Abstract
Angiotensin II (ANG II) contracts umbilical arteries and has been hypothesized to regulate fetal blood pressure primarily by altering umbilical vascular resistance. To determine whether systemic arteries in term fetal sheep are sensitive to ANG II, isometric contraction of endothelium-intact isolated fetal renal, mesenteric, and umbilical arteries in response to ANG II was studied. ANG II (10−7 M) elicited contractile responses in all three vessels (43 ± 8%, 99 ± 21%, and 105 ± 5% of the maximal response seen with 90 mM KCl for renal, mesenteric, and umbilical arteries, respectively). The time course of the contractile responses differed among the vessels: renal and mesenteric arteries exhibited rapid transient contraction followed by relaxation, whereas umbilical artery displayed a more slowly developing but sustained contraction (1 ± 0%, 3 ± 1%,and 93 ± 4% of maximal contractile response at 5 min, for renal, mesenteric, and umbilical arteries, respectively). The AT1 receptor antagonist, losartan (10−6 M), abolished contractile responses in renal and mesenteric arteries but only slowed the contraction in umbilical artery in response to ANG II and had no effect on maximal tension. AT2 receptor blockade (PD 123319, 10−7 M) had no significant effect on the response to ANG II in any vessel. Indomethacin (10−6 M) significantly potentiated contraction to ANG II in renal and mesenteric but not umbilical arteries. Northern and Western blot analyses demonstrated the presence of AT1 mRNA and protein in all three vessels. Immunostaining for the AT1 receptor was present in endothelium and the tunica media. These findings demonstrate the AT1 receptor is present and functionally active in fetal systemic arteries and are consistent with previous findings that the umbilical circulation displays a greater responsiveness to ANG II than the systemic vasculature.
Similar content being viewed by others
Main
The renin-angiotensin system is active in the fetus and newborn and is an important modulator of circulatory function (1, 2). The effects of ANG II are mediated by two distinct receptors, classified as type 1 (AT1) and type 2 (AT2) on the basis of selective antagonism by peptide and nonpeptide ligands (3, 4). The tissue distribution and expression of these receptors are developmentally regulated, changing throughout fetal and early postnatal life. In general, AT2 receptor expression is high in embryonic and fetal tissues, decreasing late in development and with postnatal maturation, whereas AT1 receptors appear later in fetal life with expression greatest in tissues regulating cardiovascular and fluid and electrolyte homeostasis (5–8). Most of the known physiologic responses to ANG II are mediated by the AT1 receptor. Far less is known about the role of the AT2 receptor, although there are reasons to believe this receptor exerts antigrowth, antihypertrophic, proapoptotic, and vasodepressor effects (9).
The sites and mechanisms by which ANG II regulates arterial pressure in the fetus are not clear. The umbilical-placental vascular bed is an important regulator of fetal total peripheral vascular resistance and may be the primary site at which circulating ANG II regulates fetal arterial pressure. The umbilical circulation is far more sensitive to infused ANG II than the fetal systemic vasculature as determined by changes in blood flow and vascular resistance (10–12). Recent studies by Kaiser et al.(12) and Cox and Rosenfeld (13) suggest that differences between umbilical and fetal systemic vascular responses to ANG II are related to differences in ANG II receptor subtype expression, AT1 receptors predominating in umbilical arteries and fetal systemic arteries expressing only AT2 receptors.
If ANG II regulates fetal blood pressure by altering umbilical vascular resistance, how then does ANG II regulate blood pressure immediately after birth? In mature animals, extensive interactions between the renin-angiotensin system and the autonomic nervous system in the maintenance of cardiovascular homeostasis are known to exist. For example, ANG II has been shown to stimulate sympathetic outflow, facilitate sympathetic neurotransmission, and modulate baroreceptor reflexes (14, 15). In a previous study, we demonstrated in newborn lambs that endogenous circulating and central ANG II acts to maintain arterial pressure and exerts a tonic effect on baroreflex control of heart rate and renal sympathetic nerve activity (16). However, the direct effects of ANG II on the systemic vasculature early in development have not been evaluated in detail. The present study was designed to determine whether renal and mesenteric arteries of term fetal sheep are sensitive to ANG II, and to compare the contractile responses of these vessels with those of the umbilical artery.
METHODS
Tissue collection.
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee. Time-dated pregnant ewes at 134–140 d gestation (term, 145 d) were obtained from a local source. Undisturbed twins of fetal sheep used for unrelated studies served as the tissue source. Because the unrelated protocol required delivery by cesarean section, pregnant ewes were given anesthetic induction with 12 mg/kg of thiopental sodium, (Abbott Laboratories, North Chicago, IL, U.S.A.), intubated, and ventilated with room air. Low spinal anesthesia (1% lidocaine, 10 mL) was administered to the ewe, after which the lamb was delivered by cesarean section. Fetuses were euthanized with i.v. pentobarbital sodium (50 mg/kg), and the renal, first-generation mesenteric, and external umbilical arteries were collected. Arterial segments, from which residual blood was expressed and fat and connective tissue were removed, were quickly placed in either ice-cold calcium-free PSS, 4% paraformaldehyde, or snap-frozen in liquid nitrogen.
Isolated vessel contractile responses.
Arterial segments were cleaned of adherent connective tissue and cut into 3- to 5-mm rings. The endothelium was left intact, and the rings were mounted in individual 18-mL isolated organ chambers and connected to an isometric force transducer by 32-gauge stainless steel wire. Contractile responses were recorded with an eight-channel MacLab 8E (ADInstruments Pty Ltd. Castle Hill, NSW, Australia) and stored on a Power Macintosh 7300 computer (Apple Computers Inc., Cupertino, CA, U.S.A.). The length-tension relationship was defined experimentally in each vessel using the response to 90 mM KCl at varying degrees of passive stretch. Passive stretch was set at 90% of the tension required to obtain peak responses to KCl (1.5 g for renal and mesenteric arteries, 3.0 g for the umbilical artery), and rings were allowed to equilibrate in PSS at 37°C for 120 min before the start of experimentation. PSS was aerated with a mixture of 95% O2/5% CO2; the composition was as follows (in mM): 130 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7 H2O, 14.9 NaHCO3, 1.6 CaCl2·H2O, 5.5 dextrose, and 0.03 CaN2-EDTA (pH 7.30). The measured osmolality was 293 mosm/kg.
Endothelium-intact isolated vessels underwent testing according to one of the following three protocols. Individual vessel rings were used for only one protocol. From any single fetus, renal, mesenteric, and umbilical arteries were studied in duplicate (two vessel rings from each artery) and simultaneously according to a specific protocol as outlined below. The duplicate responses for each vessel type were averaged to yield a specific data point for that particular vessel.
Protocol 1.
These studies determined the contractile response to ANG II. At the beginning of each experiment, a contraction to 90 mM KCl was recorded by adding KCl directly to the buffer from a 1 M stock solution. The bath was washed three times with PSS for a 10-min period, then the vessels were allowed to equilibrate again for 20 min before addition of ANG II (10−7 M). This concentration has previously been demonstrated in a number of published dose-response studies to be near the effective concentration producing 50% of the maximal response for umbilical artery responses to ANG II (17, 18). The sequence was repeated three times to evaluate the development of tachyphylaxis in the three vessel types.
Protocol 2.
To determine the ANG II receptor type mediating vasoconstriction, a different set of vessels was first contracted with KCl. After washing three times with PSS, vessels were then incubated for 20 min in the presence of the AT1 receptor antagonist, losartan (10−6 M), the AT2 receptor antagonist PD 123319 (10−6 M), or vehicle (PSS) before the contractile response to ANG II (10−7 M) was measured. The umbilical artery contractile responses to ANG II in the presence of a higher concentration of losartan (10−5 M) and the nonselective ANG II receptor antagonist saralasin (10−5 M) were also measured.
Protocol 3.
These studies determined the role of prostaglandins in regulating the contractile responses to ANG II. Arterial segments were first contracted with ANG II (10−7 M), washed three times with PSS, then incubated in indomethacin (10−8 M to 10−6 M) or vehicle for 20 min before ANG II was again added to the bath. Indomethacin was prepared as a stock solution in DMSO. To determine whether prostaglandin production participates in the development of tachyphylaxis, we elected to measure the ANG II response after incubation with indomethacin during the second exposure to ANG II. Therefore, the measured responses are presented as the percent of the contractile response seen in vehicle (DMSO)-treated vessels with the second administration of ANG II.
RNA extraction and Northern blot analysis.
Cellular RNA was isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH, U.S.A.). RNA was quantified spectrophotometrically by absorbance at 260 nm. RNA samples were stored as an ethanol precipitate at −70°C until further analysis. An ovine-specific AT1 receptor 32P-labeled antisense RNA probe previously used and described by our group was used to detect the presence of AT1 mRNA (6). We also attempted but were unsuccessful in detecting AT2 mRNA by Northern blot analysis, likely because of technical difficulties. An 18S rRNA probe prepared from an 18S cDNA clone corresponding to an 82-bp fragment of a highly conserved region of human 18S rRNA (Ambion Inc., Austin, TX, U.S.A.) was used to determine variability in loading and transfer of RNA.
Aliquots of 10 μg of RNA as measured by absorbance at 260 nm were fractionated by 1% formaldehyde-agarose gel electrophoresis. After electrophoresis, RNA was transferred to a 0.45-mm Nytran filter. The filters were prehybridized for 1 h at 60°C in a solution of 50% deionized formamide, 5× SSPE (875 mM sodium chloride, 50 mM sodium phosphate, 5 mM EDTA), 5× Denhardt's reagent, 0.5% SDS, and 200 μg/mL denatured sheared salmon sperm DNA. Hybridization of filters was performed with fresh hybridization buffer solution containing 2 × 106 counts/min/mL of the appropriate radiolabeled probe. The hybridization reaction was performed at 60°C for 12–18 h. Filters were washed with three low-stringency washes (1× SSPE, 0.5% SDS) at 68°C and a high-stringency wash (0.1× SSPE, 0.5%SDS) at 65°C and exposed to Kodak XAR film at −70°C.
Immunoblotting.
Western blot analysis for AT1 and AT2 were performed as previously described (19). Fetal renal cortex (from 140 d gestation, term 145 d) and adult adrenal were used as control tissues for AT1 and AT2 protein. The adult adrenal medulla of large mammals (human, bovine, ovine) expresses high levels of AT1 but not AT2 receptor. Both AT1 and AT2 receptors are present in fetal kidneys, although AT2 expression decreased significantly after completion of nephrogenesis (after 130–135 d gestation) (20–22). Protein concentrations were determined by the method of Lowry, as modified by Peterson (23). The AT1 receptor-specific polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) was raised in rabbits against an epitope corresponding to amino acids 306–359 of the human AT1 receptor and has previously been used for the detection of the ovine AT1 receptor (21, 24). The AT2 receptor-specific polyclonal antibody (Santa Cruz Biotechnology) was raised in rabbits against an epitope corresponding to amino acids 221–363 mapping the carboxy terminus of the human AT2 receptor and has been shown to be mouse, rat, and human reactive. Nitrocellulose blots (20 μg of protein per lane) were incubated with the primary antibody at a 1:2000 (AT1) or 1:6000 (AT2) dilution in 1% gelatin/TTBS (Tween-zo-Tris buffered saline) for 2 h at room temperature. Blots were rinsed, washed, and then incubated with a 1:8000 dilution of goat anti-rabbit horseradish peroxidase–conjugated antibody (Sigma Chemical Co., St. Louis, MO, U.S.A.) in 1% gelatin/TTBS at room temperature for 1 h. Binding of the secondary antibody was detected using a chemiluminescent system consisting of horseradish peroxidase–hydrogen peroxide oxidation of luminol (ECL, Amersham, Arlington Heights, IL, U.S.A.). Blots were then exposed to Fuji RX x-ray film for 1 min.
Immunohistochemistry.
Isolated vessels were fixed in formalin (10%) and embedded in paraffin, and sections were mounted on glass slides. Sections were deparaffinized in xylene and hydrated in an ethanol-PBS series. After a 5-min PBS rinse, sections were incubated in H2O2 (3%) in methanol for 30 min before rinsing with PBS (×2) and blocking with BSA (1% in PBS). Sections were incubated with primary antibody, either rabbit anti-AT1, 1:100 dilution or rabbit anti-AT2, 1:100 dilution overnight at 4°C. The sections were then rinsed two times for 10 min per rinse in PBS and stained using a Vectastain Elite kit (Vector Laboratories, Inc., Burlingame, CA, U.S.A.). The tissue sections were incubated for 30 min in biotinylated secondary antibody, rinsed two times in PBS, and then incubated for 45 min in avidin-peroxidase reagent. After rinsing two times in PBS, sections were incubated in diaminobenzidine for 1–3 min. Sections were rinsed in PBS for 5 min, rinsed quickly in distilled water, counterstained with hematoxylin for 1 min, dehydrated, and then mounted with glass coverslips. Incubation with secondary antibody alone was performed for each vessel type to serve as controls.
Data analysis.
One-way ANOVA was performed for comparisons among groups. When F ratios were significant, Newman-Keuls test was applied to identify which groups were significantly different. Statistical difference was defined as p < 0.05. Values are presented as mean ± SEM.
RESULTS
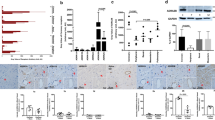
Contractile responses to ANG II were present in all three vessel types, although the character of the responses differed. The force recording shown in Figure 1 demonstrates that both the renal and mesenteric arteries exhibited rapid, transient contractions to ANG II followed by relaxation, whereas the umbilical artery displayed a slower yet far more sustained contraction. The renal artery contractile response elicited by ANG II (relative to the responses to 90 mM KCl) was less (p < 0.05) than that seen in mesenteric or umbilical arteries (Fig. 2, n = 4 for each vessel type). However, the duration of the contractile response was significantly greater in the umbilical compared with the renal and mesenteric arteries. Five minutes after addition of ANG II to the bath buffer, umbilical arteries were still maximally contracted. On the other hand, renal and mesenteric vessels had relaxed to near resting levels by this time. Tachyphylaxis also developed in all three vessels, the extent being greater in renal compared with mesenteric and umbilical arteries (Fig. 3, n = 4 for each vessel type).
Contractile responses to ANG II alone and in the presence of AT1 receptor blockade (losartan) and AT2 receptor blockade (PD 123319). R, renal artery;M, mesenteric artery;U, umbilical artery. For all experiments n = 4. *p < 0.05 compared with ANG II alone for similar vessels. †p < 0.05 compared with maximal response for similar vessels. ‡p < 0.05 compared with mesenteric and umbilical artery responses to ANG II alone.
To determine the ANG II receptor subtype mediating the contractile responses, 20-min incubations in either losartan (10−6 M), PD 123319 (10−6 M), or vehicle were performed before exposure to ANG II (Fig. 2, n = 4 for each vessel type). Losartan significantly inhibited ANG II-induced contractions in renal and mesenteric arteries but at this concentration had no effect on the umbilical artery response. At a concentration of 10−5 M, 100-fold greater than the concentration of ANG II, losartan attenuated but did not completely inhibit the contractile response in umbilical artery (Fig. 4). Contraction to ANG II was completely inhibited by saralasin (10−5 M). No significant effects of PD 123319 on the contractile responses to ANG II were seen in any vessel (Fig. 2).
ANG II responses after exposure to varying concentrations of indomethacin were measured to determine the role of prostaglandins in regulating contractility (Fig. 5). Indomethacin (10−6 M) potentiated the peak contraction to ANG II by >100% in both renal and mesenteric arteries (p < 0.05). A slight but nonsignificant increase in the ANG II response was seen in the umbilical artery. No significant effects of indomethacin at concentrations of 10−7 or 10−8 M were detected. Indomethacin did not appear to alter the time course of the responses to ANG II.
Northern and Western blotting performed on vessels from four different fetuses consistently demonstrated the presence of AT1 mRNA and protein in all three arteries (Figs. 6 and 7). AT1 receptor mRNA was detected by Northern blot analysis at the expected size of 2.4 kb. The immunoblots probed with the AT1 receptor antibody showed bands of the expected size in the renal, mesenteric, and umbilical arteries as well as in term-gestation fetal kidney cortex and adult adrenal, with the major band at 67 kD, as previously reported by Marrero et al.(25). Western blot analysis also demonstrated that AT2 receptor protein was present in all arterial segments, with a major band at approximately 78 kD. The primary band in fetal kidney was present at 44 kD, as previous reported in rat heart and kidney (26, 27). AT2 receptor protein was barely detectable in adult adrenal extracts (Fig. 7).
Western blot analysis for the AT1 and AT2 proteins in ovine fetal renal, mesenteric, and umbilical arteries, fetal kidney (cortex), and adult adrenal homogenates (20 μg loaded per lane). For this figure, tissue samples from two different animals were analyzed. AT1 receptor protein was detected in all three vessels with major bands at approzimately 67 and 40 kD. AT2 receptor protein was also detected in all three vessels and to a lesser extent in fetal kidney (cortex) and adult adrenal.
Immunostaining for AT1 and AT2 receptors was performed to localize the receptors within the arteries. Positive staining for the AT1 receptor protein is seen in all three vessels, most prominently in the outer one third of the tunica media (Fig. 8). Interestingly, staining for AT1 appears more homogeneous in vascular smooth muscle of mesenteric artery than in either renal or mesenteric arteries. AT2 receptors also appeared localized to the outer portion of the media in renal and mesenteric arteries. AT2 staining appeared less intense in umbilical artery compared with systemic arteries.
Immunostaining for AT1 (Top) and AT2 (Middle) receptor proteins in renal (Left), mesenteric (Middle), and umbilical arteries (Right). Bottom, control studies performed using secondary antibody alone. Brown staining depicts positive signal. A, B, and C, immunostaining for AT1 receptor protein in renal, mesenteric, and umbilical arteries, respectively. Magnification ×250 for renal and mesenteric arteries, ×100 for umbilical artery. D, E, and F, immunostaining for AT2 receptor protein in renal, mesenteric, and umbilical arteries, respectively. Magnification ×250 for renal and mesenteric arteries, ×100 for umbilical artery. G, H, and I, staining with secondary antibody alone in renal, mesenteric, and umbilical arteries, respectively. Magnification ×250 for renal and mesenteric arteries, ×100 for umbilical artery.
DISCUSSION
The manner by which the renin-angiotensin system participates in fetal cardiovascular homeostasis is not entirely clear; however, several lines of evidence suggest that regulation of umbilical resistance may be an important mechanism by which ANG II modulates fetal arterial pressure. The umbilical-placental vascular bed, which receives approximately 40% of the combined ventricular output, is believed to contribute substantially to total peripheral resistance in the fetus (28). Both in vitro and in vivo studies have demonstrated that the umbilical circulation is extremely sensitive to ANG II (11, 12, 18, 29, 30). Far less is known, however, regarding the functional responses of fetal systemic vessels to ANG II. We have used a variety of methods to gather data regarding the presence and function of ANG II receptors in fetal systemic arteries and have compared these findings to those seen in the external umbilical artery. Our findings demonstrate that AT1 mRNA and protein are present in isolated fetal ovine renal, mesenteric, and umbilical arteries and that this receptor mediates contraction in response to exogenous ANG II in these vessels. There were, however, inherent differences in the contractile responses of the vessels as the renal and mesenteric arteries exhibited rapid, transient contractions to ANG II followed by relaxation, whereas umbilical arteries displayed a slower and more sustained contraction. Finally, incubation with the cyclooxygenase inhibitor indomethacin significantly enhanced the magnitude of the ANG II-induced contractile response of renal and mesenteric arteries, having little effect on the response of the umbilical artery.
Recently, Cox and Rosenfeld (13) reported that the AT2 receptor appeared to be the predominant ANG II receptor in fetal systemic conduit vessels. In these studies, competitive inhibition binding assays performed in plasma membranes of fetal aorta and carotid and mesenteric arterial vascular smooth muscle failed to demonstrate the presence of a population of AT1 receptors, although AT2 receptors were present. On the other hand, AT1 receptors were present in the umbilical artery and its primary tributaries, but not subsequent branches. In contrast, our findings suggest that AT1 receptors are present in fetal sheep renal and mesenteric arteries. Using Northern blot hybridization, we demonstrated the presence of AT1 mRNA in renal and mesenteric arteries, as well as in umbilical artery. Immunoblotting as well as immunohistochemistry verified the presence of AT1 receptor protein in these vessels. The Western blots demonstrated several bands for the AT1 receptor in the fetal arteries, similar to that previously found by us and others in fetal ovine tissues (19, 22). The presence of multiple bands may be related to various degrees of protein glycosylation, protein degradation, and experimental conditions. For instance, using Western blot analysis for the AT1 receptor in ovine tissues, Wintour and colleagues (22) demonstrated that the band size and numbers differed depending on the source of primary antibody.
In addition to demonstrating the presence of AT1 receptors in fetal systemic and umbilical arteries, we showed that AT1 receptors were functional and mediated contractile responses to ANG II. ANG II-induced contractions in fetal renal and mesenteric arteries were nearly completely abolished by blockade of the AT1 receptor with losartan, a finding consistent with the generally accepted function of this receptor. Interestingly, the concentration of losartan that blocked ANG II-induced contraction in renal and mesenteric arteries had no effect on umbilical artery contraction. At a 10-fold higher concentration (10−5 M), losartan inhibited approximately 70% of the umbilical artery contraction to ANG II. Reasons for this difference in antagonist sensitivity may be related to differences in AT1 receptor number or affinity in different tissues. It also raises the possibility that contraction of the umbilical artery is mediated by a non-AT1 or -AT2 type receptor, which is blocked nonspecifically by losartan at high concentrations and by the ANG II analog saralasin.
Using immunoblotting and immunohistochemistry techniques, we were able to demonstrate expression of AT2 receptors in renal and mesenteric arteries, consistent with the binding studies of Cox and Rosenfeld (13). However, we also localized AT2 receptors to the umbilical artery by immunoblotting, unlike the findings reported by these authors. Not surprisingly, blockade of the AT2 receptors with PD 123319 had no demonstrable effect on ANG II-induced contraction in any vessel type. Although the functions of AT2 receptors are poorly understood, AT2 receptors have previously been reported to cause vasodilation in preglomerular afferent arteriole (31). Whether a similar effect occurs in fetal systemic vessels, which appear to express high concentrations of AT2 receptors, will require further studies using precontracted vessels.
We found dramatic differences in the character of the contractile response of the umbilical artery to KCl and ANG II compared with those seen in the renal or mesenteric artery. Although the initial vasoconstrictor response was of similar magnitude in the mesenteric and umbilical arteries (when expressed as percent of the response to 90 mM KCl), both were significantly greater than the renal artery response. Contraction was slower in onset and far more sustained in the umbilical artery than in the other vessels. Five minutes after addition of ANG II, umbilical artery contraction was still at its maximal response, whereas renal and mesenteric arteries had undergone significant relaxation. Interestingly, Adamson et al.(11) found in fetal sheep that the increase in arterial pressure associated with administration of ANG II occurs before an increase in placental vascular resistance, and they speculated that placental constriction probably contributes more to the maintenance than to the onset of the pressor response. Our data support these conclusions and suggest that vasoconstriction of fetal systemic arteries is responsible for the initial ANG II-induced increase in arterial pressure, whereas the slower but sustained contraction of the umbilical vasculature contributes to sustaining the hypertensive response.
The different characteristics of the contraction responses in renal and mesenteric arteries compared with umbilical artery are intriguing and suggest fundamental differences exist among the vessels. Structural differences among the vessels are one obvious factor that could contribute to the observed differences in responses of the vessels. Because the umbilical artery is a much thicker-walled vessel than the renal or mesenteric artery, the ability of ANG II to reach ANG II receptors may be relatively delayed. Furthermore, Arens et al.(32) found vascular smooth muscle from fetal femoral arteries and aorta to be biochemically and functionally immature compared with umbilical and adult arteries. Specifically, actin and total myosin content were greater in the umbilical artery, and myosin heavy chain (MHC) isoform composition also differed. These differences in contractile protein composition could alter the contractile capacities of the different vessels. However, both renal and mesenteric arteries underwent stable, sustained contractile responses to KCl.
ANG II is known to induce the release of arachidonic acid metabolites, and cyclooxygenase and lipoxygenase inhibitors have been shown to alter ANG II-mediated contractile responses in umbilical and systemic arteries (33–35). We therefore postulated that generation of vasoactive prostanoids might contribute to the different types of contractile responses among the arteries studied. At the highest concentration tested, indomethacin (10−6 M) significantly potentiated the responses to ANG II in renal and mesenteric, but not umbilical, arteries. The transient nature of the ANG II-induced responses was not altered by indomethacin. These findings suggest differences in basal production or ANG II-induced release of vasoactive arachidonic acid metabolites may contribute more to regulating fetal systemic than umbilical artery responsiveness to ANG II. In vivo, prostacyclin infusion has no effect on ovine umbilical-placental resistance, possibly because of the lack of functional receptors (36, 37). Yoshimura et al.(38, 39) have demonstrated that basal prostaglandin production is greater in mesenteric artery than placental vessels, although ANG II increased placental but not mesenteric artery production of prostaglandin E and prostacyclin derivatives. In rats, participation of prostanoids in regulating the vascular response to ANG II is also dependent on developmental stage and blood vessel type (35). Thus, regional differences in the balance between vasodilating and vasoconstricting prostaglandins may directly affect vascular tone in these fetal vessels. Finally, indomethacin, at high concentrations, may directly inhibit prostacyclin-mediated vasodilation and thereby potentiate vasoconstrictive effects of ANG II independent of changes in prostaglandin synthesis (40).
The mechanisms by which ANG II modulates cardiovascular function and blood pressure during fetal life and immediately after birth remain to be fully elucidated. We have previously shown in newborn lambs that both systemic and intracerebroventricular infusion of losartan decreases arterial pressure and alters efferent sympathetic activity, suggesting involvement of peripheral and central AT1 receptors in neural control of circulatory function (16). The finding of a sustained contractile response of the umbilical artery to ANG II is consistent with previous findings that the umbilical artery displays a greater responsiveness to ANG II than the fetal systemic vasculature and supports the hypothesis that ANG II regulates fetal blood pressure by modulating the umbilical-placental vascular bed. The decreased responsiveness of systemic arteries to ANG II may be related to ANG II-stimulated release of vasodilating prostaglandins or paracrine release of other vasoactive substances. Although our results demonstrate that the AT1 receptor is present and functionally active in large systemic arteries of term fetal sheep, these findings may not be representative of responses of the whole vascular bed. Additional studies of resistance vessels are needed to determine whether a direct effect of ANG II on peripheral vascular AT1 receptors contributes to modulation of arterial pressure after the transition from fetal to newborn life, when the umbilical circulation no longer contributes to total peripheral resistance.
Abbreviations
- ANG II:
-
angiotensin II
- PSS:
-
physiologic salt solution
References
Iwamoto HS 1998 Endocrine regulation of the fetal circulation. In: Polin RA, Fox WW (eds). Fetal and Neonatal Physiology, 2nd Ed, WB Saunders, Philadelphia, 961–970.
Davidson D 1987 Circulating vasoactive substances and hemodynamic adjustments at birth in lambs. J Appl Physiol 63: 676–684
Chiu AT, Herblin WF, Ardecky RJ, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL, Timmermans PBMWM 1989 Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun 165: 196–203
Smith RD, Chiu AT, Wong PC, Herblin WF, Timmermans PBMWM 1992 Pharmacology of nonpeptide angiotensin II receptor antagonists. Annu Rev Pharmacol Toxicol 32: 135165 135-1–65
Tsutsumi K, Saavedra JM 1991 Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol 261: R216–R216
Robillard JE, Schutte BC, Page WV, Fedderson JA, Porter CC, Segar JL 1994 Ontogenic changes and regulation of renal angiotensin II type 1 (AT1) receptor gene expression during fetal and newborn life. Pediatr Res 36: 755–762
Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE 1991 Expression of AT2 receptors in the developing rat fetus. J Clin Invest 88: 921–933
Shanmugam S, Sandberg K 1996 Ontogeny of angiotensin II receptors. Cell Biol Int 20: 169–176
Horiuchi M, Akishita M, Dzau VJ 1999 Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 33: 613–621
Iwamoto HS, Rudolph AM 1981 Effects of angiotensin II on the blood flow and its distribution in fetal lambs. Circ Res 48: 183–189
Adamson SL, Morrow RJ, Bull SB, Langille BL 1989 Vasomotor responses of the umbilical circulation in fetal sheep. Am J Physiol 256: R1056–R1062
Kaiser JR, Cox BE, Roy TA, Rosenfeld CR 1998 Differential development of umbilical and systemic arteries: I. ANG II receptor subtype expression. Am J Physiol 274: R807–R807
Cox BE, Rosenfeld CR 1999 Ontogeny of vascular angiotensin II receptor subtype expression in ovine development. Pediatr Res 45: 414–424
Phillips MI 1987 Functions of angiotensin in the central nervous system. Annu Rev Physiol 49: 413–435
Reid IA 1992 Interactions between ANG II, sympathetic nervous system and baroreceptor reflex in regulation of blood pressure. Am J Physiol 262: E763–E778
Segar JL, Minnick A, Nuyt AM, Robillard JE 1997 Role of endogenous ANG II and AT1 receptors in regulating arterial baroreflex responses in newborn lambs. Am J Physiol 272: R1862–R1873
Bjøro K 1985 Effects of angiotensin I and II and their interactions with some prostanoids in perfused human umbilical arteries. Prostaglandins 30: 989–998
Nair X, Dyer DC 1974 Responses of guinea pig umbilical vasculature to vasoactive drugs. Eur J Pharmacol 27: 294–304
Segar JL, Scholz TD, Bedell KA, Smith OM, Huss DJ, Guillery EN 1997 Angiotensin AT1 receptor blockade fails to attenuate pressure-overload cardiac hypertrophy in fetal sheep. Am J Physiol 273: R1501–R1508
Butkus A, Albiston A, Alcorn D, Giles M, McCausland J, Moritz K, Zhou J, Wintour EM 1997 Ontogeny of angiotensin receptors, types 1 and 2, in ovine mesonephros and metanephros. Kidney Int 52: 628–636
Coulter CL, Myers DA, Nathanielsz PW, Bird IM 2000 Ontogeny of angiotensin II type 1 receptor and cytochrome P450c11 in the sheep adrenal gland. Biol Reprod 62: 714–719
Wintour EM, Mortiz K, Butkus A, Baird R, Albiston A, Tenis N 1999 Ontogeny and regulation of the AT1 and AT2 receptors in the ovine fetal adrenal gland. Mol Cell Endocrinol 157: 161–170
Peterson GL 1977 A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83: 346–356
Bird IM, Zheng J, Corbin CJ, Magness RR, Conley AJ 1996 Immunohistochemical analysis of AT1 receptor versus P450c17 and 3 beta HSD expression in ovine adrenals. Endocr Res 22: 349–353
Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE . 1995 Direct stimulation of Jak/STAT pathway by the angiotensin AT1 receptor. Nature 375: 247–250
Wang ZQ, Moore AF, Ozono R, Siragy HM, Carey RM 1998 Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension 32: 78–83
Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM 1997 Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 30: 1238–1246
Dawes GS 1962 The umbilical circulation. Am J Obstet Gynecol 84: 1634–1648
Altura BM, Malaviya D, Reich CF, Orkin LR 1972 Effects of vasoactive agents on isolated human umbilical arteries and veins. Am J Physiol 222: 345–355
Clark KE, Irion GL, Mack CE 1990 Differential responses of uterine and umbilical vasculatures to angiotensin II and norepinephrine. Am J Physiol 259: H197–H203
Zhi-Qin W, Millatt LJ, Heiderstadt NT, Siragy HM, Johns RA, Carey RM 1999 Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension 33: 96–101
Arens Y, Chapados RE, Cox BE, Kamm KE, Rosenfeld CR 1998 Differential development of umbilical and systemic arteries: II. Contractile proteins. Am J Physiol 274: R1815–R1823
Stern N, Golub M, Nozawa K, Berger M, Knoll E, Yanagawa N, Natarajan R, Nadler JL, Tuck ML 1989 Selective inhibition of angiotensin II-mediated vasoconstriction by lipoxygenase blockade. Am J Physiol 257: H434–H443
Bjøro K 1986 Prostacyclin and thromboxane formation in human umbilical arteries following stimulation with vasoactive autacoids. Prostaglandins 31: 699–714
Konishi C, Saito Y, Ohara N, Ono H 1997 Age-related differences and roles of endothelial nitric oxide and prostanoids in angiotensin II responses of isolated, perfused mesenteric arteries and veins of rats. Eur J Pharmacol 320: 175–181
Rankin JH, Phernetton TM, Anderson DF, Berssenbrugge AD 1979 Effect of prostaglandin I2 on ovine placental vasculature. J Dev Physiol 1: 151–160
Paulick RP, Meyers RL, Rudolph AM 1991 Vascular responses of umbilical-placental circulation to vasodilators in fetal lambs. Am J Physiol 261: H9–H14
Yoshimura T, Rosenfeld CR, Magness RR 1991 Angiotensin II and α-agonist: III. In vitro fetal-maternal placental prostaglandins. Am J Physiol 260: E8–E13
Yoshimura T, Magness RR, Rosenfeld CR 1990 Angiotensin II and α-agonist: II. Effects on ovine fetoplacental prostaglandins. Am J Physiol 259: H473–H479
Parfenova H, Zuckerman S, Leffler CW 1995 Inhibitory effect of indomethacin on prostacyclin receptor-mediated cerebral vascular responses. Am J Physiol 268: H1884–H1890
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Segar, J., Barna, T., Acarregui, M. et al. Responses of Fetal Ovine Systemic and Umbilical Arteries to Angiotensin II. Pediatr Res 49, 826–833 (2001). https://doi.org/10.1203/00006450-200106000-00019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200106000-00019