changes in pH gap and CO2 gap can occur without alteration in pHa. Abnormalities in pHi might predict gastrointestinal complications in infants <1500 g.
Similar content being viewed by others
Main
Mesenteric ischemia has been implicated in the pathogenesis of NEC (1, 2), which remains a significant cause of morbidity and mortality in VLBW infants (3). Hypoxia, either ante- or postnatally, may lead to redistribution of the cardiac output away from nonessential organs, including the intestine, to preserve oxygenation of essential organs. This may result in an imbalance of oxygen supply and demand in the gut, which may be further exacerbated by the introduction of enteral nutrition.
The ability to study intestinal perfusion at the mucosal level might benefit both the researcher attempting further to understand NEC and the clinician determining the optimal time to introduce milk feeds (4, 5). Previously the only method available for the study of intestinal perfusion in the human newborn infant has been the study of blood flow velocity in the SMA and celiac artery using Doppler ultrasound. Although this may give insight into the resistance in the intestinal vascular bed, it cannot give information about the health of the intestinal mucosa.
Gastric pHi, which will be influenced by oxygenation and perfusion, has been measured in animals and humans using the minimally invasive technique of gastric tonometry (6). The presence of large quantities of carbonic anhydrase in gastric mucosal cells enables rapid equilibration of CO2 from the submucosa to the gastric lumen. This technique is dependent on being able to measure CO2 at the mucosal surface (mCO2) by equilibration with sterile saline in an intragastric gas-permeable Silastic balloon and substituting the result together with a concurrent estimate of arterial bicarbonate into the Henderson-Hasselbach equation.
In animals this measurement of pHi has been shown to fall in response to gut ischemia induced by mechanical (7), physiologic (8, 9), and pharmacologic (10) methods. In newborn piglets a relationship has been shown between pHi and SMA blood flow (10). These studies have shown that pHi may change in response to ischemia or regional hypoxia in the absence of changes in systemic acid-base balance or hemodynamics.
In adult critical care, sustained low gastric pHi has been associated with an increased risk of mortality and multiple organ dysfunction (11). Changes in mucosal blood flow both in the small intestine and the stomach measured using scanning laser Doppler have been correlated with pHi(12, 13) measurements.
In limited data from the pediatric population, low values of pHi have been demonstrated to be predictive of mortality in septic shock (14) and independently to predict mortality in intensive care patients (15). In a population of children undergoing extracorporeal life support, the CO2 gap (Paco2−mCO2) had greater predictive power for mortality than pHi, the predictive value for death with a CO2 gap of >10 mm Hg being 92%(16).
The objectives of the present study were to assess whether tonometry could be successfully performed in a population of VLBW infants; to establish ranges of values for pHi and mCO2 in this population in the early neonatal period, and to explore relationships between measured variables and systemic acid-base to quantify imbalances in O2 supply and demand at the mucosal level. In addition, pilot data were collected to examine for associations between the measured and calculated variables and the development of major GI complications and death.
METHODS
This was a prospective observational clinical study of pHi in infants <1500 g birth weight in the early neonatal period.
Inclusion criteria were a birth weight <1500 g and the presence of an indwelling arterial catheter. All catheters were placed for clinical purposes at the discretion of the responsible clinician. Infants with life-threatening congenital malformations or chromosomal anomalies were excluded. Recruitment only occurred when M.E.C. was available to perform measurements.
The study protocol was approved by the East London and City Health Authority Research Ethics Committee, and informed parental consent was obtained before study entry. Infants were recruited into the study as early as possible after delivery up to 24 h of age from the population admitted to the Neonatal Intensive Care Unit at the Homerton Hospital, London, during the period September 1998 to December 1999 inclusive. All intensive care management was conducted according to standard unit protocol. Decisions on the timing of commencement of enteral feed were made by the attending clinicians, who were not informed of pHi and mCO2 values.
After recruitment, measurements were taken at 3, 12, 24, and 48 h after study entry and thereafter on a daily basis until death or removal of the indwelling arterial line. Tonometry measurements were performed using a standard protocol. A single-use size 5F dual-lumen nasogastric tonometry catheter (Tonometrics Inc., Worchester, MA, U.S.A.) was passed into the infant's stomach, and correct placement was confirmed at the time of routine abdominal radiography for arterial line position. At each sampling time the catheter balloon was instilled with 1.0 mL of sterile 0.9% saline and left to equilibrate for 45 min. After equilibration, 0.3 mL of saline was withdrawn and discarded. The remaining 0.7-mL sample was withdrawn anaerobically and immediately analyzed for CO2 content using a standard blood gas analyser (ABL Radiometer 520, Radiometer Medical A/S, DK-2700, Brønshøj, Denmark). The tonometry catheter used contained a gas-permeable Silastic balloon for which the correction factors for equilibration time for CO2 had been predetermined by the manufacturers. mCO2 levels were calculated using the correction factor for a 45-min dwell time, mCO2 = saline CO2 × 1.13. Concurrent arterial blood samples of 0.2-mL volume were drawn for measurement of blood gases and serum lactate (YSI 2300 Glucose/Lactate Analyzer, YSI, Inc., Yellow Springs, OH, U.S.A.). A single observer (M.E.C.) performed all measurements.
The nasogastric lumen of the catheters was left on free drainage and aspirated every 4–6 h. The gastric contents were aspirated before performing all measurements. Infants were not routinely given H2 receptor antagonists.
Intramucosal pH (pHi) was calculated using the Henderson-Hasselbalch equation: pHI = 6.1 + log [HCO3]/(mCO2 × 0.031). Where 6.1 is the negative logarithm of the dissociation coefficient of carbonic acid and 0.031 is the solubility of CO2 in plasma, mCO2 is the mucosal CO2 measured tonometrically and HCO3 is the bicarbonate concentration of a concurrent arterial blood gas. The following variables were calculated: pH gap = pHa − pHi, CO2 ratio = mCO2/Paco2, and CO2 gap = mCO2 − Paco2.
Repeatability of saline CO2 measurements was assessed by aspirating two samples simultaneously after removal of the catheter dead space. Samples were sealed and then immediately analyzed for CO2 content.
The following clinical data were collected:
Gestational age, birth weight, length, and cord gases as available.
Arterial blood gas and serum lactate were estimated concurrently with the tonometry saline CO2 measurements.
CRIB (severity of illness) scores, which has been shown to predict mortality in this birth weight group (17), were calculated for all subjects at 12 h of age. The total possible score of 22 is contributed to by birth weight (>1350 g, 0; 851–1350 g, 1; 701–850 g, 4; ≤700 g, 7), gestational age (>24 wk, 0; ≤24 wk, 1), congenital malformations (none, 0; not acutely life-threatening, 1; acutely life-threatening, 3), maximum base excess (>−7.0 mM, 0−7.0 to −9.9 mM, 1; −10.0 to −14.9 mM, 2; ≤−15.0 mM, 3), minimum appropriate inspired O2 (≤40%, 0; 41–60%, 2; 91–100%, 3), and maximum appropriate inspired O2 (≤40%, 0; 41–80%, 1; 81–90%, 3; 91–100%, 5).
Neonatal complications including overt signs of NEC.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS; Chicago, IL, U.S.A.). Statistical significance was set at the 95% level. Data are presented as mean ±SD except when populations were not normally distributed, in which case data are presented as median (interquartile ranges).
Differences among groups were analyzed using ANOVA, t test, and Mann-Whitney when normality tests failed. Differences in proportions between two groups were analyzed using Fisher's exact test and for more than two groups using χ2 for trend analysis. The CV was calculated using the following formula: CV (%) = SD/mean × 100.
Correlation of mean values from individual subjects was used to assess the strength of the relationship between variables. Correlations were performed using Pearson's parametric correlation.
The clinical outcomes of death and major GI complications (NEC proven by the presence of intramural gas or perforation) were assessed in relation to the following tonometry-derived variables:
-
pHi, with the subjects divided into three groups according to the lowest recorded pHi value (group A lowest pHI ≥ 7.25, group B lowest pHI < 7.25, and group C lowest pHI < 7.2 more than one occasions). These three groups were mutually exclusive.
-
A CO2 gap >10 mm Hg.
-
A CO2 ratio ≥ 1.29; this ratio represents the upper limit of a CO2 gap of >10 mm Hg within the normal Paco2 range of 35–45 mm Hg.
RESULTS
During the study period, 38 infants were recruited (Table 1). Thirty-seven infants had umbilical arterial lines, and one infant had a radial arterial line inserted.
Gastric tonometry was successfully performed in all 38 infants; in one infant the tonometry catheter was found on radiography to be in the right main bronchus and was replaced. There were no complications encountered with tube dislodgement or balloon leakage. A total of 211 paired measurements of pHa and pHi were performed in the 38 subjects.
Repeatability of saline CO2 measurements under stable ventilatory and acid-base conditions were assessed on 26 occasions, the CV of CO2 was 5.6% (95% CI, 4.3–7.1%).
Mean values for pHa, pHi, Paco2, mCO2, pH gap, CO2 gap, and arterial base excess from all measurements (n = 211) are summarized in Table 2. The mean difference between pHa and pHi was 0.064 ± 0.046 (95%CI, 0.058–0.07;p < 0.001) and between Paco2 and mCO2 was 5.41 ± 4.6 mm Hg (95% CI, 4.79–6.03 mm Hg;p < 0.001). Mean pHa and pHi at each time point measured during the first 5 d of life are illustrated in Figure 1.
Changes in arterial and intramucosal pH. Mean values (±SD) for arterial and intramucosal pH at each time point during the first 5 d of life are presented; the number of subjects from which measurements were taken at each time point are presented in parentheses. pHi values are significantly lower than pHa values at each time point.
Relationships between variables were examined using correlation analysis by calculating the mean values of each variable for each subject (n = 38) (Table 3).
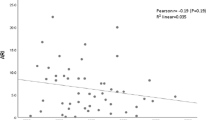
mCO2 values correlated significantly with Paco2 (r = 0.9;p < 0.001; (Fig. 2) and pHa (r = −0.83;p < 0.01). Similarly the calculated pHi values correlated significantly with Paco2 (r = −0.635;p < 0.001) and pHa (r = 0.88;p < 0.001;Fig. 3). There were no significant relationships between pHa and pH gap or CO2 gap. Arterial base excess correlated significantly with mCO2 (r = −0.35;p = 0.03), pHi (r = 0.79;p < 0.0001;Figs. 4 and 5), and pH gap (r = −0.21;p < 0.001). There was no significant relationship between base excess and CO2 gap or CO2 ratio.
Lactate levels were high at birth; the mean value at 3 h was 1.44 mM (95% CI, 1.16–1.72 mM), then decreased significantly (p < 0.0001). There was no significant relationship demonstrated between lactate and pHi or mCO2.
Birth weight and gestation were not significantly different among groups A, B, and C, although there was a nonsignificant trend toward lower gestation and birth weight in group C (Table 4). There was a significant trend toward an increased incidence of major GI complications in group C (p = 0.038, df = 1,χ2=4.5). There were no differences among groups in survival to discharge.
Eighteen infants had a CO2 gap of >10mm Hg. There was a trend toward an increased incidence of GI complications in these infants (5 of 18) in comparison to those with a gap of <10mm Hg (1 of 20), but this did not achieve statistical significance (p = 0.08).
A CO2 ratio of ≥1.29 was present in 18 infants, six of whom developed major GI complications; there were no GI complications in the 20 infants with a ratio of <1.29 (p = 0.007).
There were no statistically significant associations between low pHi, a raised CO2 gap, or CO2 ratio and death.
DISCUSSION
There are few previous published data on gastric tonometry in the pediatric population, and there are none in preterm infants.
We have performed a total of 211 tonometry measurements in a population of 38 VLBW infants without complication. Measurement errors were minimized by having all measurements performed by a single operator. Saline CO2 levels were highly reproducible with a CV of 5.6%. Problems relating to the instability of CO2 in saline were minimized by immediate analysis of saline samples. The underestimation of saline CO2 levels by standard blood gas analyzers (18) has been shown to be improved by the use of a phosphate-buffered solution (19). We elected to use a normal saline solution in the tonometry balloon as the blood gas analyzer used in this study (ABL 520) has been demonstrated to have the smallest bias in saline CO2 measurement (18). That no measurement of pHi was higher than concurrent pHa suggests that there was little CO2 diffusion before measurement in the blood gas analyzer. Errors may occur as a result of CO2 being generated in the stomach by the neutralization of gastric acid by duodenal bicarbonate. This effect may be minimized by administration of H2 receptor antagonists (20, 21) or by aspiration of gastric contents before performing tonometry measurements (22). In a population of critically ill patients, pHi values were not affected by the use of ranitidine (23). In this study H2 receptor antagonists were not routinely administered; however, nasogastric tubes were kept on free drainage and aspirated before the tonometry measurements. The removal of the arterial line coincided with the commencement of enteral nutrition in all but one infant, who received 0.5 mL/6 h expressed maternal milk for a 24-h period while being studied. None of these infants were receiving H2 antagonists; premature infants demonstrate little unstimulated acid secretion, and acid-neutralization is unlikely to be a source of significant error. Theoretically tonometry measurements can be performed in infants being fed provided there is a sufficient time interval between feeds to allow for saline equilibration and all gastric contents are aspirated before study, but there are no published data in this population.
The time taken for CO2 equilibration using saline tonometry may mask acute changes occurring in mCO2 generation. This problem may be overcome using the technique of recirculating gas tonometry (24, 25), but currently suitable size 5F catheters are not available.
Using the total set of 211 measurements, we have established ranges for pHi, pH gap, mCO2, and CO2 gap in this population of infants (Table 2). pHi values have previously been reported in healthy children undergoing general anesthesia [mean pHi = 7.35 ± 0.06;(26)] and in a population of children with sepsis receiving intensive care [pHi = 7.32 ± 0.18 in nonsurvivors and 7.48 ± 0.07 in survivors;(27)]. The mean value reported in the present study (pHi = 7.27 ± 0.078) is lower than in these pediatric populations.
This difference is likely to be contributed to by the tight control of ventilation achieved by clinical protocols in older children, by increased buffering capacity in comparison with this preterm population, and by the severity of illness in the study population as indicated by high CRIB scores (Table 1).
In previous studies relationships have been found between pHi and serum lactate (28, 29). In this study the high early lactate levels reflect the obstetric complications leading to preterm delivery. These elevated levels may not be attributable only to anaerobic glycolysis but also to direct effects of epinephrine on lactate metabolism in a stressed population (30, 31). The high lactate levels in the first 48 h may be masking an underlying relationship with pHi. Unfortunately there are insufficient data beyond this time to explore associations satisfactorily.
The pH gap, CO2 gap, and CO2 ratio were all independent of pHa. Calculated pH gap and CO2 gap provide an indication of the adequacy of oxygenation at the mucosal level, widening gaps reflecting progressive imbalance in oxygen delivery and mucosal cell metabolism. The absence of significant relationships between these variables and systemic acid-base status demonstrate the ability of the technique of gastric tonometry to detect problems at the mucosal level that might not be suspected from routine measurements of arterial blood gases.
The potential clinical usefulness of tonometry in VLBW infants has been confirmed by recurrent low values of pHi and a CO2 ratio of ≥1.29 both being associated with major GI complications. The association between low pHi and major GI complications has been previously reported in two term infants with hypoplastic left heart syndrome, who developed low levels of pHi before the onset of NEC (32).
Changes in arterial CO2 levels affect the small-caliber resistance vessels producing constriction and dilation in response to systemic hypocapnia or hypercapnia (33). In experimental animals, Guzman et al.(34) have shown that systemic hypocapnia results in ileal mucosal and serosal hypoperfusion. Paco2 levels in the study population varied in response to episodes of acute respiratory deterioration. To adjust for these effects, we calculated CO2 ratio (mCO2/Paco2), in a neonatal model. CO2 ratio has been shown to increase with decreasing intestinal blood flow (10). A ratio of ≥1.29 was significantly associated with increased incidence of major GI complications (p = 0.007), with a sensitivity of 1.0 (95% CI, 0.54–1.0) and specificity of 0.625 (95% CI, 0.44–0.79) for identifying infants who had GI complications.
In contrast to reported adult (11, 29) and pediatric data (14–16, 27) from critically ill patients, we have not found statistically significant associations between death and recurrent low pHi, CO2 ratio of ≥1.29, and CO2 gap of >10 mm Hg in this heterogeneous group of VLBW infants. A larger study would be required to confirm associations between these measurements and death in the newborn. If size 5F catheters for recirculating gas tonometry become available, the sensitivity and specificity of tonometry to predict adverse neonatal outcomes may increase.
Tonometry provides a simple, reproducible way of assessing mucosal metabolic status. This study provides data that suggest that pHi is independently predictive of major GI complications in the neonatal intensive care unit setting. The extent of its usefulness in the research and clinical fields on its own or in combination with other techniques such as Doppler ultrasound needs further evaluation.
Abbreviations
- pHi:
-
intramucosal pH
- pHa:
-
arterial pH
- mCO2:
-
mucosal CO2
- GI:
-
gastrointestinal
- NEC:
-
necrotizing enterocolitis
- VLBW:
-
very low birth weight
- CI:
-
confidence interval
- CV:
-
coefficient of variability
- SMA:
-
superior mesenteric artery
- CRIB:
-
clinical risk index for babies
- Paco2:
-
arterial Pco2
References
Nowicki PT, Nankeveris CA 1994 The role of the circulation in the pathogenesis of necrotizing enterocolitis. Clin Perinatol 219: 219–234
Coombs RC, Morgan ME, Durbin GM, Booth IW, McNeish AS 1992 Abnormal gut blood flow velocities in neonates at risk of necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 15: 13–19
Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL 1991 Necrotizing enterocolitis in very low birthweight infants: biodemographic and clinical correlates. J Pediatr 119: 630–638
Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM 1999 Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 103: 434–439
LaGamma EF, Browne LE 1994 Feeding practices for infants weighing <1500 g at birth and the pathogenesis of necrotizing enterocolitis. Clin Perinatol 21: 271–306
Antonsson JB, Boyle CC III, Kruithoff KL, Wang H, Sacristan E, Rothschild HR, Fink MP 1990 Validation of tonometric measurement of gut intramural pH during endotoxemia and mesenteric occlusion in pigs. Am J Physiol 259: G519–G523
Boros M, Kaszaki J, Ordogh B, Nagy S 1994 Intramucosal pH changes following complete segmental small intestinal ischaemia, as compared with the effects of superior mesenteric artery occlusion. Eur Surg Res 26: 76–86
Hartmann M, Montgomery A, Jonsson K, Haglund U 1991 Tissue oxygenation in hemorrhagic shock measured as transcutaneous oxygen tension, subcutaneous oxygen tension, and gastrointestinal intramucosal pH in pigs. Crit Care Med 19: 205–210
Montgomery A, Hartmann M, Jonsson K, Haglund U 1989 Intramucosal pH measurement with tonometers for detecting gastrointestinal ischemia in porcine hemorrhagic shock. Circ Shock 29: 319–327
Campbell ME, VanAerde JE, Cheung PY, Mayes D 1999 Tonometry to estimate intestinal perfusion in the newborn piglet. Arch Dis Child 81: 105–109
Maynard N, Bihari D, Beale R, Smithies M, Baldock G, Mason R, McColl I 1993 Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA 270: 1203–1210
Boyle NH, Pearce A, Hunter D, Owen WJ, Mason RC 1998 Scanning laser Doppler flowmetry and intraluminal recirculating gas tonometry in the assessment of gastric and jejunal perfusion during oesophageal resection. Br J Surg 85: 1407–1411
Elizalde JI, Hernandez C, Llach J, Monton C, Bordas JM, Pique JM, Torres A 1998 Gastric intramucosal acidosis in mechanically ventilated patients: role of mucosal blood flow. Crit Care Med 26: 827–832
Hatherhill M, Tibby SM, Evans R, Murdoch IA 1998 Gastric tonometry in septic shock. Arch Dis Child 78: 155–158
Casado-Flores J, Mora E, Perez-Corral F, Martinez-Azagra A, Garcia-Teresa MA, Ruiz-Lopez MJ 1998 Prognostic value of gastric intramucosal pH in critically ill children. Crit Care Med 26: 1123–1127
Duke T, Butt W, South M, Shann F 1997 The DCO2 measured by gastric tonometry predicts survival in children receiving extracorporeal life support: comparison with other hemodynamic and biochemical information. Chest 111: 174–179
The International Neonatal Network 1993 The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and for comparing performance of neonatal intensive care units. Lancet 342: 193–198
Takala J, Parviainen I, Siloaho M, Ruokonen E, Hamalainen E 1994 Saline pCO2 is an important source of error in the assessment of gastric intramucosal pH. Crit Care Med 22: 1877–1879
Knichwitz G, Kuhmann M, Brodner G, Mertes N, Goeters C, Brussel T 1996 Gastric tonometry: precision and reliability are improved by a phosphate buffered solution. Crit Care Med 24: 512–516
Kolkman JJ, Groenveld AB, Meuwissen SG 1994 Effect of ranitidine on basal and bicarbonate enhanced intragastric pCO2: a tonometric study. Gut 35: 737–741
Heard SO, Helsmoortel CM, Kent JC, Shahnarian A, Fink MP 1991 Gastric tonometry in healthy volunteers: effects of ranitidine on calculated intramural pH. Crit Care Med 19: 271–274
Parviainen I, Vaisanen O, Ruokonen E, Takala J 1996 Effect of nasogastric suction and ranitidine on the calculated gastric intramucosal pH. Intensive Care Med 22: 319–323
Calvet X, Baigorri F, Duarte M, Saura P, Royo C, Joseph D, Mas A, Artigas A 1998 Effect of ranitidine on gastric intramucosal pH in critically ill patients. Intensive Care Med 24: 12–17
Guzman JA, Kruse JA 1996 Development and validation of a technique for continuous monitoring of gastric intramucosal pH. Am J Respir Crit Care Med 153: 694–700
Heinonen PO, Jousela IT, Blomqvist KA, Olkkola KT, Takkunen OS 1997 Validation of air tonometric measurement of gastric regional concentration of CO2 in critically ill septic patients. Intensive Care Med 23: 524–529
Reinoso-Barbero F, Calvo C, Lopez-Herce J, Bueno M, Garcia S 1998 Reference values of gastric intramucosal pH in children. Paed Anaesthesia 8: 135–138
Krafte-Jacobs B, Carver J, Wilkinson JD 1995 Comparison of gastric intramucosal pH and standard perfusional measurements in pediatric septic shock. Chest 108: 220–225
Duke TD, Butt W, South M 1997 Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med 23: 684–692
Friedman G, Berlot G, Kahn RJ, Vincent JL 1995 Combined measurements of blood lactate concentrations and gastric intramucosal pH in patients with severe sepsis. Crit Care Med 23: 1184–1193
Reverte M, Garcia-Barrada MJ, Moratinos J 1991 Change in plasma glucose and lactate evoked by alpha and beta-2-adrenoreceptor stimulation in conscious fasted rabbits. Fund Clin Pharmacol 5: 663–676
James JH, Luchette FA, McCarter FD, Fischer JE 1999 Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 354: 505–508
Hatherhill M, Tibby SM, Denver L, Marsh MJ, Murdoch I 1998 Early detection of necrotizing enterocolitis by gastrointestinal tonometry. Acta Paediatr 87: 344–345
Cullen J, Eger EI 1974 Cardiovascular effects of carbon dioxide in man. Anaesthesiology 41: 345–349
Guzman JA, Kruse JA 1999 Splanchnic hemodynamics and gut mucosal-arterial Pco2 gradient during systemic hypocapnia. J Appl Physiol 87: 1102–1106
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by a grant from the Joint Research Board of St. Bartholomew's Hospital (M.E.C.).
Rights and permissions
About this article
Cite this article
Campbell, M., Costeloe, K. Measuring Intramucosal pH in Very Low Birth Weight Infants. Pediatr Res 50, 398–404 (2001). https://doi.org/10.1203/00006450-200109000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200109000-00016