Abstract
Reduced fetal growth is known to be associated with a reduced ovarian fraction of primordial follicles, with ovarian hyperandrogenism and anovulation in late adolescence. In this study, we examined whether adolescent girls born small for gestational age also present an abnormality in uterine or ovarian size. Standardized ultrasound measurements of the internal genitalia were performed in 36 healthy post-menarcheal girls (mean age 14 y) born with a size that was either appropriate for gestational age (AGA) or small (SGA), birth weight averaging 0.1 and −3.0 SD, respectively; clinical and endocrine characteristics were documented concomitantly. Compared with AGA girls, the SGA girls had a smaller uterus (mean difference of 20%;p < 0.006) and a reduced ovarian volume (mean difference of 38%;p < 0.0002). In conclusion, the gynecological correlates of prenatal growth restriction are herewith extended to include a reduced size of the uterus and the ovaries.
Similar content being viewed by others
Main
Reduced fetal growth is known to be associated with a reduced ovarian fraction of primordial follicles (1), ovarian hyperandrogenism (2), and anovulation in late adolescence (3). In this study, we examined whether adolescent girls born small for gestational age also present an abnormality in uterine or ovarian size.
SUBJECTS AND METHODS
Study population and design.
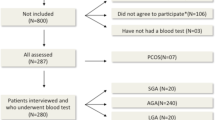
The study population consisted of 36 healthy girls (age 14.2 ± 1.2 y; range 12.6–17.0 y) who had been recruited for this study at the time of discharge from the hospital after an intercurrent, minor illness.
The inclusion criteria were:1) A birth weight for gestational age that was either appropriate (AGA) or small [SGA; below 10th percentile (4)], 2) menarche between 1.5 and 3 y before study, 3) regular menstrual cycles with a duration between 21 and 45 d, and 4) normal body mass index (5).
The exclusion criteria were: evidence for a syndromic, chromosomal, or infectious etiology of low birth weight; hirsutism [defined as a score of 8 or more on the Ferriman and Gallwey scale (6)]; thyroid dysfunction; Cushing syndrome; hyperprolactinemia; previous or current use of oral contraceptive medication.
Pelvic ultrasound examinations were performed together with blood sampling for measurement of plasma LH, FSH, estradiol, testosterone, and sex hormone-binding globulin (SHBG) in the follicular phase (range: day 5 ± 3) of the menstrual cycle.
Birth weight and gestational age data were obtained from hospital records and transformed into SD scores, as previously described (2).
Ultrasonography.
The ultrasound examinations were performed with a full bladder. Scans were obtained by a single observer (GE) using the Acuson 128 XP10 model (Mountain View, CA) with a 5-MHz sector probe. The observer remained blinded for the girls' birth weight throughout the study.
Longitudinal and transverse views of the uterus were obtained and measurements made of uterine length (from the top of the fundus to the cervix), anteroposterior diameters of the cervix and fundus, and uterine cross-sectional area (uterine length × uterine anteroposterior diameter).
Longitudinal and transverse views of the ovaries were obtained for measurement of length, breadth, and depth of each ovary. Ovarian volume (right and left) was calculated using the formula for a modified prolate ellipsoid (depth × breadth × length/2) (7–9). For comparisons between AGA and SGA groups, average volumes of right and left ovary of each girl were used.
Endocrinology.
LH and FSH were measured using a commercially available microparticle enzyme immunoassay (IMX System, Abbott, Chicago, IL). The intra-assay and inter-assay coefficients of variation (CVs) were 3.0% and 5.4% for LH and, respectively, 4.1% and 6.9% for FSH. Serum testosterone and estradiol concentrations were determined by RIA (10) and SHBG levels by enzymo-immuno-chemiluminiscence (11).
Statistics and ethics.
Results are expressed as mean ± SEM, unless stated otherwise. Independent variables were compared by Mann-Whitney test;p values < 0.05 were considered statistically significant.
The study protocol was approved by the Institutional Review Board of the Barcelona Hospital. Informed consent was obtained from the parents and assent from the girls.
RESULTS
Table 1 summarizes clinical characteristics and endocrine results. SGA girls had higher serum FSH levels than AGA girls (p = 0.03), while LH, estradiol, testosterone, and SHBG concentrations were similar.
As shown in Figure 1, SGA girls had a markedly reduced uterine cross-sectional area (mean difference 20%;p < 0.006) and strikingly reduced ovarian volumes (mean difference 38%;p < 0.0002). Uterine cervix dimensions were comparable (data not shown).
The differences in uterine and ovarian size between AGA and SGA girls remained similar and significant after matching AGA and SGA subpopulations for height, weight, and BMI, suggesting that the reduced size of the internal genitalia in SGA girls is essentially independent of the reduction of total body size.
DISCUSSION
Adolescent girls born small were found to have a reduced size of the uterus and the ovaria. This observation indicates either that fetal growth restriction may have a long-term impact on the size of the female internal genitalia, or that a common mechanism participates in the regulation of prenatal growth and the subsequent development of the internal genitalia.
The finding of reduced ovarian size after prenatal growth restriction complements recent insights linking growth before birth to ovarian function later in life. Reduced fetal growth has been associated with a reduced fraction of primordial follicles (1), with ovarian hyperandrogenism in adolescent girls (2), and with anovulation, particularly dating from late adolescence (3).
It has been firmly established that the prime factor accounting for the variability of birth weight of infants is the birth weight of the mother (12–14), and that women born SGA are at risk for delivering SGA and preterm infants (13, 14). However, the mechanisms governing these intergenerational effects remain elusive. Reduced prenatal growth was now found to be followed by a reduced size of the uterus. This may be one of the mechanisms underpinning the intergenerational effects of maternal birth weight. Indeed, mammalian embryo transfer and cross-breeding experiments have shown that the size of the conceptus at birth relates to the size of the maternal environment in which it grows (reviewed in Ref. (15). Conceivably, such a nongenomic mechanism provides a survival advantage, as it allows to adapt fetal growth in each generation, according to the growth conditions that the mother herself experienced as fetus.
Ovarian size was found to be reduced in SGA girls in the presence of elevated serum FSH concentrations, a combination pointing toward impaired ovarian responsiveness rather than to a lack of gonadotropic drive.
In conclusion, the gynecological correlates of prenatal growth restriction are herewith extended to include a reduced size of the uterus and the ovaries, and slightly increased serum concentrations of FSH. Disorders such as ovarian hyperandrogenism and ovulatory dysfunction in the presence of a small uterus and small ovaries may have a prenatal origin, particularly when occurring after precocious pubarche and in a context of short stature, hyperinsulinism, and dyslipidemia (2, 3, 16, 17).
Abbreviations
- AGA:
-
appropriate for gestational age
- SGA:
-
small for gestational age
- SHBG:
-
sex hormone-binding globulin
References
de Bruin JP, Dorland M, Bruinse HW, Spliet W, Nikkels PGJ, Te Velde ER 1998 Fetal growth retardation as a cause of impaired ovarian development. Early Human Dev 51: 39–46.
Ibáñez L, Potau N, Francois I, de Zegher F 1998 Precocious pubarche, hyperinsulinism and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 83: 3558–3662.
Ibáñez L, de Zegher F, Potau N 1999 Anovulation after precocious pubarche: early markers and time course in adolescence. J Clin Endocrinol Metab 84: 2691–2695.
Battaglia FC, Lubchenco LO 1967 A practical classification of newborn infants by weight and gestational age. J Pediatr 71: 159–163.
Hammer LD, Kraemer HC, Wilson DM, Reiter PL, Dornbusch SM 1991 Standardized percentile curves of body-mass index for children and adolescents. Arch Pediatr Adolesc Med 145: 259–263.
Ferriman D, Gallwey JD 1961 Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 21: 1440–1447.
Sample WF, Lippe BM, Gyepes MT 1977 Gray-scale ultrasonography of the normal female pelvis. Radiology 125: 477–483.
Griffin IJ, Cole TJ, Duncan KA, Hollman AS, Donaldson MDC 1995 Pelvic ultrasound measurements in normal girls. Acta Paediatr 84: 536–543.
Buzi F, Pilotta A, Dordoni D, Lombardi A, Zaglio S, Adlard P 1998 Pelvic ultrasonography in normal girls and in girls with pubertal precocity. Acta Paediatr 87: 1138–1145.
Ibáñez L, Potau N, Zampolli M, Prat N, Gussinyé M, Saenger P, Vicens-Calvet E, Carrascosa A 1994 Source localization of androgen excess in adolescent girls. J Clin Endocrinol Metab 79: 1778–1784.
Potau N, Ibáñez L, Sentis M, Carrascosa A 1999 Sexual dimorphism in the maturation of the pituitary-gonadal axis, assessed by gonadotropin-releasing hormone agonist challenge. Eur J Endocrinol 141: 27–34.
Ounsted M, Ounsted C 1968 Rate of intrauterine growth. Nature 220: 599–600.
Klebanoff MA, Meirik O, Berendes HW 1989 Second-generation consequences of small-for-dates birth. Pediatrics 84: 343–347.
Hennessy E, Alberman E 1998 Intergenerational influences affecting birth outcome. I. Birthweight for gestational age in the children of the 1958 British birth cohort. Paediatr Perinat Epidemiol 12( suppl 1): 60.
Brooks AA, Johnson MR, Steer PJ, Pawson ME, Abdalla HI 1995 Birth weight: nature or nurture?. Early Hum Dev 42: 29–35.
Ibáñez L, Potau N, de Zegher F 1999 Precocious pubarche, dyslipidemia and low IGFBP-1 in girls: relation to reduced prenatal growth. Pediatr Res 46: 320–322.
de Zegher F, Francois I, Ibáñez L 1999 Pediatric endocrinopathies related to reduced fetal growth. Growth Genet Horm 15: 1–5.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ibáñez, L., Potau, N., Enriquez, G. et al. Reduced Uterine and Ovarian Size in Adolescent Girls Born Small for Gestational Age. Pediatr Res 47, 575–577 (2000). https://doi.org/10.1203/00006450-200005000-00003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200005000-00003
This article is cited by
-
Assessment of Cytogenetic Findings and Risk Factors in Females with Primary Amenorrhea
Gynäkologie in der Praxis (2024)
-
Effects of early life adversity on maternal effort and glucocorticoids in wild olive baboons
Behavioral Ecology and Sociobiology (2021)
-
The Auxological and Metabolic Consequences for Children Born Small for Gestational Age
Indian Journal of Pediatrics (2021)
-
Relation of Birthweight and Ovarian and Uterine Size Prior to Menarche
Reproductive Sciences (2021)
-
Birth size and morphological femininity in adult women
BMC Evolutionary Biology (2020)